Abstract
1. 45Ca fluxes were studied in normal and potassium-depolarized goldfish ventricles as a function of the external Na concentration. Some of the experiments were also performed on guinea-pig auricles.
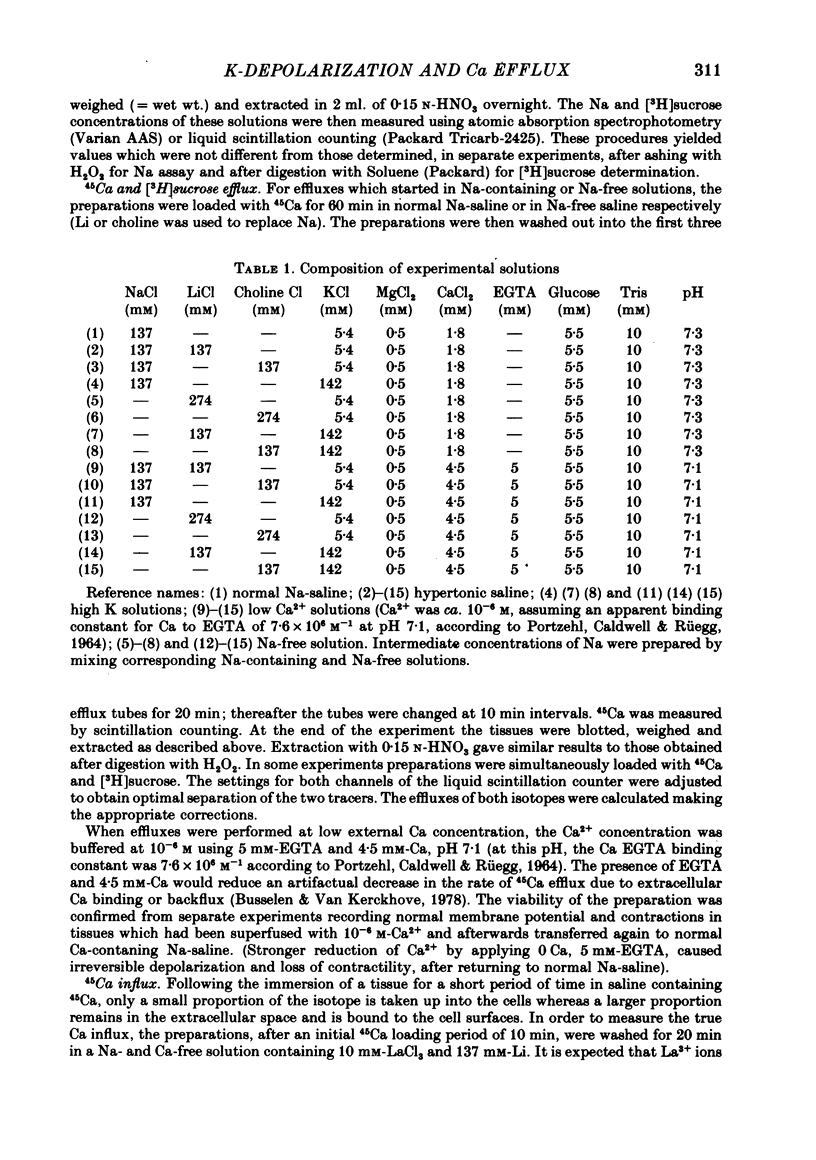
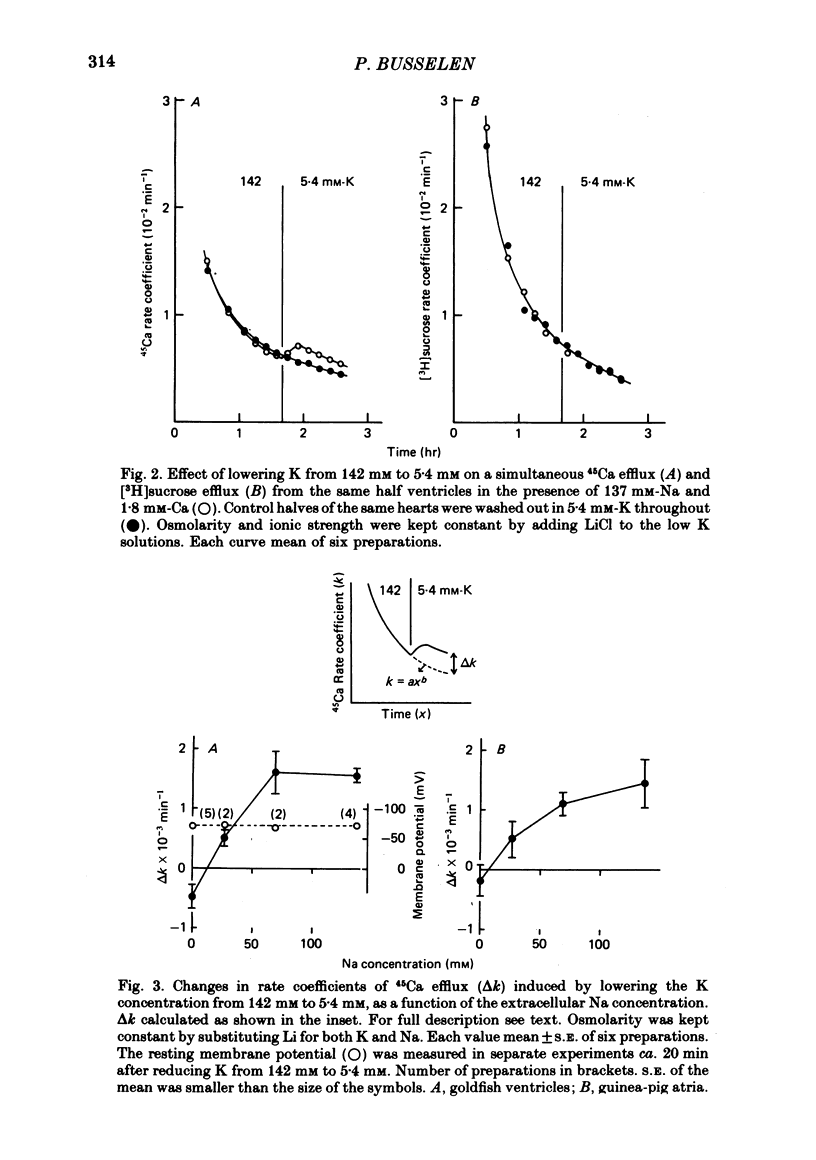
2. When the external K concentration was increased from 5·4 to 142 mM, keeping osmolarity constant by adding 137 mM-Li or choline (hyperosmotically) to the low K solution, the 45Ca efflux was reversibly inhibited, whereas the [3H]sucrose efflux was unaffected.
