Abstract
Excess activation of glutamate receptors and production of free radicals including nitric oxide may result in severe and irreversible damage to the mammalian central nervous system (CNS), but endogenous defense systems that protect neurons from these insults are poorly understood. Here, we purified and isolated a neuroprotective substance, which has been named “serofendic acid,” from a lipophilic fraction of FCS based on the ability to protect rat primary cortical neurons against nitric oxide cytotoxicity. Mass spectrometry and NMR spectroscopy revealed the chemical structure of serofendic acid (15-hydroxy-17-methylsulfinylatisan-19-oic acid) as a sulfur-containing atisane-type diterpenoid, which is unique among known endogenous substances. Synthetic serofendic acid exhibited potent protective actions on cortical neurons against cytotoxicity of a nitric oxide donor as well as of glutamate, although it did not show appreciable influences on glutamate receptor-mediated responses in these neurons. Electron spin resonance analysis demonstrated that serofendic acid had no direct scavenging activity on nitric oxide radicals but was capable of inhibiting the generation of hydroxyl radical, a presumed “executor” radical in the nitric oxide-mediated neurotoxic cascade. These findings suggest that serofendic acid is a low-molecular-weight bioactive factor that promotes survival of CNS neurons, probably through the attenuation of free radical-mediated insults.
Glutamate, a major excitatory neurotransmitter in the central nervous system (CNS), is well known to exert detrimental effects on CNS neurons under pathophysiological conditions. Glutamate neurotoxicity is associated with various neurological disorders, including hypoxic-ischemic brain injury (1, 2), Alzheimer's disease (3, 4), Huntington's disease, and Parkinson's disease (5). CNS neurons are also vulnerable to insults caused by reactive oxygen species (ROS) and nitric oxide (NO), and these radicals are considered to play crucial roles in glutamate neurotoxicity associated with ischemic brain injury and a wide range of neurodegenerative disorders (6–9). In cultured cortical neurons, glutamate induces Ca2+ influx via the N-methyl-d-aspartate (NMDA) subtype of glutamate receptor channels. Elevation of intracellular Ca2+ triggers the formation of NO in NO synthase (NOS)-containing neurons. NO then diffuses out of NOS-containing cells and reaches the surrounding neurons, where it exerts potent cytotoxic actions with the aid of ROS (10–13). In fact, either pharmacological inhibition of NOS activity or scavenging of NO markedly attenuates glutamate-induced neurotoxicity (14, 15), suggesting that NO is a key molecule in NMDA receptor-mediated glutamate neurotoxicity in the cerebral cortex.
Considering the crucial roles of glutamate as a major neurotransmitter despite its robust neurotoxic actions, one would assume that various kinds of regulatory mechanisms in the CNS might serve to maintain physiological functions and prevent the pathogenic effects of glutamate. In this context, we have previously demonstrated that glutamate neurotoxicity on cultured CNS neurons is attenuated by diverse sets of endogenous substances, including nicotinic acetylcholine (16), prostanoids (17), and neurotrophins (18). We further hypothesized that, in addition to these established signaling molecules whose actions are probably mediated by membrane-associated receptors, distinct groups of yet unidentified substances may also play an important role in regulating glutamate neurotoxicity through mechanisms involving, for example, direct attenuation of NO and/or ROS-mediated cytotoxic consequences. Based on these assumptions, we explored neuroprotective substances of mammalian origin, and detected potent neuroprotective activity in the ether extract of fetal calf serum (FCS) that markedly attenuates cell death induced by glutamate and an NO donor, S-nitrosocysteine (SNOC), in cortical cultures (19). The present study was designed to isolate a neuroprotective factor from an ether extract of FCS, and here we identified a lipophilic low-molecular-weight substance that exerts potent neuroprotective actions at submicromolar concentrations.
Methods
Cell Culture and Bioassay.
Near-pure neuronal cultures were obtained from the cerebral cortices of fetal rats (17–19 days of gestation) as described (18). Each fraction obtained from the HPLC preparation was dissolved with diethyl ether and added into the culture medium before incubation at 37°C for 1 h to vaporize diethyl ether. SNOC and samples were simultaneously added to cultures for 1 h. The viability of cultures was evaluated by trypan blue exclusion (18, 19). Synthetic serofendic acid was dissolved in methanol (<0.1%) and diluted with the incubation medium immediately before the experiments. Methanol at this concentration does not affect cell viability under control conditions nor cytotoxicity induced by SNOC or glutamate. Glutamate neurotoxicity was assessed as described previously (18).
Chemical Analyses.
