Abstract
1. We recorded extracellularly from 1045 neurones in area 17 of seven monocularly deprived kittens and we employed careful sampling techniques to examine the effects of removing the signals from the non-deprived eye on the proportion of cells responding to stimulation of the deprived eye.
2. Monocular deprivation itself produced a pronounced over-all change in the ocular dominance of neurones in favour of the experienced eye, but both between animals and even between different samples of cells in individual animals there were marked variations in the magnitude of the effect.
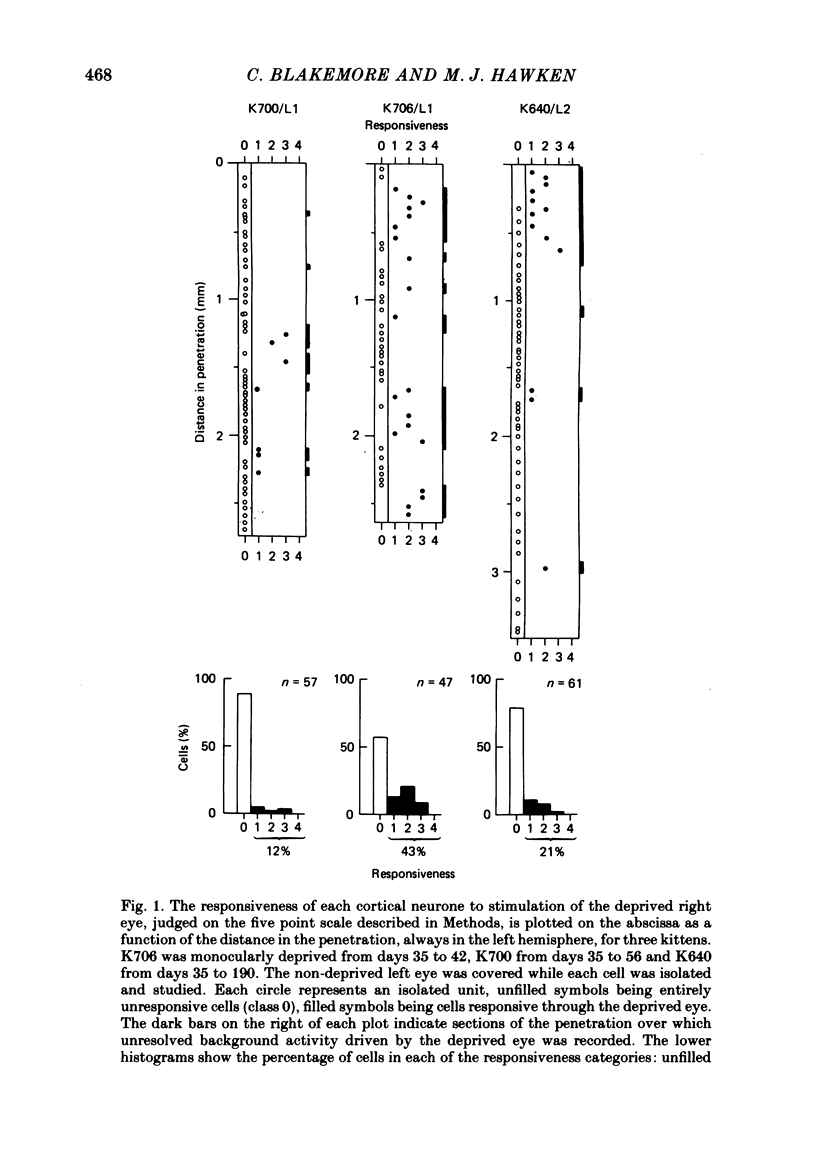
3. Monocular deprivation starting immediately at the time of natural eye opening and lasting for several weeks reduced to about 10% the proportion of cortical neurones influenced through the deprived eye. Enucleation of the experienced eye did not then produce a significant increase in the proportion of cells responsive to the deprived eye.
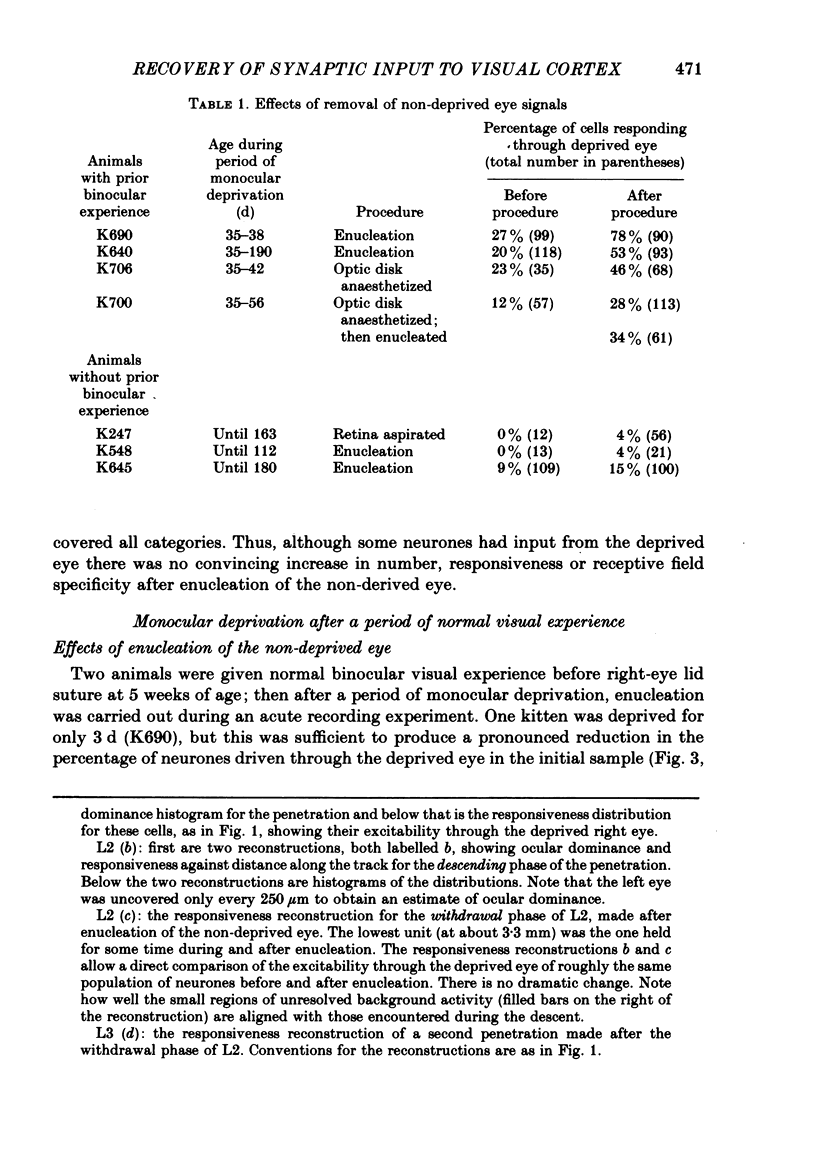
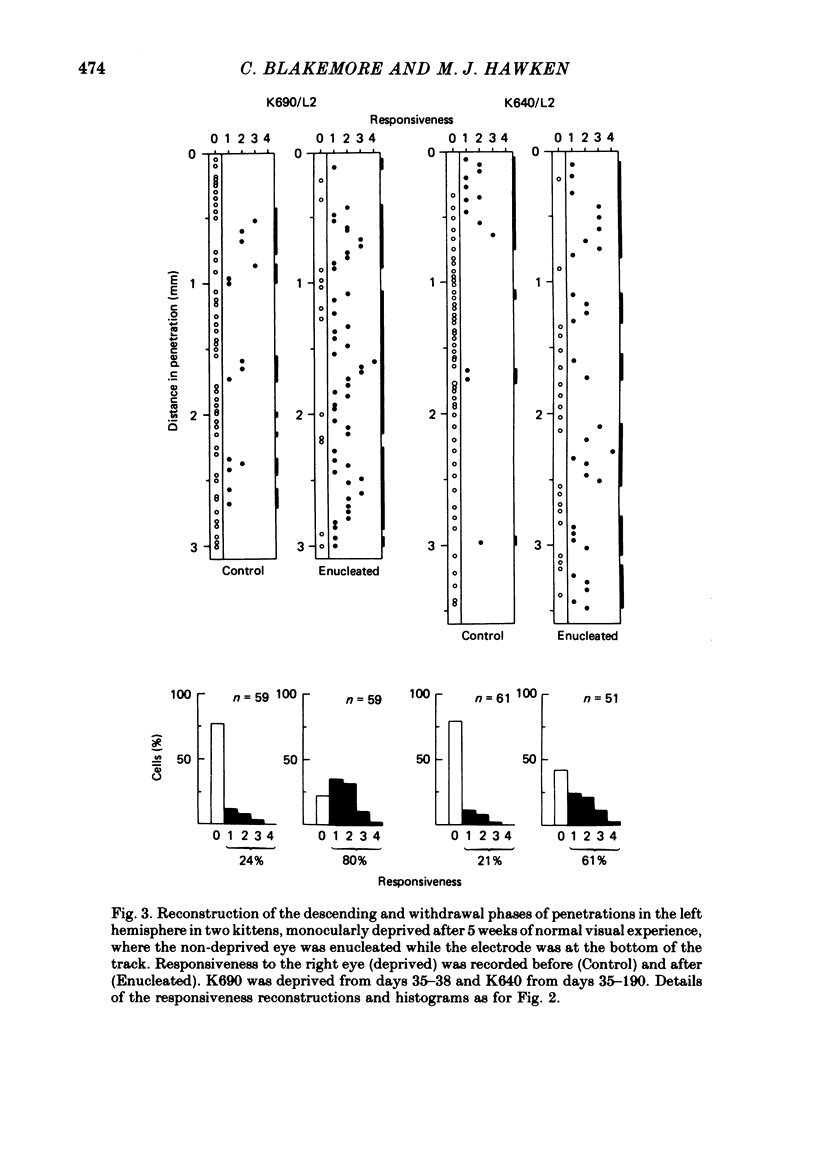
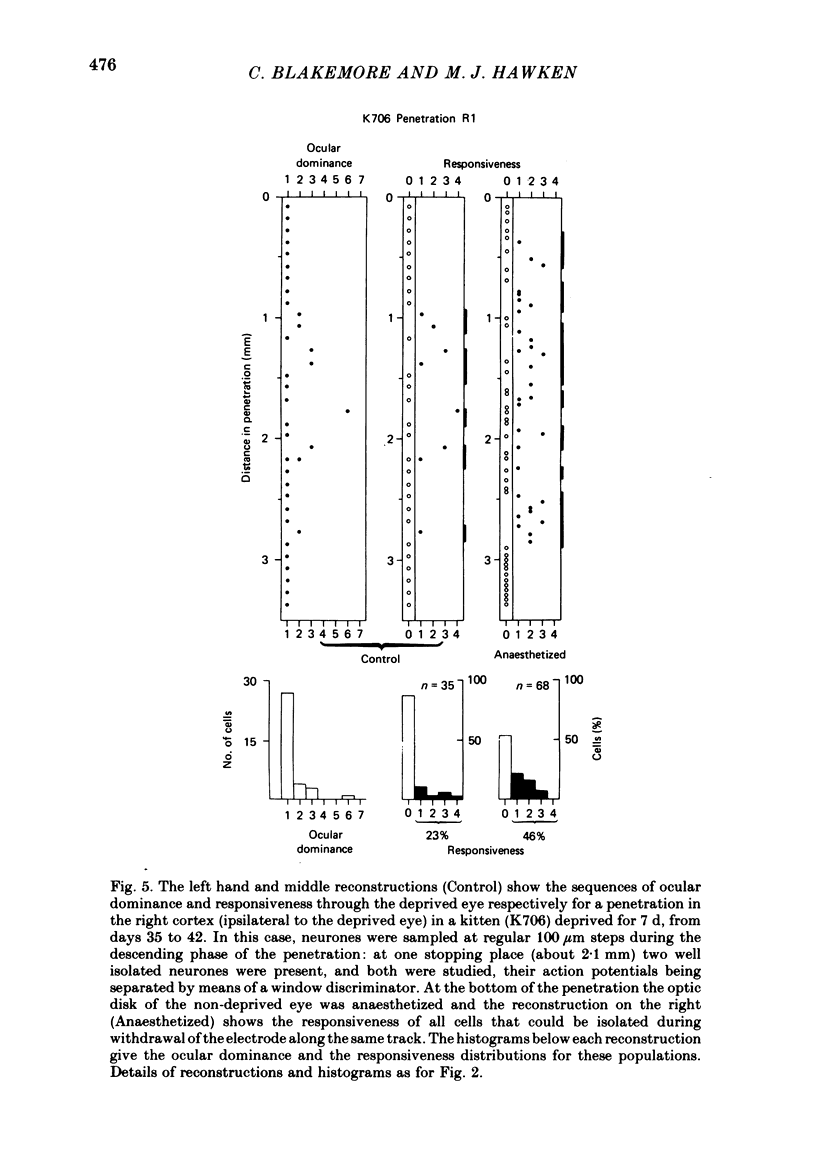
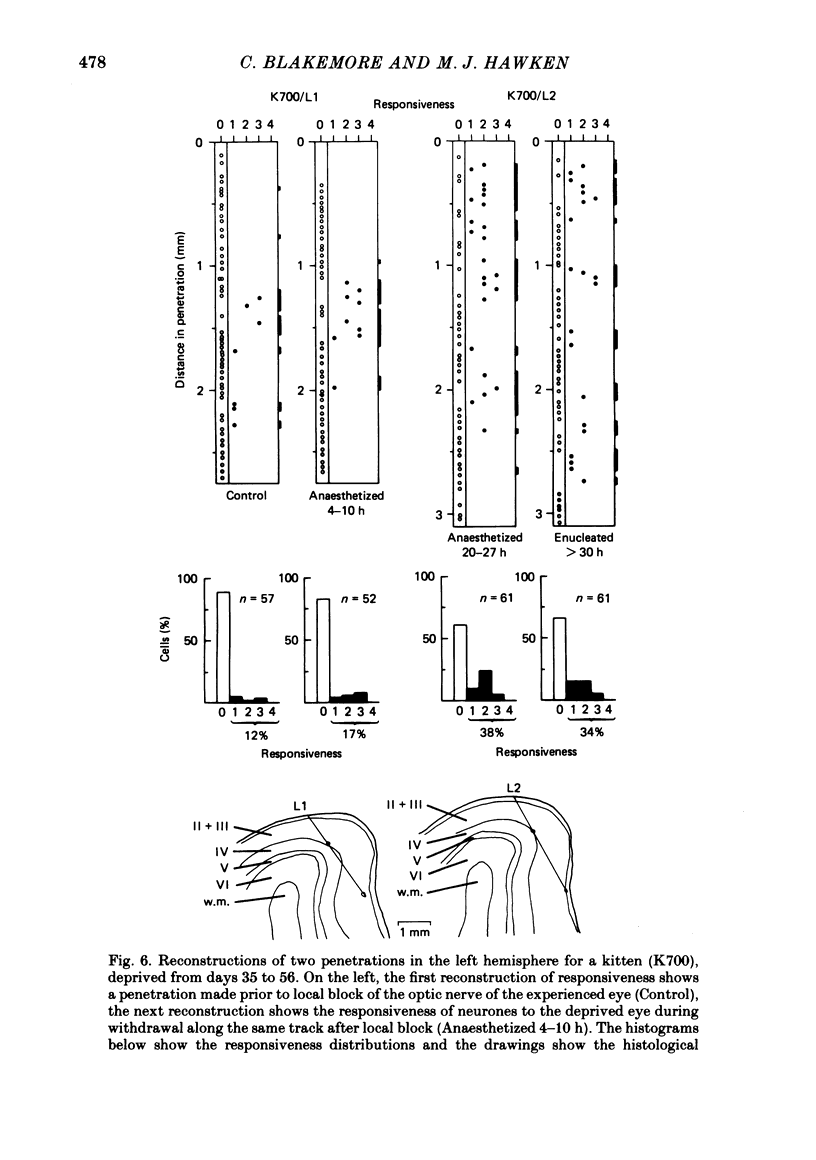
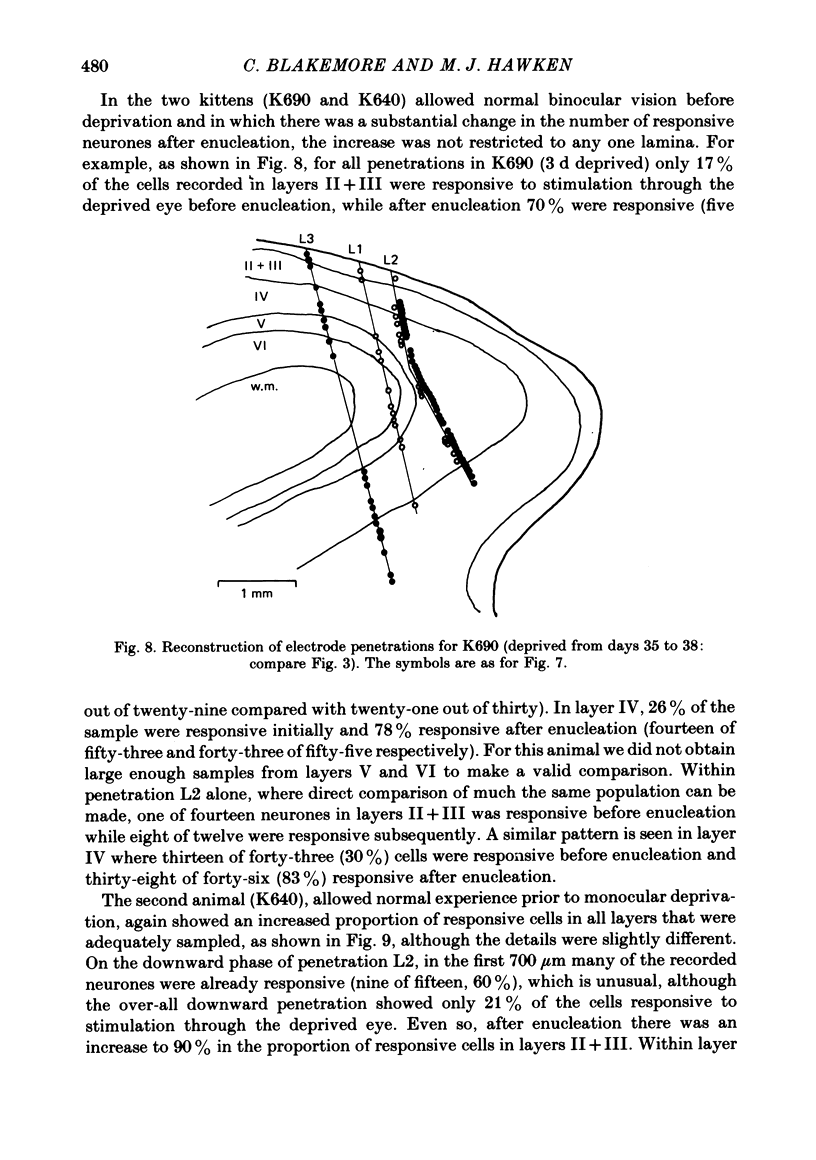
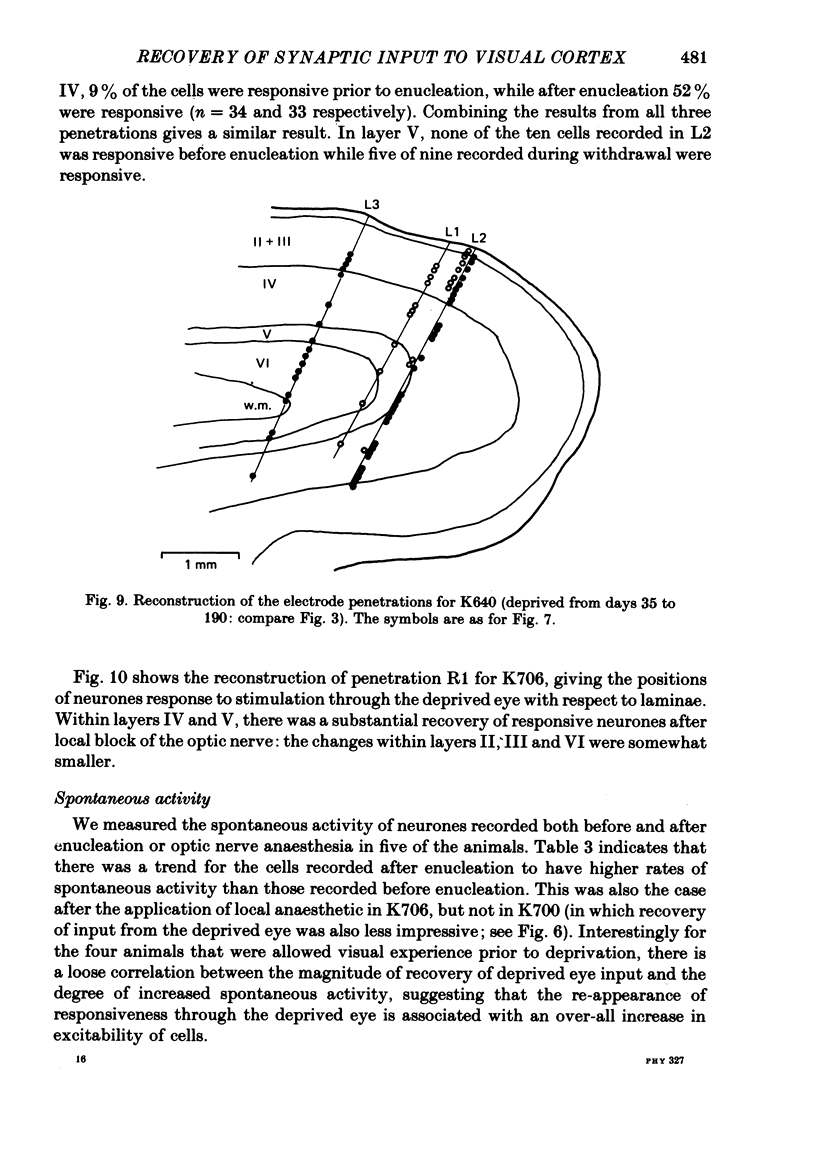
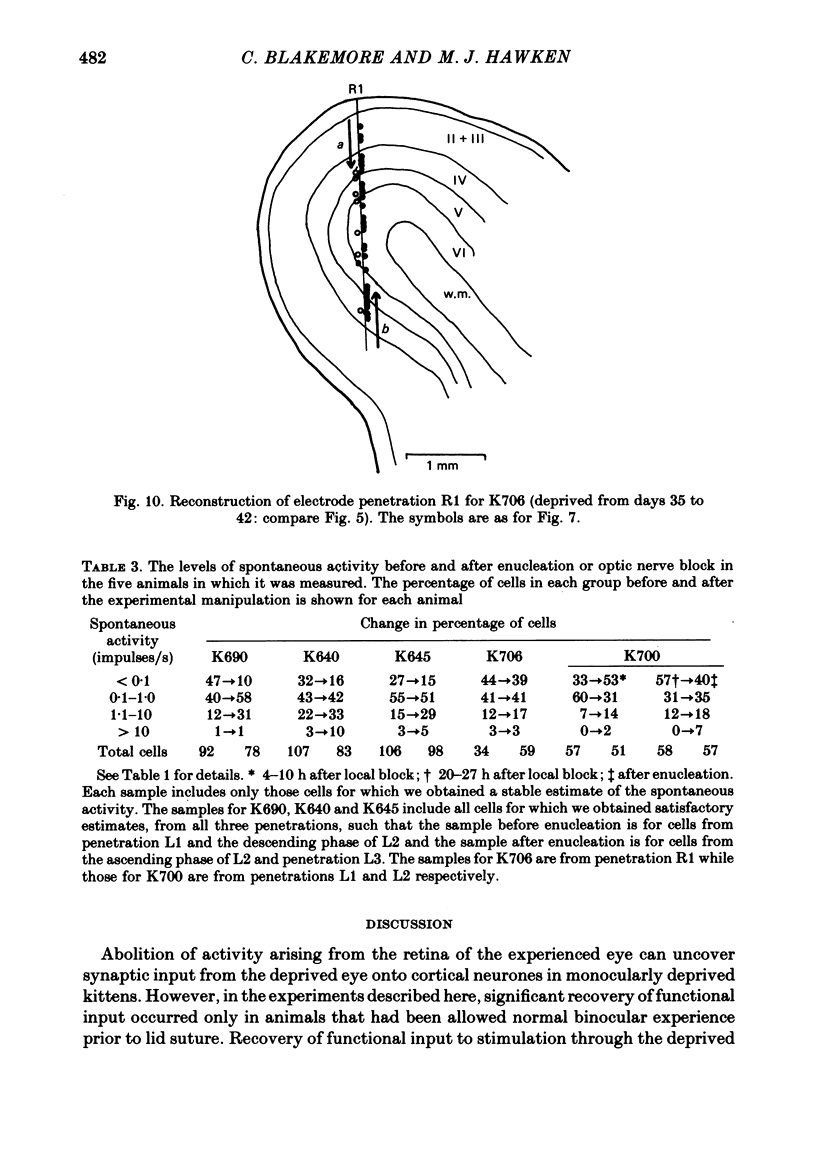