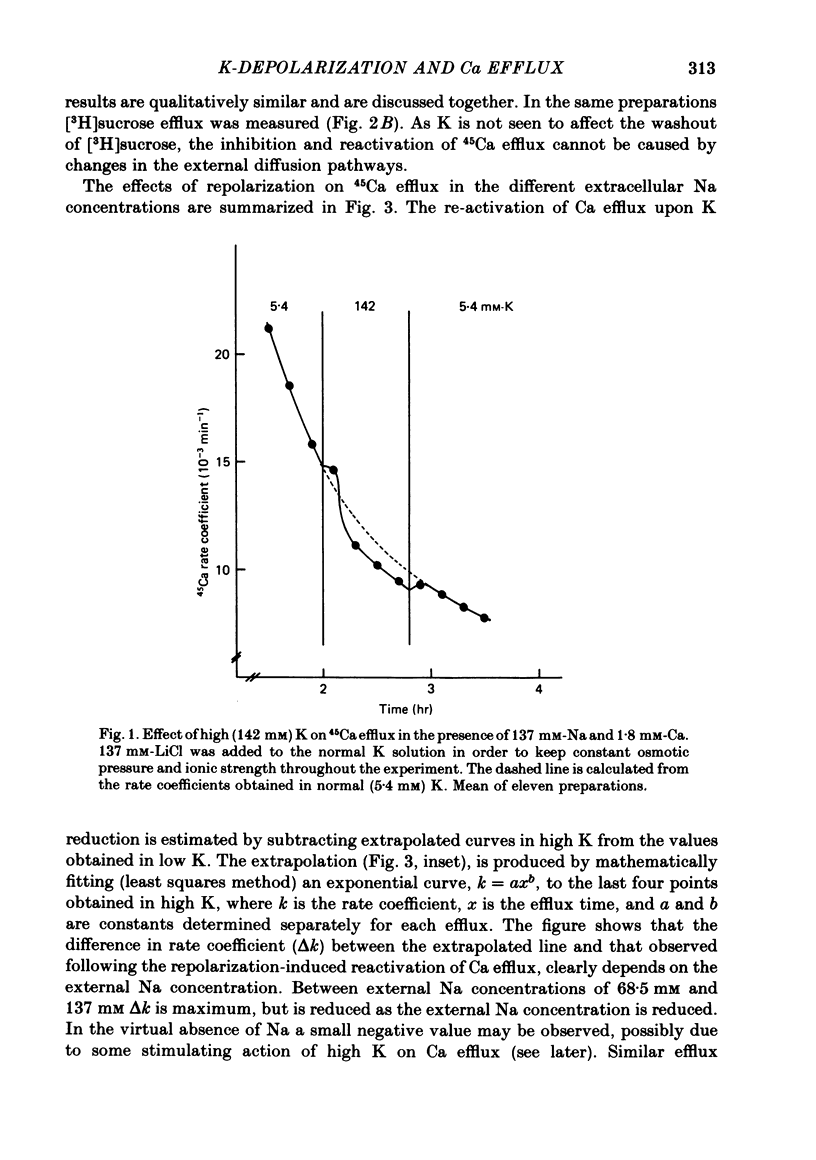
3. Goldfish ventricles, which have been depolarized with 142 mM-K for 100 min, repolarized within 20 min, from ca. -15 mV to ca. -70 mV, following the application of 5·4 mM-K. This repolarization was independent of the presence of external Na. During the repolarization the 45Ca efflux was reactivated. This reactivation, however, depended on the external Na concentration. Comparable results were obtained in guinea-pig atria.