The infrared absorption spectra were obtained on an FT/IR-620 spectrometer (Jasco, Tokyo). The electrospray ionization (ESI) MS and ESI-MS/MS were performed on an API-III plus mass spectrometer (Applied Biosystems). HPLC separations were performed with an HP model 1090 (Agilent, Palo Alto, CA) or an LC-10A (Shimadzu) liquid chromatograph system. HPLC-MS experiments were conducted with the above HPLC systems interfaced with an API-III plus mass spectrometer. High-resolution mass spectra were measured on an LCmate (JEOL) mass spectrometer equipped with the ESI interface. The following NMR spectra were recorded either on a Unity INOVA 500 spectrometer (Varian) or a JNM-a600 spectrometer (JEOL) by using standard pulse sequences: 1H NMR spectrum; 13C NMR spectrum, COSY (chemical shift correlated spectroscopy) spectrum; NOESY (nuclear Overhauser enhancement and exchange spectroscopy) spectrum; TOCSY (total correlated spectroscopy); HMQC (heteronuclear multiple quantum coherence) spectrum; and HMBC (heteronuclear multiple bond correlation) spectrum. The NOESY spectrum was obtained at a mixing time of 1,000 ms. The HMBC experiment was performed to measure the coupling signals of 8 Hz. All of the data were collected at 30°C by using methanol-d4 as a solvent. Chemical shifts were referred to the solvent peaks: δH 3.35 for CD2HOD and δC 49.0 for CD3OD.
Large-Scale Purification.
After deactivation of enzymes, FCS (250 liters, lot no. 9G4001, JRH Biosciences, Lenexa, KS) was extracted twice with an equivalent volume of ethyl acetate. The ethyl acetate soluble portion was then concentrated in vacuo and partitioned between n-hexane and aqueous 90% methanol. The aqueous methanol layer was further partitioned between ethyl acetate and water to remove water-soluble ingredients. The lipid-soluble fraction was subjected to reversed-phase HPLC (YMC-pack ODS-AM, 20 mm × 250 mm, 5-μm particle size; YMC, Kyoto) using an acetonitrile/water with 0.1% trifluoroacetic acid (10→80% acetonitrile). Each of the resulting fractions was monitored by HPLC-MS, and the fractions containing the serofendic acids were pooled and concentrated. For the HPLC-MS analysis, semimicro condition (Inertsil ODS-3, 1.5 mm × 15 cm, 5-μm particle size; GL-Science, Tokyo) was used at a 0.1 ml/min flow rate of methanol/water with 2 mM ammonium acetate (63→89% methanol). Finally, the combined fraction was purified by preparative HPLC-MS (YMC-pack ODS-AM, 10 mm × 25 cm, 5-μm particle size; YMC, Kyoto) using methanol/water with 5 mM ammonium acetate (46→58% methanol). HPLC effluent (2.5 ml/min) was interfaced in the mass spectrometer with a 500:1 flow split for ion-spray spectrometry (selected ion monitoring on m/z 383). Fractions containing the serofendic acids were manually collected to yield 1.7 mg of serofendic acid A (1) and 1.4 mg of serofendic acid B (2).
Serofendic acid A (1).
HRESI(+)MS: (M+H)+ m/z 383.2256 (C21H35O4S, Δ 0.0 milli mass unit (mmu); ESI(+)MS/MS: m/z 383, 365, 347, 319, 301, 283, 255; IR (KBr, cm−1): 3432 (νO-H), 2927 (νC-H), 1698 (νC = O); 1H NMR (600 MHz, CD3OD): 2.99 (1H, dd, J = 13.0, 9.3 Hz, H17a), 2.97 (1H, d, J = 3.7 Hz, H15), 2.87 (1H, dd, J = 13.0, 6.1 Hz, H17b), 2.72 (3H, s, H21), 2.17 (1H, brd, J = 14.2 Hz, H3α), 2.06 (1H, ddd, J = 14.2, 11.7, 2.9 Hz, H14α), 1.96 (1H, m, H16), 1.93 (1H, m, H2β), 1.90 (1H, m, H6α), 1.83 (1H, m, H6β), 1.74 (1H, ddd, J = 13.2, 13.2, 4.5 Hz, H7β), 1.71 (1H, m, H12), 1.68 (1H, m, H13α), 1.66 (1H, m, H1α), 1.63 (1H, m, H11β), 1.61 (1H, m, H9), 1.45 (1H, m, H11α), 1.42 (1H, m, H2α), 1.42 (1H, m, H13β), 1.24 (3H, s, H18), 1.11 (1H, J = ddd, 13.2, 2.9, 2.9 Hz, H7α), 1.07 (1H, m, H3β), 1.07 (1H, m, H5), 1.00 (1H, m, H1β), 0.99 (3H, s, H20), 0.87 (1H, ddd, J = 14.2, 12.0, 6.6 Hz, H14β); 13C NMR (150 MHz, CD3OD): 181.7 (C19), 81.3 (C15), 60.9 (C17), 57.8 (C5), 44.7 (C4), 44.5 (C16), 42.8 (C9), 41.2 (C1), 39.3 (C3), 39.2 (C10), 38.8 (C21), 37.7 (C8), 34.3 (C7), 32.2 (C12), 30.0 (C11), 29.5 (C18), 28.2 (C14), 22.2 (C13), 20.9 (C6), 20.0 (C2), 13.4 (C20).
Serofendic acid B (2).