4. Monocular deprivation lasting 3 days or more and beginning at 5 weeks of age, after normal binocular vision, also shifted ocular dominance substantially: 11-27% of neurones responded through the deprived eye. Enucleation of the experienced eye or topical anaesthesia of the optic nerve resulted in a substantial recovery of input from the deprived eye: up to 78% of the cells responded.
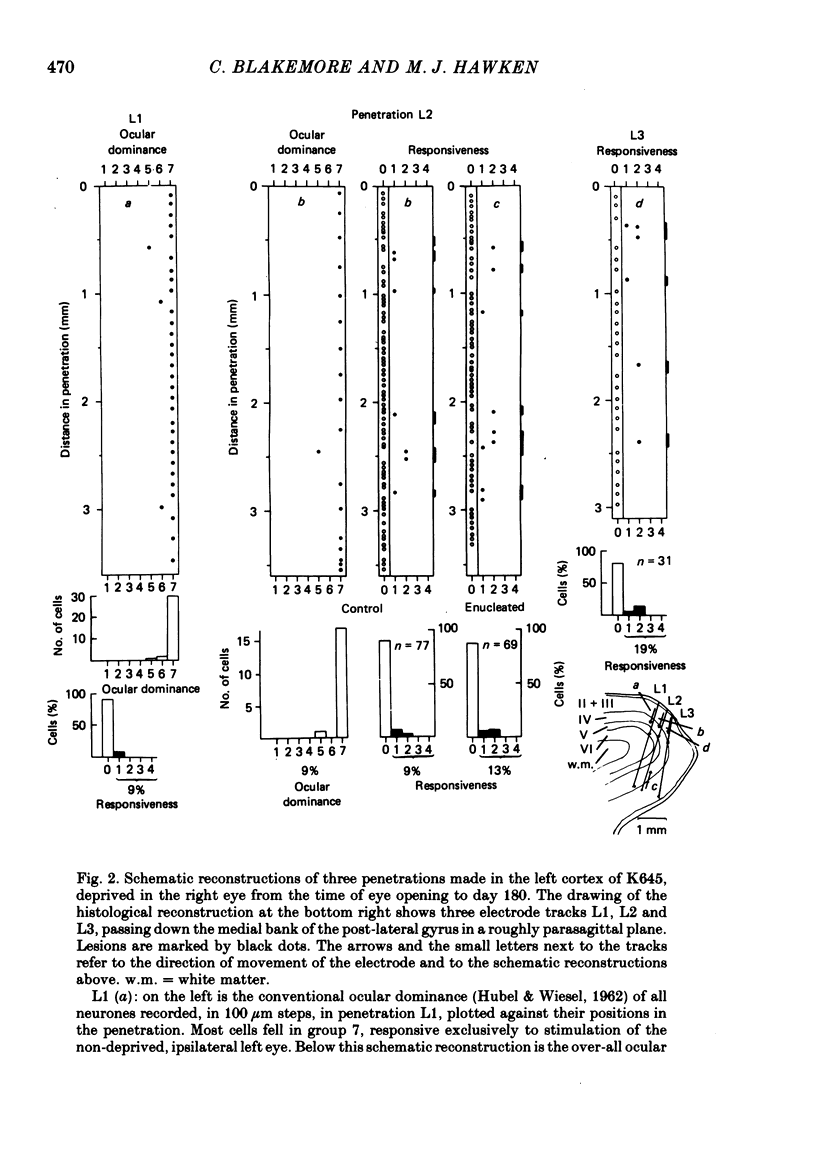
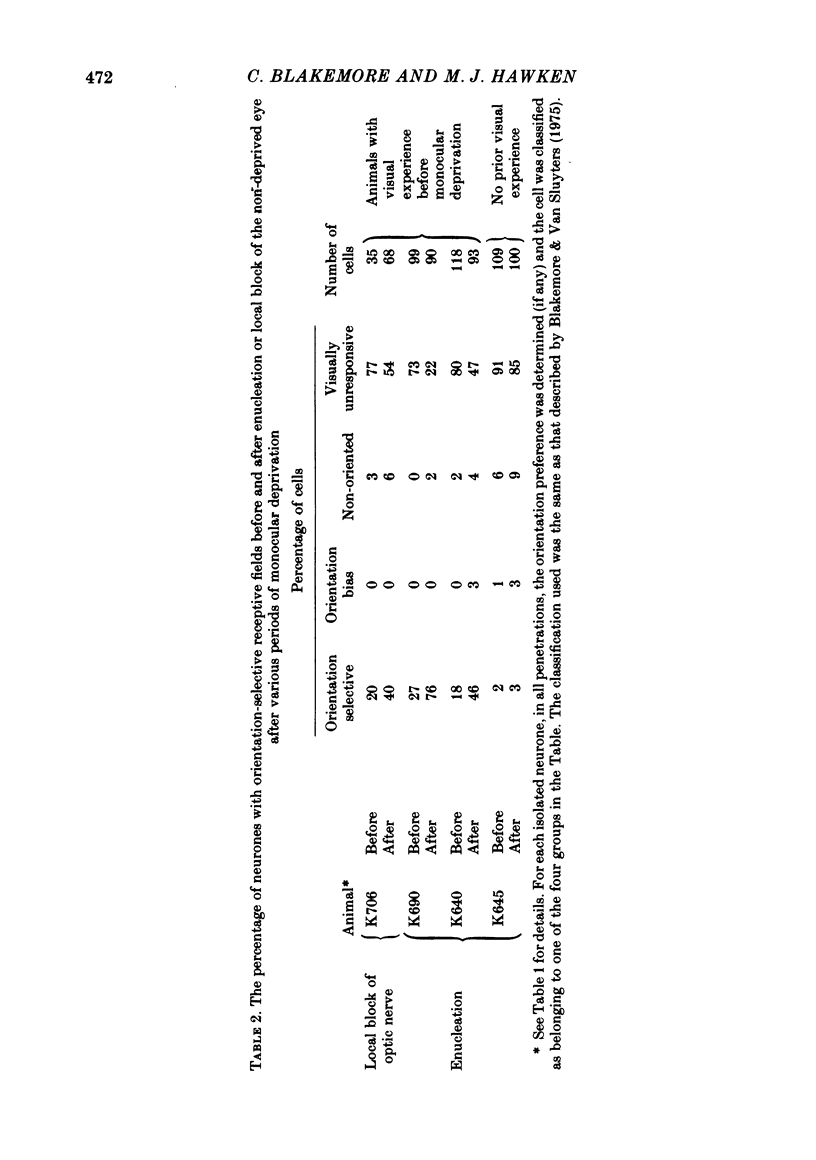
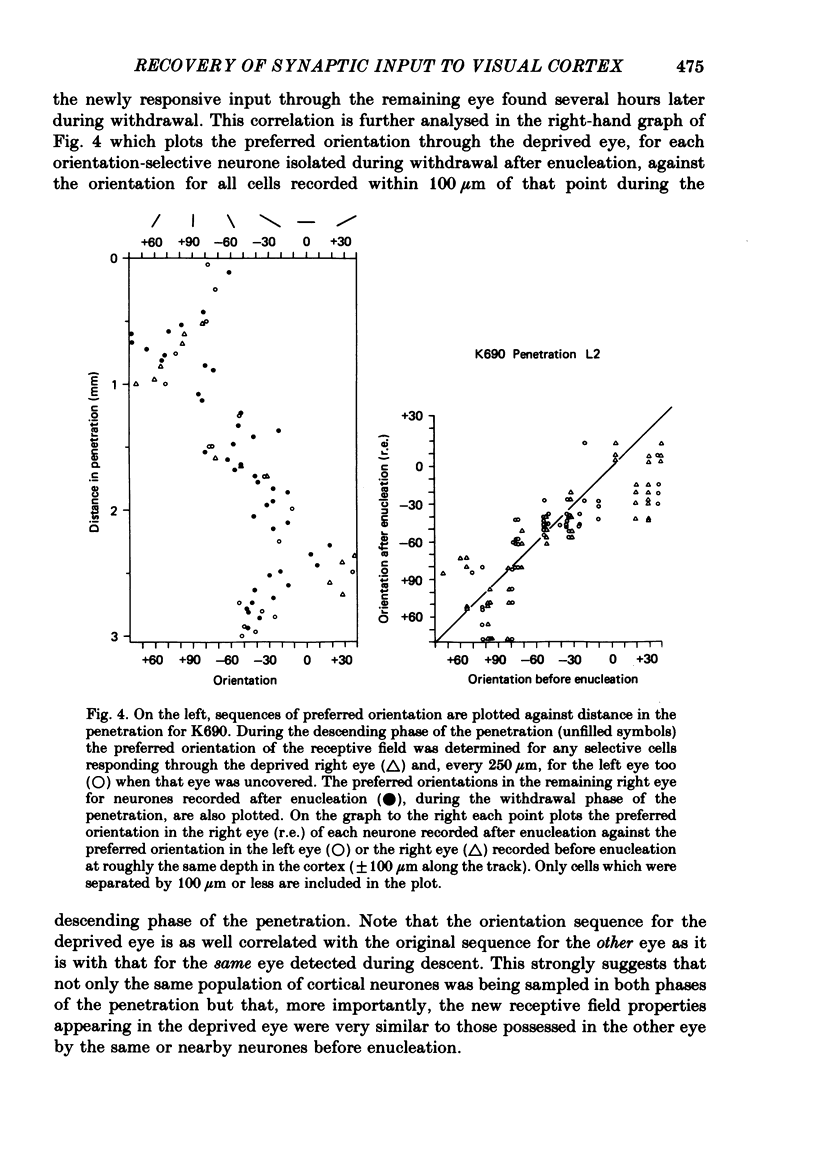
5. In such animals the majority of neurones that recovered input from the deprived eye had receptive field properties qualitatively similar to those of normal cortical cells. Recording in a single penetration both before and after enucleation (or optic nerve block) suggested that the orientation preferences of cells with recovered input followed the same sequence as was originally present for the non-deprived eye.
6. Recovery of input occurred in all cortical laminae in which cells were recorded, even in layer IV, and mainly took the form of an expansion of already existing clusters of cells driven by the deprived eye.
7. Spontaneous activity tended to increase after enucleation.