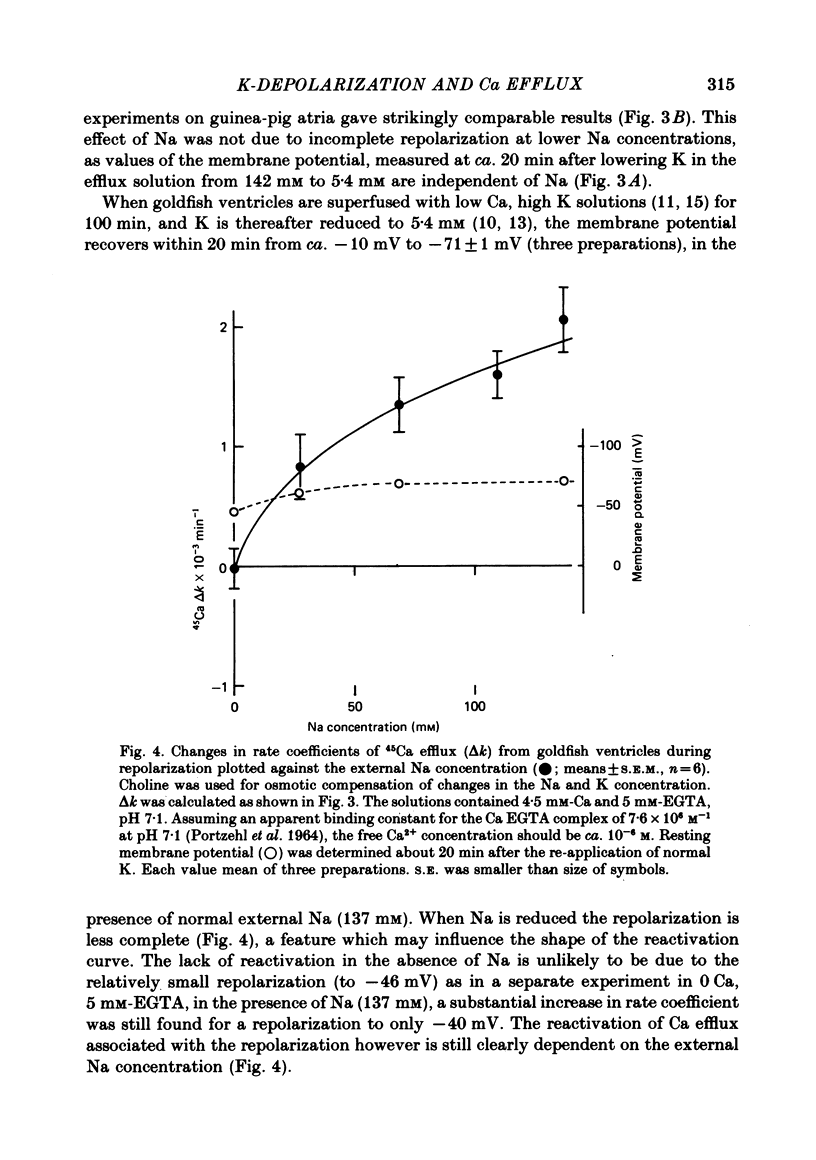
4. A similar repolarization and Na-dependent reactivation of 45Ca efflux was obtained in goldfish ventricles superfused with 10-6 M-Ca2+ (4·5 mM-Ca, 5 mM-EGTA, pH 7·1), provided that the 45Ca washout was started in high K.