HRESI(+)MS: (M+H)+ m/z 383.2261 (C21H35O4S, Δ +0.5 mmu); ESI(+)MS/MS: m/z 383, 365, 347, 319, 301, 283, 255; IR (KBr, cm−1): 3420(νO-H), 2927 (νC-H), 1699 (νC = O); 1H NMR (500 MHz, CD3OD): 3.04 (1H, dd, J = 13.2, 6.8, H17a), 2.95 (1H, d, J = 4.4 Hz, H15), 2.91 (1H, dd, J = 13.2, 9.0 Hz, H17b), 2.73 (3H, s, H21), 2.18 (brd, J = 13.2 Hz, H3α), 2.08 (1H, ddd, J = 14.0, 11.7, 3.0 Hz, H14α), 1.96 (1H, m, H2β), 1.92 (1H, m, H16), 1.90 (1H, m, H6α), 1.83 (1H, m, H6β), 1.79 (1H, m, H12), 1.73 (1H, ddd, J = 13.2, 13.2, 4.4 Hz, H7β), 1.66 (1H, m, H1α), 1.66 (1H, m, H13α), 1.63 (1H, m, H11β), 1.62 (1H, m, H9), 1.46 (1H, m, H11α), 1.44 (1H, m, H13β), 1.42 (1H, m, H2α), 1.23 (3H, s, H18), 1.10 (1H, ddd, J = 13.2, 2.9, 2.9 Hz, H7α), 1.06 (1H, m, H3β), 1.06 (1H, m, H5), 1.00 (1H, m, H1β), 0.99 (3H, s, H20), 0.86 (1H, ddd, J = 14.0, 12.2, 6.5 Hz, H14β); 13C NMR (125 MHz, CD3OD): 181.0 (C19), 81.2 (C15), 59.8 (C17), 57.9 (C5), 44.8 (C4), 44.3 (C16), 42.9 (C9), 41.3 (C1), 39.4 (C3), 39.2 (C10), 38.3 (C21), 37.7 (C8), 34.3 (C7), 30.8 (C12), 29.9 (C11), 29.6 (C18), 28.4 (C14), 21.9 (C13), 21.0 (C6), 20.1 (C2), 13.5 (C20).
Electrophysiology.
Recordings of whole cell currents were performed as described (20). The bathing solution contained (in mM) 145 NaCl, 5 KCl, 2 CaCl2, 10 Hepes, 10 d-glucose at pH 7.3, whereas the micropipette (internal) solution was composed of (in mM) 145 CsCl, 11 cesium ethylene glycol bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid (CsEGTA), 4 MgATP, and 10 Hepes at pH 7.3. Patch microelectrodes had a resistance of 5–6 MΩ. Fast application of the drug was rendered possible by the use of an array of seven capillary tubes (280-μm inner diameter) placed near the cell. Glycine (10 μM) was added to the NMDA-containing solution. The whole cell currents were recorded at 20–25°C.
ESR Analysis.
NO⋅ and hydroxyl radical (⋅OH) were detected by ESR spectrometry in an in vitro system generating NO⋅ and ⋅OH as described (21). The NO⋅ donor 3-(2-hydroxy-1-methyl-2-nitrosohydrazino)-N-methyl-1-propanamin (NOC7; 5 μM) and 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazole-1-oxyl 3oxide (carboxy-PTIO; 5 μM), as a spin-trapping agent, were used for detection of NO⋅. The amount of carboxy-PTI spin adduct was measured 90 min after mixing the drugs dissolved in methanol with the solution containing NOC7 and carboxy-PTIO. For detection of ⋅OH, the signal intensity was evaluated by the relative peak of the second signal of the quartet of the 5,5-dimethyl-1-pyrroline-N-oxide (DMPO)/OH spin adduct to the intensity of Mn2+ used as an internal standard 3 min after mixing solutions containing FeSO4 (25 μM), H2O2 (50 μM), and DMPO (5 mM).
Results
Purification of Serofendic Acids.
We first explored the active compounds in a relatively small amount of FCS, by testing the effects of HPLC fractions from the ether extracts of FCS in an in vitro bioassay with cultured cortical neurons. Attention was focused on the acute SNOC neurotoxicity assay, because neuroprotective action can be more potently detected than in the glutamate neurotoxicity assay. Lipophilic substances in 5 liters of heat-inactivated FCS (JRH Biosciences) were extracted with diethyl ether, and the collected organic layer was evaporated. The residue was subjected to reversed-phase HPLC with a C18 column and a water/acetonitrile solvent system (mobile-phase A, 990:10; mobile-phase B, 10:990) containing 0.1% trifluoroacetic acid under a gradient of 0–100% of mobile-phase B. The active fraction eluted with ≈45–55% of mobile-phase B was collected and subjected to a second purification step. In the second purification by HPLC with a water/methanol solvent system (mobile-phase A, 900:100; mobile-phase B, 10:990) containing 2 mM ammonium acetate under a gradient of 25–100% concentration of mobile-phase B, activity was observed in the fraction with ≈40–50% of mobile-phase B (Fig. 1A). As distinct peaks of m/z 383 under positive ESI [ESI(+)] were detected by HPLC-MS analysis (Fig. 1B), this fraction was subjected to further purification steps. After the third preparative HPLC, where the conditions were similar to the second purification step except the gradient was 40–50%, the final purification was performed by preparative HPLC-MS (22) with an isocratic mode of 20% acetonitrile containing 0.1% trifluoroacetic acid. A pair of peaks, both of which showed prominent ions at m/z 383 under ESI(+), were detected. The results revealed the presence of two compounds with the same molecular weight of 382 in the active fraction.