8. The results indicate that monocular deprivation after a period of normal binocular vision leaves subthreshold but functionally organized synaptic input from the deprived eye on cortical cells, which is revealed when activity arising in the retina of the non-deprived eye is abolished.
Full text
PDF













Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blakemore C., Garey L. J., Vital-Durand F. The physiological effects of monocular deprivation and their reversal in the monkey's visual cortex. J Physiol. 1978 Oct;283:223–262. doi: 10.1113/jphysiol.1978.sp012498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore C., Hawken M. J., Mark R. F. Brief monocular deprivation leaves subthreshold synaptic input on neurones of the cat's visual cortex. J Physiol. 1982 Jun;327:489–505. doi: 10.1113/jphysiol.1982.sp014244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore C., Hillman P. An attempt to assess the effects of monocular deprivation and strabismus on synaptic efficiency in the kitten's visual cortex. Exp Brain Res. 1977 Nov 24;30(2-3):187–202. doi: 10.1007/BF00237250. [DOI] [PubMed] [Google Scholar]
- Blakemore C., Van Sluyters C. V., Movshon J. A. Synaptic competition in the kitten's visual cortex. Cold Spring Harb Symp Quant Biol. 1976;40:601–609. doi: 10.1101/sqb.1976.040.01.056. [DOI] [PubMed] [Google Scholar]