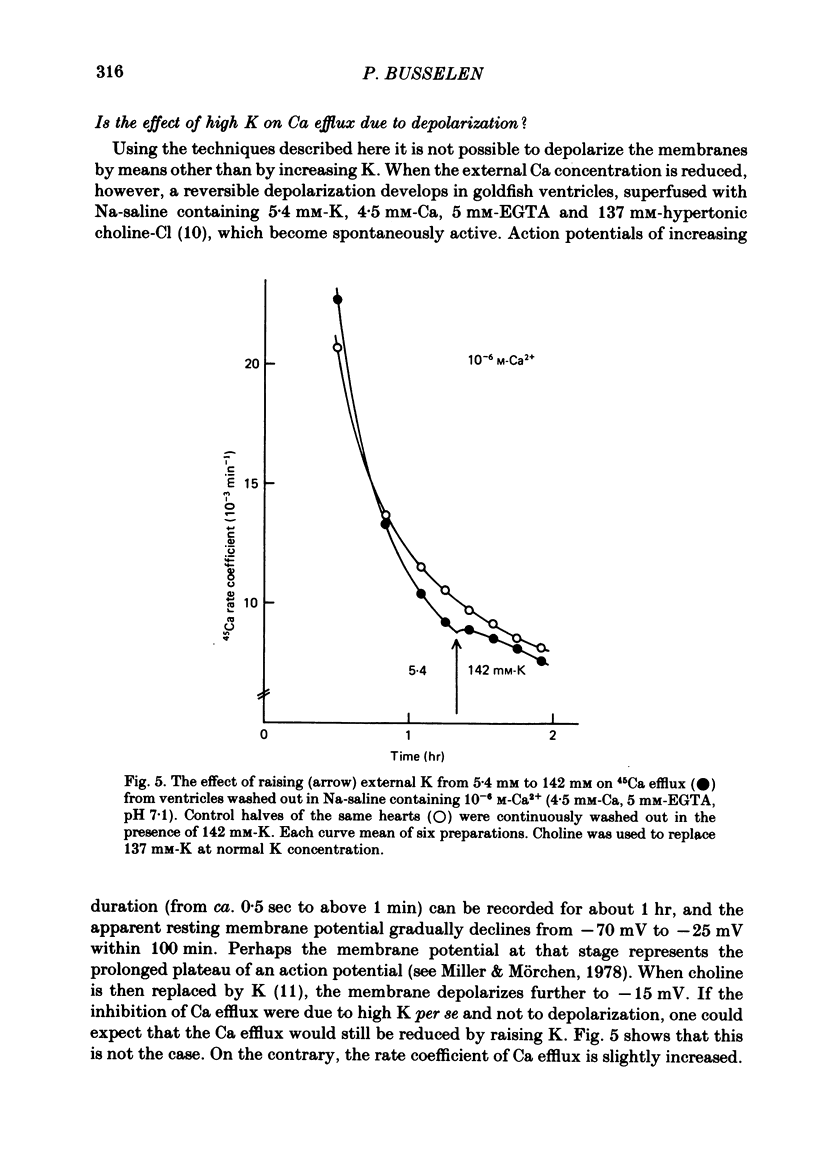
5. In 10-6 M-Ca2+, 137 mM-Na, 5·4 mM-K and 137 mM-choline goldfish ventricles depolarized to about -25 mV within 80 min. If the choline was now replaced by 137 mM-K, the membrane potential moved to ca. -15 mV, and under these conditions the 45Ca efflux was slightly increased.