Figure 1.
HPLC purification and MS analysis of active fraction from ether extract of FCS. (A) HPLC-UV (250 nm) chromatogram of the second purification step and the protective activity of fractions 10–14. For measurements of protective activity, FCS was extracted with ether and then subjected to the two-step purification. The residue from each fraction, whose amount corresponded to that derived from 300 ml of FCS, was dissolved in 2.5 ml of culture medium and added to the culture for 1 h with SNOC (300 μM). C, control. Values are expressed as the mean ± SE. **, P < 0.01, compared with SNOC alone (Dunnett's two-tailed test). (B) HPLC-MS (m/z 383) chromatogram of the second purification step.
To determine the chemical structure of the isolated compounds, we performed a large-scale extraction from 250 liters of FCS as described in Methods. Two peaks of prominent ions at m/z 383 under ESI(+) in the purified fraction with final yields of 1.7 mg and 1.4 mg were confirmed. The compounds were named “serofendic acid” because they were isolated from serum (sero-), displaying cytoprotective effect (-fend) while possessing carboxylic acid. The two compounds were tentatively called serofendic acid A (1) and B (2).
Structure Elucidation of Serofendic Acid A.
The molecular formula of serofendic acid A (1) was determined to be C21H34O4S by high-resolution ESI-MS, indicating five degrees of unsaturation. The 1H NMR spectrum revealed the alicyclic nature of 1, and one of the characteristic features was a singlet methyl signal observed at δH 2.72 ppm. This signal was assigned to a methylsulfinyl group (CH3SO–) based on a chemical shift and the elemental composition of 1. The 1H NMR, 13C NMR, and HMQC spectra (23) of 1 showed the presence of three methyl, nine methylene, five methine, and four quaternary carbon groups, accounting for 21 carbons and 32 protons. One of the methine carbons was assigned to a secondary alcohol on the basis of chemical shift (δC 81.3 ppm) and the neutral loss of H2O (m/z 365) observed in the ESI-MS/MS. In addition, the presence of carbonyl group was indicated by the 13C NMR spectrum (δC 181.7 ppm) and the IR stretching band at 1698 cm−1. These observations account for C21H33O3S of the molecular formula (C21H34O4S). Because the 13C NMR spectrum of 1 excluded the possibility of additional hydroxyl or ether groups, the remaining OH unit was assigned to a carboxyl group. Because only one of five unsaturations was explained by these functional groups, 1 must be tetracyclic.
Interpretation of NMR data from 1H, 13C, COSY, TOCSY, and HMQC experiments led to proton and carbon assignments of three structural fragments a–c (outlined in bold face in Fig. 2A). In the HMBC spectrum, the methyl proton (δH 2.72 ppm) showed a correlation to one of the terminal carbons of fragment c (δC 60.9 ppm); thus, the methylsulfinyl group was located on C17. HMBC correlations also demonstrated the connection of three termini of fragment c (C9, C14, C15) via a quaternary carbon (δC 37.7 ppm, C8), indicating the presence of a bicyclo[2.2.2]octane ring. In a similar manner, interpretation of HMBC data established the connectivity of the partial structures and led to the planar structure of 1 (Fig. 2C). The structure of serofendic acid A was thus characterized to be an atisane-type diterpenoid (24), 15-hydroxy-17-methylsulfinylatisan-19-oic acid.
Figure 2.
Structure elucidation of serofendic acid A. (A) Construction of planar structure. Partial structures a–c established by 1H NMR, 13C NMR, COSY, TOCSY, and HMQC data sets are outlined in bold faces. Bold arrows represent the observed HMBC correlations (selected). (B) Determination of relative stereochemistry. Broken arrows represent the observed NOESY correlations (selected). (C) Chemical structures of serofendic acid A (1) and B (2); 1 and 2 are the epimer at the sulfoxide group.
The relative stereochemistry of 1 was inferred from the data of NOESY and the 1H–1H coupling constants. A vicinal coupling constant of 3.7 Hz observed for H15–H16 indicated the transsubstitution of the methylsulfinylmethyl and the hydroxyl groups. Key NOESY correlations are schematically represented in Fig. 2B. These correlations unambiguously established the relative configuration at the tetracyclic moiety of 1.
Structure Elucidation of Serofendic Acid B.