- Blakemore C., Van Sluyters R. C. Innate and environmental factors in the development of the kitten's visual cortex. J Physiol. 1975 Jul;248(3):663–716. doi: 10.1113/jphysiol.1975.sp010995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore C., Van Sluyters R. C. Reversal of the physiological effects of monocular deprivation in kittens: further evidence for a sensitive period. J Physiol. 1974 Feb;237(1):195–216. doi: 10.1113/jphysiol.1974.sp010478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasdel G. G., Pettigrew J. D. Effect of prior visual experience on cortical recovery from the effects of unilateral eyelid suture in kittens. J Physiol. 1978 Jan;274:601–619. doi: 10.1113/jphysiol.1978.sp012169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cragg B. G. The development of synapses in the visual system of the cat. J Comp Neurol. 1975 Mar 15;160(2):147–166. doi: 10.1002/cne.901600202. [DOI] [PubMed] [Google Scholar]
- Crewther D. P., Crewther S. G., Pettigrew J. D. A role for extraocular afferents in post-critical period reversal of monocular deprivation. J Physiol. 1978 Sep;282:181–195. doi: 10.1113/jphysiol.1978.sp012456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldridge J. L. A reversible ophthalmoscope using a corner-cube [proceedings]. J Physiol. 1979 Oct;295:1P–2P. [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. Receptive fields, binocular interaction and functional architecture in the cat's visual cortex. J Physiol. 1962 Jan;160:106–154. doi: 10.1113/jphysiol.1962.sp006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann K. P., Cynader M. Functional aspects of plasticity in the visual system of adult cats after early monocular deprivation. Philos Trans R Soc Lond B Biol Sci. 1977 Apr 26;278(961):411–424. doi: 10.1098/rstb.1977.0051. [DOI] [PubMed] [Google Scholar]
- Hubel D. H., Wiesel T. N. Ferrier lecture. Functional architecture of macaque monkey visual cortex. Proc R Soc Lond B Biol Sci. 1977 Jul 28;198(1130):1–59. doi: 10.1098/rspb.1977.0085. [DOI] [PubMed] [Google Scholar]
- Hubel D. H., Wiesel T. N., LeVay S. Plasticity of ocular dominance columns in monkey striate cortex. Philos Trans R Soc Lond B Biol Sci. 1977 Apr 26;278(961):377–409. doi: 10.1098/rstb.1977.0050. [DOI] [PubMed] [Google Scholar]
- Hubel D. H., Wiesel T. N. Sequence regularity and geometry of orientation columns in the monkey striate cortex. J Comp Neurol. 1974 Dec 1;158(3):267–293. doi: 10.1002/cne.901580304. [DOI] [PubMed] [Google Scholar]