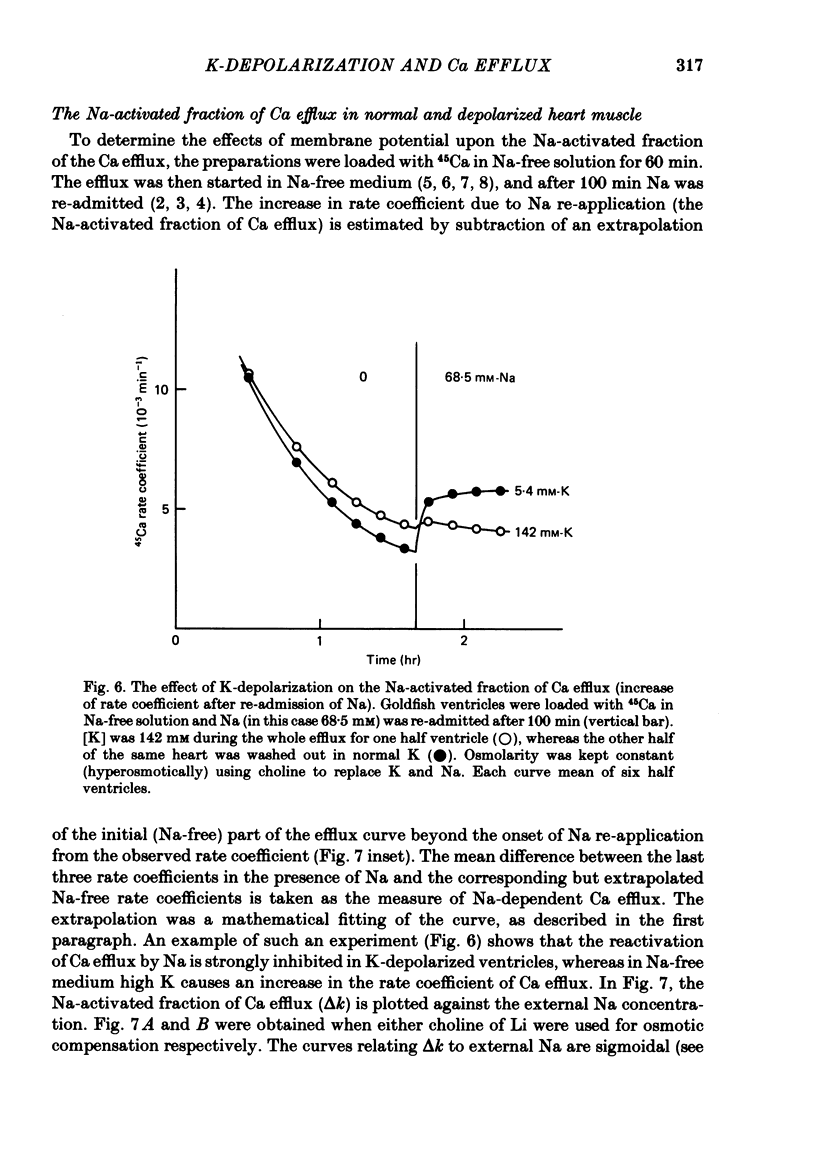
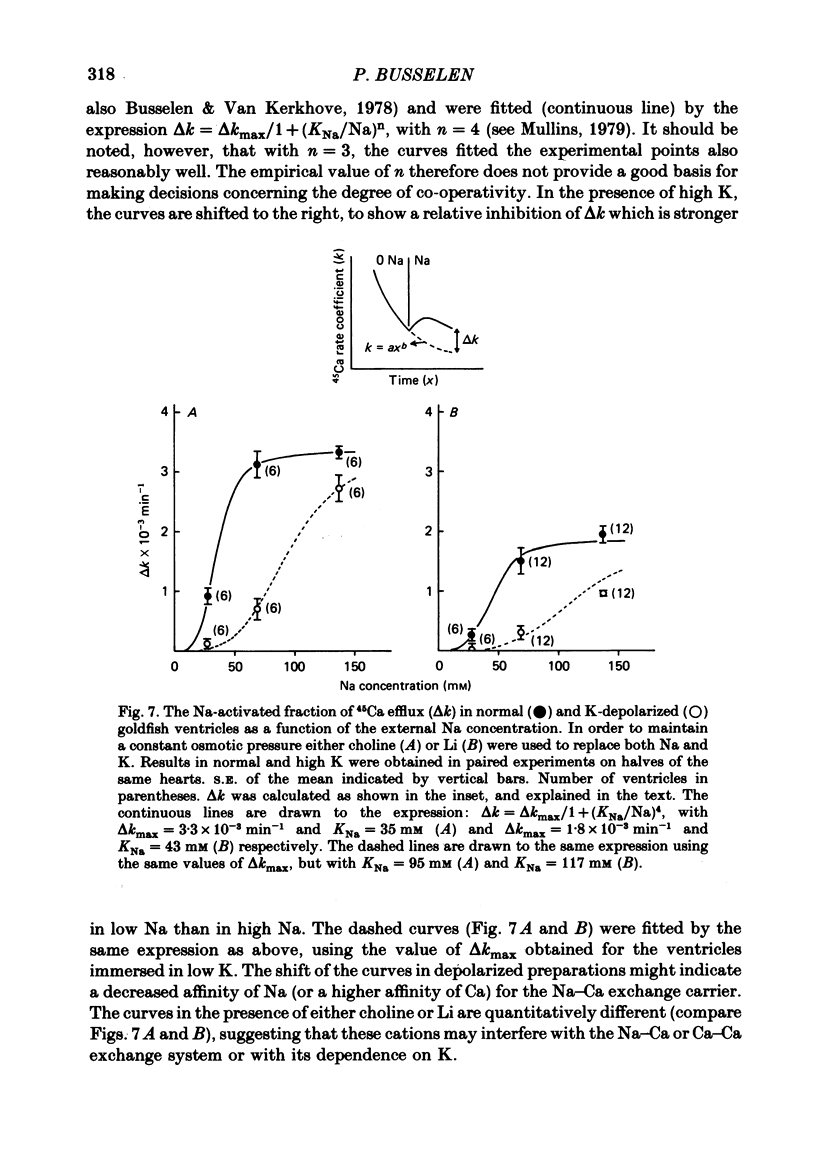
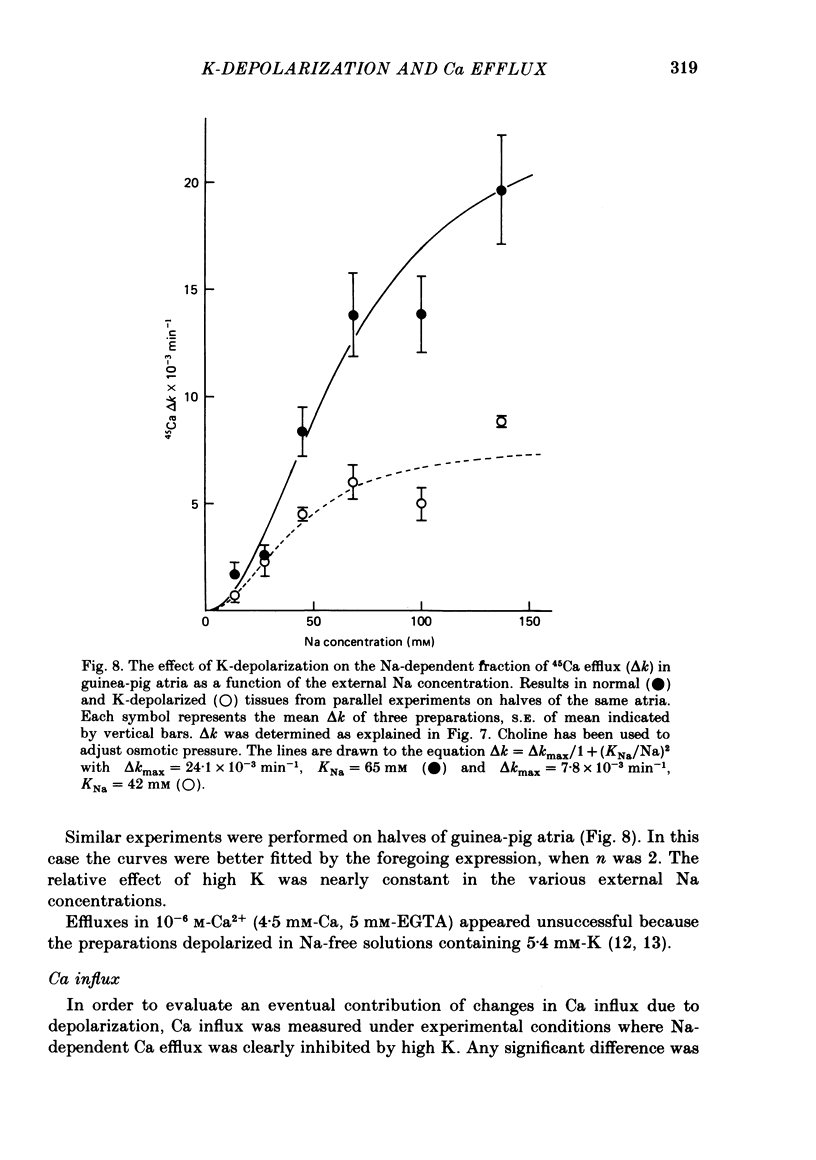
6. Following Na-free perfusion for 100 min, and at normal external Ca concentrations, the 45Ca efflux from goldfish ventricles was stimulated by the addition of Na. The curve relating this stimulation to the external Na concentration had a sigmoidal shape and was shifted to the right by K-depolarization. In guinea-pig atria the inhibition of the Na-stimulated Ca efflux by depolarization was of a non-competitive type.
7. Following a Na-free incubation of 100 min and a subsequent period of 20 min in 137 mM-Na, the intracellular Na content of goldfish ventricular cells was some 20% lower in K-depolarized cells than in cells at the resting potential.