High resolution ESI-MS indicated that 2 was isomeric to serofendic acid A (1). The 1H NMR spectrum of 2 was almost identical with that of 1 but slightly different in the proton signals adjacent to the sulfoxide group. NMR studies (1H, 13C, COSY, TOCSY, HMQC, and HMBC) confirmed that 2 had the same planar structure with that of 1. Furthermore, interpretation of NOESY data indicated that 1 and 2 had the same relative configuration at the atisane-skeleton. Therefore, it was suggested that 1 and 2 are epimers having the opposite configuration at the sulfoxide group.
Synthesis.
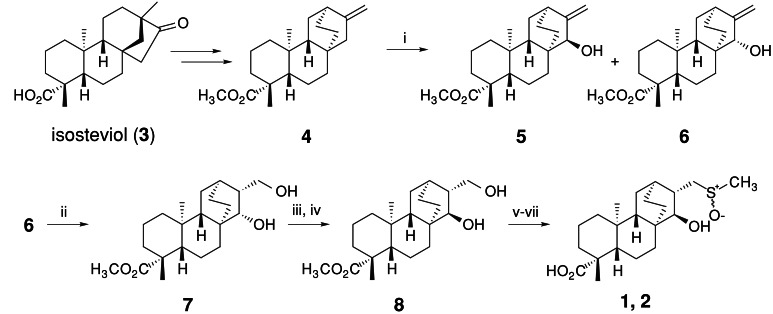
The synthesis of serofendic acids is outlined in Scheme S1. Starting from (−)-isosteviol, 10-step derivatizations according to Coates's protocol (25) yielded the intermediate 4. The Coates's exo-olefin (4) was then oxidized to a mixture of alcohols 5 and 6 by selenium oxide. The stereochemistries of 5 and 6 were confirmed by NOESY experiments, and isomer 6 was then hydroborated to give desirable syn-diastereomer 7 in 84% yield. Inversion of the C15-hydroxyl group in 7 was achieved by selective oxidation of the secondary alcohol by using NaBrO3-NaSO3 (26), followed by NaB(OAc)3H reduction to produce diol 8 in 67% overall yield. The primary alcohol in 8 was tosylated and then converted to methylsulfide by NaSMe in 68% overall yield. Under these conditions, the methyl ester group was hydrolyzed to give carboxylic acid. Finally, the sulfide was oxidized by Davis's oxaziridine (27), which quantitatively yielded serofendic acid A (1) and B (2) (1:2 = 1:2). These isomers were separated by HPLC, and the physicochemical properties of synthetic isomers were found to be identical with natural isomers, thus confirming the structure of serofendic acids. Details of synthetic studies will be published elsewhere.
Scheme 1.
Synthesis of serofendic acids. i: SeO2, TBHP, CH2Cl2, rt, 5 h, 5 (26%), 6 (28%); ii: BH3-THF/THF, rt, 3 h, then H2O2, NaOH aq (84%); iii: NaBrO3-NaHSO3, CH3CN, H2O, rt, 2 h; iv: NaB(OAc)3H, AcOH, CH3CN, 0°C, 5 h, (67% from 6); v: TsCl, DMAP, pyridine, rt, 24 h; vi: NaSMe/HMPA, 80°C, 48 h; vii: Davis's oxaziridine, CHCl3, 0°C, 30 min (68% from 8)
Neuroprotective Activities.
The effect of synthetic serofendic acid on the acute neurotoxicity induced by SNOC was examined. Treatment of cortical cultures with 100 μM SNOC for 1 h resulted in a marked decrease in the percentage of viable neurons. Concurrent application of serofendic acid A and B, as well as the epimeric mixture of serofendic acid at a concentration of 10 μM, potently and significantly attenuated SNOC neurotoxicity with a similar potency (Fig. 3A). Therefore, the epimeric mixture was used in the following experiments. We have previously shown that a marked reduction of cell viability is induced by exposure of cortical cultures to glutamate for 10 min followed by 24-h incubation with glutamate-free medium, and that a NOS inhibitor prevents the glutamate neurotoxicity (18). Accordingly, we assessed potential protective actions of serofendic acid against glutamate neurotoxicity with this procedure. We observed marked neuroprotective activity of serofendic acid when it was applied to cortical cultures for 1 h before, during 10-min glutamate application, and after 24-h incubation with glutamate-free medium. Serofendic acid at a concentration as low as 100 nM exhibited a prominent protective effect against glutamate insults (Fig. 3 B and C).
Figure 3.
Protective action of synthetic serofendic acid in cultured cortical neurons. (A) Effect of serofendic acid on SNOC neurotoxicity. Cultures were exposed to SNOC (100 μM) alone or SNOC plus serofendic acid (10 μM) for 1 h. **, P < 0.01, compared with SNOC alone. C, control; S-A, serofendic acid A; S-B, serofendic acid B; Mix, epimeric mixture of serofendic acid. (B) Protective effect of the epimeric mixture of serofendic acid on glutamate (Glu) neurotoxicity. Cultures were exposed to Glu (500 μM) for 10 min and then incubated in Glu-free medium for 24 h. Cultures were treated with serofendic acid for 1 h before, 10 min during, and 24 h after Glu exposure. **, P < 0.01, compared with Glu alone. (C) Hoffman modulation contrast photomicrographs showing the effects of serofendic acid (S) at 100 nM on Glu neurotoxicity. Cultures treated as in B were photographed after trypan blue staining followed by fixation with formalin. Note a marked decrease of trypan blue-stained cells in the culture treated with Glu plus S, compared with that treated with Glu alone. Calibration bar = 50 μm.