- Hubel D. H., Wiesel T. N. The period of susceptibility to the physiological effects of unilateral eye closure in kittens. J Physiol. 1970 Feb;206(2):419–436. doi: 10.1113/jphysiol.1970.sp009022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kratz K. E., Spear P. D. Postcritical-period reversal of effects of monocular deprivation on striate cortex cells in the cat. J Neurophysiol. 1976 May;39(3):501–511. doi: 10.1152/jn.1976.39.3.501. [DOI] [PubMed] [Google Scholar]
- LeVay S., Wiesel T. N., Hubel D. H. The development of ocular dominance columns in normal and visually deprived monkeys. J Comp Neurol. 1980 May 1;191(1):1–51. doi: 10.1002/cne.901910102. [DOI] [PubMed] [Google Scholar]
- Merrill E. G., Ainsworth A. Glass-coated platinum-plated tungsten microelectrodes. Med Biol Eng. 1972 Sep;10(5):662–672. doi: 10.1007/BF02476084. [DOI] [PubMed] [Google Scholar]
- Mitchell D. E., Cynader M., Movshon J. A. Recovery from the effects of monocular deprivation in kittens. J Comp Neurol. 1977 Nov 1;176(1):53–63. doi: 10.1002/cne.901760104. [DOI] [PubMed] [Google Scholar]