8. 45Ca influx in goldfish ventricles in the presence of 137 or 68·5 mM-Na was not significantly changed by K-depolarization.
9. The results show that the Na-dependent fraction of Ca efflux is inhibited by high external K. The effect is probably due to depolarization, which may be an argument in favour of electrogenic n Na+-1 Ca2+ exchange, with n ≥ 3.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker P. F., Blaustein M. P., Hodgkin A. L., Steinhardt R. A. The influence of calcium on sodium efflux in squid axons. J Physiol. 1969 Feb;200(2):431–458. doi: 10.1113/jphysiol.1969.sp008702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., McNaughton P. A. Kinetics and energetics of calcium efflux from intact squid giant axons. J Physiol. 1976 Jul;259(1):103–144. doi: 10.1113/jphysiol.1976.sp011457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benninger C., Einwächter H. M., Haas H. G., Kern R. Calcium-sodium antagonism on the frog's heart: a voltage-clamp study. J Physiol. 1976 Aug;259(3):617–645. doi: 10.1113/jphysiol.1976.sp011486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bers D. M., Philipson K. D., Nishimoto A. Y. Sodium-calcium exchange and sidedness of isolated cardiac sarcolemmal vesicles. Biochim Biophys Acta. 1980 Sep 18;601(2):358–371. doi: 10.1016/0005-2736(80)90540-4. [DOI] [PubMed] [Google Scholar]
- Blaustein M. P., Russell J. M. Sodium-calcium exchange and calcium-calcium exchange in internally dialyzed squid giant axons. J Membr Biol. 1975 Jul 24;22(3-4):285–312. doi: 10.1007/BF01868176. [DOI] [PubMed] [Google Scholar]
- Busselen P. The effect of high external K on the Na-activated fraction Ca efflux in goldfish ventricles. Arch Int Pharmacodyn Ther. 1981 Feb;249(2):309–311. [PubMed] [Google Scholar]
- Busselen P., Van Kerkhove E. Proceedings: The effects of low external sodium on contracture tension and Ca movements in goldfish ventricle. Arch Int Physiol Biochim. 1975 May;83(2):337–340. [PubMed] [Google Scholar]
- Busselen P., van Kerkhove E. The effect of sodium, calcium and metabolic inhibitors on calcium efflux from goldfish heart ventricles. J Physiol. 1978 Sep;282:263–283. doi: 10.1113/jphysiol.1978.sp012462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caroni P., Carafoli E. An ATP-dependent Ca2+-pumping system in dog heart sarcolemma. Nature. 1980 Feb 21;283(5749):765–767. doi: 10.1038/283765a0. [DOI] [PubMed] [Google Scholar]
- Chapman R. A. Excitation-contraction coupling in cardiac muscle. Prog Biophys Mol Biol. 1979;35(1):1–52. doi: 10.1016/0079-6107(80)90002-4. [DOI] [PubMed] [Google Scholar]
- Chapman R. A., Tunstall J. The interaction of sodium and calcium ions at the cell membrane and the control of contractile strength in frog atrial muscle. J Physiol. 1980 Aug;305:109–123. doi: 10.1113/jphysiol.1980.sp013353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman R. A., Tunstall J. The tension-depolarization relationship of frog atrial trabeculae as determined by potassium contractures. J Physiol. 1981 Jan;310:97–115. doi: 10.1113/jphysiol.1981.sp013539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croaboeuf E., Gautier P., Giuraudou P. Potential and tension changes induced by sodium removal in dog Purkinje fibres: role of an electrogenic sodium-calcium exchange. J Physiol. 1981 Feb;311:605–622. doi: 10.1113/jphysiol.1981.sp013607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis D. The effects of external cations and ouabain on the intracellular sodium activity of sheep heart Purkinje fibres. J Physiol. 1977 Dec;273(1):211–240. doi: 10.1113/jphysiol.1977.sp012090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Contractions induced by a calcium-triggered release of calcium from the sarcoplasmic reticulum of single skinned cardiac cells. J Physiol. 1975 Aug;249(3):469–495. doi: 10.1113/jphysiol.1975.sp011026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glitsch H. G., Reuter H., Scholz H. The effect of the internal sodium concentration on calcium fluxes in isolated guinea-pig auricles. J Physiol. 