An NMDA subtype of glutamate receptors is considered to play a predominant role in triggering glutamate neurotoxicity (7, 9). Numerous mechanisms are known to regulate the activities of NMDA receptor channels, which possess several binding sites for ligands and modulator molecules in their structure (28). Therefore, it is possible that serofendic acid directly binds to NMDA receptors and inhibits the activity of receptor channels, thereby attenuating glutamate neurotoxicity. Conventional whole-cell recordings demonstrated a robust inward current after application of 30 μM NMDA to cortical neurons voltage-clamped at −60 mV (Fig. 4). Application of 10 μM serofendic acid for 10 s before and during NMDA exposure had no significant effects on NMDA-evoked currents (97.3 ± 1.5% of control, n = 4). We also examined the effects of serofendic acid on whole-cell currents evoked by selective agonists of other ionotropic glutamate receptors, α-amino-3-hydroxy-5-methylisoxazole-4-propionate (AMPA) and kainite. Serofendic acid showed no effects on either AMPA- or kainate-evoked currents (99.7 ± 1.7% of control for AMPA-evoked currents, n = 4; 100.18 ± 2.0% of control for kainate-evoked currents, n = 4). These results indicate that the neuroprotective effects of serofendic acid do not involve the inhibition of the activities of glutamate receptor channels.
Figure 4.
Null effects of serofendic acid on glutamate receptor-mediated current responses in cultured cortical neurons. Whole cell currents induced by 10-s application of NMDA (30 μM) were recorded at a holding potential of −60 mV in the absence and presence of serofendic acid (10 μM).
Because isolation and identification of serofendic acid were based on its ability to attenuate neurotoxicity of NO, one of the most plausible mechanisms of the neuroprotective actions of serofendic acid is direct scavenging of NO by the compound. To examine this possibility, we performed a cell-free assay to detect potential NO-scavenging activity of serofendic acid by using ESR spectroscopy. We used carboxy-PTIO as an NO-selective probe. Addition of an NO donor, NOC7, to carboxy-PTIO solution caused prominent changes in ESR signals, indicating the generation of NO from NOC7 and its reaction with carboxy-PTIO. Importantly, the presence of serofendic acid in the mixture did not influence the ESR signals, arguing against the proposal that serofendic acid may scavenge NO (Fig. 5A).
Figure 5.
Effects of serofendic acid on NO and hydroxyl radical formation. (A) Spin trapping ESR signals obtained in the reaction mixture of 5 μM NOC7 and 5 μM carboxy-PTIO for evaluating NO-scavenging effect of serofendic acid. NOC7-induced changes in ESR signals of carboxy-PTIO (Left and Center) were not affected by addition of serofendic acid (Right). (B) Traces show typical spectra of a spin adduct (DMPO/OH) obtained by reaction of 25 μM Fe2+ and 50 μM H2O2. Addition of serofendic acid in the reaction mixture markedly attenuated the signal of DMPO/OH spin adducts. The graph summarizes the effects of serofendic acid on the signal intensity. **, P < 0.01, compared with control (C).
It has been postulated that NO yields peroxynitrite anion (ONOO−) by reaction with superoxide anion, and that rapid degradation of ONOO− produces ⋅OH, which in turn induces potent cytotoxicity (7, 29, 30). Therefore, scavenging of ⋅OH may represent a mechanism of the protective actions of serofendic acid. ESR spectroscopy was again used for examining the possible reaction of serofendic acid with ⋅OH. In this experiment, ⋅OH radicals generated by a Fenton reaction involving Fe2+ and H2O2 were trapped with DMPO, and the DMPO/OH signal was analyzed. As shown in Fig. 5B, addition of serofendic acid to the reaction mixture markedly and significantly reduced the intensity of DMPO/OH signal in a concentration-dependent manner.
Discussion
In this study, we isolated neuroprotective compounds named serofendic acid from FCS. It is a low-molecular-weight substance (mw 382) of atisane-type diterpenoid bearing a methylsulfoxide group. To our knowledge, the discovery of the atisane-derivative serofendic acid has not previously been documented in animals, although natural atisane-derivatives contained in plants have been reported (31, 32). Large-scale extraction of serofendic acid from 250 liters of FCS yielded 1.7 mg and 1.4 mg of the purified epimers. Even if we ignored possible losses during purification processes, the concentrations of the epimers in FCS were estimated to be no less than 18 and 15 nM, respectively. Therefore, the concentration of the active compound contained in FCS was probably higher than the order of some tens of nM. In our in vitro bioassay, serofendic acid showed a marked inhibition of glutamate neurotoxicity when applied at a concentration as low as 100 nM. Thus, this lipophilic low-molecular-weight substance is likely to play a significant role in preventing glutamate neurotoxicity in the brain at embryonic stages. The discovery of serofendic acid from FCS as a cyclic diterpenoid in mammals indicates the possibility that this substance is a member of a novel family of endogenous products of fetal mammalian organs. The responsible organ(s) for production and the biosynthetic pathways of serofendic acid remain to be determined.