- Movshon J. A., Blakemore C. Functional reinnervation in kitten visual cortex. Nature. 1974 Oct 11;251(5475):504–505. doi: 10.1038/251504a0. [DOI] [PubMed] [Google Scholar]
- Movshon J. A., Dürsteler M. R. Effects of brief periods of unilateral eye closure on the kitten's visual system. J Neurophysiol. 1977 Nov;40(6):1255–1265. doi: 10.1152/jn.1977.40.6.1255. [DOI] [PubMed] [Google Scholar]
- Movshon J. A. Reversal of the physiological effects of monocular deprivation in the kitten's visual cortex. J Physiol. 1976 Sep;261(1):125–174. doi: 10.1113/jphysiol.1976.sp011551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Movshon J. A., Van Sluyters R. C. Visual neural development. Annu Rev Psychol. 1981;32:477–522. doi: 10.1146/annurev.ps.32.020181.002401. [DOI] [PubMed] [Google Scholar]
- OTSUKA R., HASSLER R. [On the structure and segmentation of the cortical center of vision in the cat]. Arch Psychiatr Nervenkr Z Gesamte Neurol Psychiatr. 1962;203:212–234. doi: 10.1007/BF00352744. [DOI] [PubMed] [Google Scholar]
- Olson C. R., Freeman R. D. Monocular deprivation and recovery during sensitive period in kittens. J Neurophysiol. 1978 Jan;41(1):65–74. doi: 10.1152/jn.1978.41.1.65. [DOI] [PubMed] [Google Scholar]