1970 Jul;209(1):25–43. doi: 10.1113/jphysiol.1970.sp009153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horackova M., Vassort G. Sodium-calcium exchange in regulation of cardiac contractility. Evidence for an electrogenic, voltage-dependent mechanism. J Gen Physiol. 1979 Apr;73(4):403–424. doi: 10.1085/jgp.73.4.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jundt H., Porzig H., Reuter H., Stucki J. W. The effect of substances releasing intracellular calcium ions on sodium-dependent calcium efflux from guinea-pig auricles. J Physiol. 1975 Mar;246(1):229–253. doi: 10.1113/jphysiol.1975.sp010888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamers J. M., Stinis J. T. An electrogenic Na+/Ca2+ antiporter in addition to the Ca2+ pump in cardiac sarcolemma. Biochim Biophys Acta. 1981 Jan 22;640(2):521–534. doi: 10.1016/0005-2736(81)90476-4. [DOI] [PubMed] [Google Scholar]
- Miller D. J., Mörchen A. On the effects of divalent cations and ethylene glycol-bis-(beta-aminoethyl ether) N,N,N',N'-tetraacetate on action potential duration in frog heart. J Gen Physiol. 1978 Jan;71(1):47–67. doi: 10.1085/jgp.71.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morad M., Reeck S., Rao M. Potassium chloride versus voltage clamp contractures in ventricular muscle. Science. 1981 Jan 30;211(4481):485–487. doi: 10.1126/science.7455687. [DOI] [PubMed] [Google Scholar]
- Morcos N. C. Isolation of frog heart sarcolemma possessing (Ca2+ + Mg2+)-ATPase and Ca2+ pump activities. Biochim Biophys Acta. 1981 Apr 22;643(1):55–62. doi: 10.1016/0005-2736(81)90218-2. [DOI] [PubMed] [Google Scholar]
- Mullins L. J., Brinley F. J., Jr Sensitivity of calcium efflux from squid axons to changes in membrane potential. J Gen Physiol. 1975 Feb;65(2):135–152. doi: 10.1085/jgp.65.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins L. J. The generation of electric currents in cardiac fibers by Na/Ca exchange. Am J Physiol. 1979 Mar;236(3):C103–C110. doi: 10.1152/ajpcell.1979.236.3.C103. [DOI] [PubMed] [Google Scholar]
- NIEDERGERKE R. MOVEMENTS OF CA IN FROG HEART VENTRICLES AT REST AND DURING CONTRACTURES. J Physiol. 1963 Jul;167:515–550. doi: 10.1113/jphysiol.1963.sp007166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PORTZEHL H., CALDWELL P. C., RUEEGG J. C. THE DEPENDENCE OF CONTRACTION AND RELAXATION OF MUSCLE FIBRES FROM THE CRAB MAIA SQUINADO ON THE INTERNAL CONCENTRATION OF FREE CALCIUM IONS. Biochim Biophys Acta. 1964 May 25;79:581–591. doi: 10.1016/0926-6577(64)90224-4. [DOI] [PubMed] [Google Scholar]
- Philipson K. D., Nishimoto A. Y. Efflux of Ca2+ from cardiac sarcolemmal vesicles. Influence of external Ca2+ and Na+. J Biol Chem. 1981 Apr 25;256(8):3698–3702. [PubMed] [Google Scholar]
- Pitts B. J. Stoichiometry of sodium-calcium exchange in cardiac sarcolemmal vesicles. Coupling to the sodium pump. J Biol Chem. 1979 Jul 25;254(14):6232–6235. [PubMed] [Google Scholar]
- Reuter H., Seitz N. The dependence of calcium efflux from cardiac muscle on temperature and external ion composition. J Physiol. 1968 Mar;195(2):451–470. doi: 10.1113/jphysiol.1968.sp008467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjodin R. A., Abercrombie R. F. The influence of external cations and membrane potential on Ca-activated Na efflux in Myxicola giant axons. J Gen Physiol. 1978 Apr;71(4):453–466. doi: 10.1085/jgp.71.4.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trumble W. R., Sutko J. L., Reeves J. P. ATP-dependent calcium transport in cardiac sarcolemmal membrane vesicles. Life Sci. 1980 Jul 21;27(3):207–214. doi: 10.1016/0024-3205(80)90139-3. [DOI] [PubMed] [Google Scholar]
- Van Breemen C., McNaughton E. The separation of cell membrane calcium transport from extracellular calcium exchange in vascular smooth muscle. Biochem Biophys Res Commun. 1970 May 22;39(4):567–574. doi: 10.1016/0006-291x(70)90241-x. [DOI] [PubMed] [Google Scholar]
- Zimmerman A. N., Hülsmann W. C. Paradoxical influence of calcium ions on the permeability of the cell membranes of the isolated rat heart. Nature. 1966 Aug 6;211(5049):646–647. doi: 10.1038/211646a0. [DOI] [PubMed] [Google Scholar]