The striking property of serofendic acid as a bioactive factor is that it showed marked inhibition of delayed neuronal death induced by brief glutamate exposure at low concentrations. Obviously, further studies are warranted to determine the detailed mechanisms of neuroprotective actions of serofendic acid. Although serofendic acid exists in FCS as an epimeric mixture, both isomers showed similar protective activities against SNOC neurotoxicity, indicating that the opposite configuration at the sulfoxide group does not exert an influence on neuroprotective activity. In addition, serofendic acid did not block glutamate receptor-mediated currents in cortical neurons despite its pronounced activity in preventing glutamate neurotoxicity. However, suppression of intracellular ROS generation may constitute an important mechanism of neuroprotective actions of serofendic acid, because the compound exhibited ⋅OH-scavenging activity in ESR analysis. This finding is consistent with the fact that the compound possesses a methylsulfoxide group in its structure that is reported to have the ability to scavenge ⋅OH (33, 34). An important finding is that serofendic acid did not show any direct scavenging activity against NO. These observations provide an intriguing possibility that serofendic acid selectively attenuates NO-triggered neurotoxic events without interfering in the physiological functions of NO as an important signaling molecule.
In conclusion, we propose that serofendic acid functions as a potent, endogenous neuroprotective factor. This substance may play a crucial role in the CNS by attenuating cytotoxic consequences induced by radical stress on overactivation of neuronal glutamate receptor channels. The discovery of serofendic acid may provide a new scope for the investigation of low-molecular-weight bioactive factors that promote survival of CNS neurons.
Acknowledgments
We are grateful to Dr. Takahashi, Y. of JEOL Co. Ltd. for assistance with the HRMS measurements. This study was supported in part by a Grant-in-Aid for Scientific Research and that on priority areas from the Ministry of Education, Culture, Science, Sports and Technology, Japan.
Abbreviations
- CNS
central nervous system
- NMDA
N-methyl-d-aspartate
- SNOC
S-nitrosocysteine
- ESI
electrospray ionization
- ROS
reactive oxygen species
- NOS
NO synthase
- COSY
chemical shift correlated spectroscopy
- NOESY
nuclear Overhauser enhancement and exchange spectroscopy
- TOCSY
total correlated spectroscopy
- HMQC
heteronuclear multiple quantum coherence
- HMBC
heteronuclear multiple bond correlation
- FCS
fetal calf serum
References
- 1.Choi D W. Trends Neurosci. 1988;11:465–469. doi: 10.1016/0166-2236(88)90200-7. [DOI] [PubMed] [Google Scholar]
- 2.Meldrum B, Garthwaite J. Trends Pharmacol Sci. 1990;11:379–387. doi: 10.1016/0165-6147(90)90184-a. [DOI] [PubMed] [Google Scholar]
- 3.Koh J-Y, Yang L L, Cotman C W. Brain Res. 1990;533:315–420. doi: 10.1016/0006-8993(90)91355-k. [DOI] [PubMed] [Google Scholar]
- 4.Le W-D, Colom L V, Xie W-J, Smith R G, Alexianu M, Appel S H. Brain Res. 1995;686:49–60. doi: 10.1016/0006-8993(95)00450-5. [DOI] [PubMed] [Google Scholar]
- 5.Sawada H, Shimohama S, Tamura Y, Kawamura T, Akaike A, Kimura J. J Neurosci Res. 1996;43:55–62. doi: 10.1002/jnr.490430107. [DOI] [PubMed] [Google Scholar]
- 6.Coyle J T, Puttfarcken P. Science. 1993;262:689–695. doi: 10.1126/science.7901908. [DOI] [PubMed] [Google Scholar]
- 7.Dawson V L, Dawson T M, Bartley D A, Uhl G R, Snyder S H. J Neurosci. 1993;13:2651–2261. doi: 10.1523/JNEUROSCI.13-06-02651.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Olanow C W. Trends Neurosci. 1993;16:439–444. doi: 10.1016/0166-2236(93)90070-3. [DOI] [PubMed] [Google Scholar]