- Olson C. R., Freeman R. D. Progressive changes in kitten striate cortex during monocular vision. J Neurophysiol. 1975 Jan;38(1):26–32. doi: 10.1152/jn.1975.38.1.26. [DOI] [PubMed] [Google Scholar]
- Shatz C. J., Stryker M. P. Ocular dominance in layer IV of the cat's visual cortex and the effects of monocular deprivation. J Physiol. 1978 Aug;281:267–283. doi: 10.1113/jphysiol.1978.sp012421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer W. Control of thalamic transmission by corticofugal and ascending reticular pathways in the visual system. Physiol Rev. 1977 Jul;57(3):386–420. doi: 10.1152/physrev.1977.57.3.386. [DOI] [PubMed] [Google Scholar]
- WIESEL T. N., HUBEL D. H. SINGLE-CELL RESPONSES IN STRIATE CORTEX OF KITTENS DEPRIVED OF VISION IN ONE EYE. J Neurophysiol. 1963 Nov;26:1003–1017. doi: 10.1152/jn.1963.26.6.1003. [DOI] [PubMed] [Google Scholar]
- Wall P. D. The presence of ineffective synapses and the circumstances which unmask them. Philos Trans R Soc Lond B Biol Sci. 1977 Apr 26;278(961):361–372. doi: 10.1098/rstb.1977.0048. [DOI] [PubMed] [Google Scholar]
- van Sluyters R. C. Reversal of the physiological effects of brief periods of monocular deprivation in the kitten. J Physiol. 1978 Nov;284:1–17. doi: 10.1113/jphysiol.1978.sp012524. [DOI] [PMC free article] [PubMed] [Google Scholar]