- 9.Dugan L L, Sensi S L, Canzoniero L M, Handran S D, Rothman S M, Lin T S, Goldberg M P, Choi D W. J Neurosci. 1995;15:6377–6688. doi: 10.1523/JNEUROSCI.15-10-06377.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Akaike A, Tamura Y, Terada K, Nakata N. Prog Brain Res. 1994;103:391–403. doi: 10.1016/s0079-6123(08)61153-x. [DOI] [PubMed] [Google Scholar]
- 11.Dawson V L, Dawson T M, London E D, Bredt D S, Snyder S H. Proc Natl Acad Sci USA. 1991;88:6368–6371. doi: 10.1073/pnas.88.14.6368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang Z, Huang P L, Panahian N, Dalkara T, Fishman M C, Moskowitz M A. Science. 1994;265:1883–1885. doi: 10.1126/science.7522345. [DOI] [PubMed] [Google Scholar]
- 13.Garthwaite G, Garthwaite J. Neuropharmacology. 1994;33:1431–1438. doi: 10.1016/0028-3908(94)90046-9. [DOI] [PubMed] [Google Scholar]
- 14.Tamura Y, Sato Y, Akaike A, Shiomi H. Brain Res. 1992;592:317–325. doi: 10.1016/0006-8993(92)91691-7. [DOI] [PubMed] [Google Scholar]
- 15.Dawson T M, Dawson V L, Snyder S H. Ann Neurol. 1992;32:297–311. doi: 10.1002/ana.410320302. [DOI] [PubMed] [Google Scholar]
- 16.Akaike A, Tamura Y, Yokota T, Shimohama S, Kimura J. Brain Res. 1994;644:181–187. doi: 10.1016/0006-8993(94)91678-0. [DOI] [PubMed] [Google Scholar]
- 17.Akaike A, Kaneko S, Tamura Y, Nakata N, Shiomi H, Ushikubi F, Narumiya S. Brain Res. 1994;663:237–243. doi: 10.1016/0006-8993(94)91268-8. [DOI] [PubMed] [Google Scholar]
- 18.Kume T, Kouchiyama H, Kaneko S, Maeda T, Kaneko S, Akaike A, Shimohama S, Kihara T, Kimura J, Wada K, Koizumi S. Brain Res. 1997;756:200–204. doi: 10.1016/s0006-8993(97)00195-9. [DOI] [PubMed] [Google Scholar]
- 19.Kume T, Kohchiyama H, Nishikawa H, Maeda T, Kaneko S, Akaike A, Noda N, Fujita T. Jpn J Pharmacol. 1997;73:371–374. doi: 10.1254/jjp.73.371. [DOI] [PubMed] [Google Scholar]
- 20.Ujihara H, Albuquerque E X. J Pharmacol Exp Ther. 1992;263:859–867. [PubMed] [Google Scholar]
- 21.Gomez-Vargas M, Nishibayashi-Asanuma S, Asanuma M, Kondo Y, Iwata E, Ogawa N. Brain Res. 1998;790:202–228. doi: 10.1016/s0006-8993(97)01521-7. [DOI] [PubMed] [Google Scholar]
- 22.Oda Y, Mano N, Asakawa N. Anal Biochem. 1995;231:141–150. doi: 10.1006/abio.1995.1513. [DOI] [PubMed] [Google Scholar]
- 23.Croasmun W A, Carlson R M K. Two-Dimensional NMR spectroscopy. New York: VCH; 1994. [Google Scholar]
- 24.Devon T K, Scott A I. Handbook of Naturally Occurring Compounds. II. New York: Academic; 1972. [Google Scholar]
- 25.Coates R M, Bertram E F. J Org Chem. 1971;36:2625–2631. [Google Scholar]
- 26.Sakaguchi S, Kikuchi D, Ishii Y. Bull Chem Soc Jpn. 1997;70:2561–2566. [Google Scholar]
- 27.Davis F A, Lamendola J J, Nadir U, Kliger E W, Sedergan T C, Panunto T W, Billmers R, Jenkins J R, Turchi I J, Watson W H, Chen J S, Kimura M. J Am Chem Soc. 1980;102:2000–2005. [Google Scholar]
- 28.Cull-Candy S, Brickley S, Farrant M. Curr Opin Neurobiol. 2001;11:327–335. doi: 10.1016/s0959-4388(00)00215-4. [DOI] [PubMed] [Google Scholar]
- 29.Beckman J S, Beckman T W, Chen J, Marshall P A, Freeman B A. Proc Natl Acad Sci USA. 1990;87:1620–1164. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lipton S A, Choi Y B, Pan Z H, Lei S Z, Chen H S, Sucher N J, Loscalzo J, Singel D J, Stamler J S. Nature (London) 1993;364:626–632. doi: 10.1038/364626a0. [DOI] [PubMed] [Google Scholar]
- 31.Appendino G, Belloro E, Tron G C, Jakupovic J, Ballero M. Fitoterapia. 2000;71:134–142. doi: 10.1016/s0367-326x(99)00134-3. [DOI] [PubMed] [Google Scholar]
- 32.Lal A R. Tetrahedron Lett. 1989;30:3205–3208. [Google Scholar]
- 33.Littlefield L G, Joiner E E, Colyer S P, Sayer A M, Frome E L. Int J Radiat Biol. 1988;53:875–890. doi: 10.1080/09553008814551241. [DOI] [PubMed] [Google Scholar]
- 34.Watanabe M, Suzuki M, Suzuki K, Hayakawa Y, Miyazaki T. Radiat Res. 1990;124:73–78. [PubMed] [Google Scholar]