Abstract
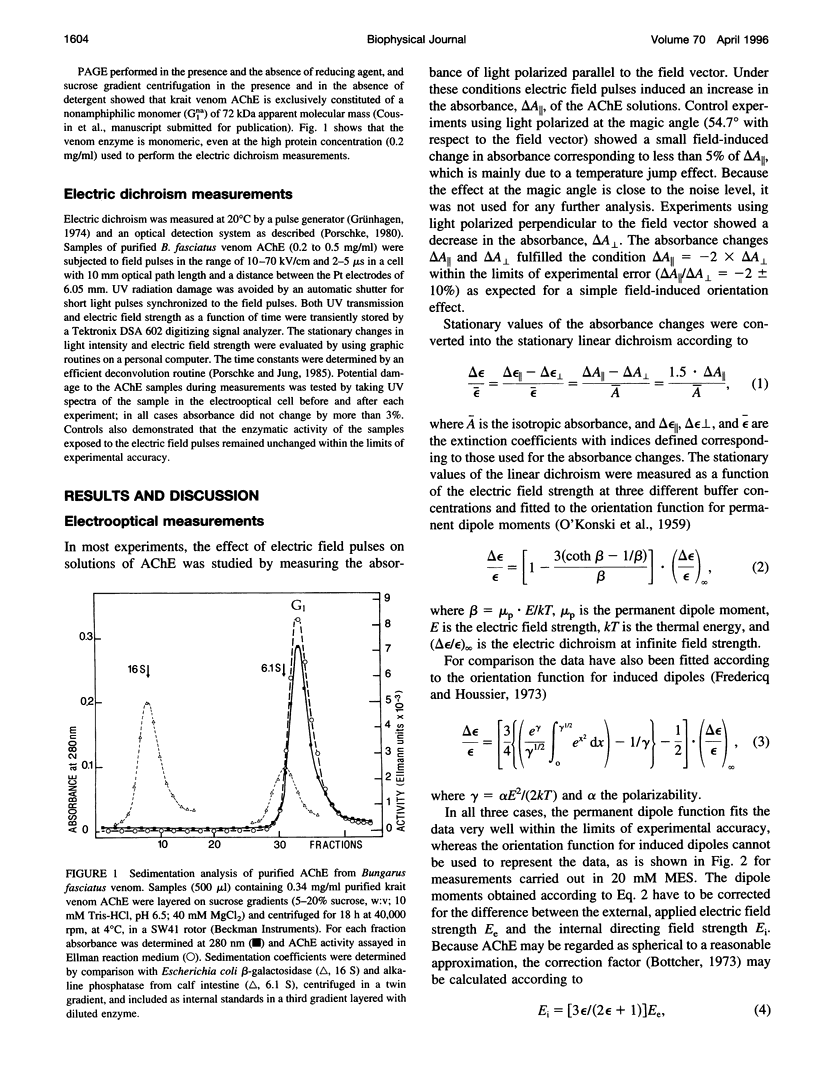
Acetylcholinesterase (AChE) from krait (Bungarus fasciatus) venom is a soluble, nonamphiphilic monomer of 72 kDa. This snake venom AChE has been analyzed by measurements of the stationary and the transient electric dichroism at different field strengths. The stationary values of the dichroism are consistent with the orientation function for permanent dipoles and are not consistent with the orientation function for induced dipoles. The permanent dipole moment obtained by least-squares fits for a buffer containing 5 mM MES is 1000 D, after correction for the internal directing field, assuming a spherical shape of the protein. The dipole moment decreases with increasing buffer concentration to 880 D at 10 mM MES and 770 D at 20 mM MES. The dichroism decay time constant is 90 ns (+/- 10%) which is clearly larger than the value expected from the size/shape of the protein and indicates contributions from sugar residues attached to the protein. The dichroism rise times observed at low field strengths are larger than the decay times and, thus, support the assignment of a permanent dipole moment, although it has not been possible to approach the limit where the energy of the dipole in the electric field is sufficiently low compared to kT. The experimental value of the permanent dipole moment is similar to that calculated for a model structure of Bungarus fasciatus AChE, which has been constructed from its amino and acid sequence, in analogy to the crystal structure of AChE from Torpedo californica.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antosiewicz J., Gilson M. K., Lee I. H., McCammon J. A. Acetylcholinesterase: diffusional encounter rate constants for dumbbell models of ligand. Biophys J. 1995 Jan;68(1):62–68. doi: 10.1016/S0006-3495(95)80159-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antosiewicz J., Porschke D. An unusual electrooptical effect observed for DNA fragments and its apparent relation to a permanent electric moment associated with bent DNA. Biophys Chem. 1989 Mar;33(1):19–30. doi: 10.1016/0301-4622(89)80003-1. [DOI] [PubMed] [Google Scholar]
- Antosiewicz J., Porschke D. The nature of protein dipole moments: experimental and calculated permanent dipole of alpha-chymotrypsin. Biochemistry. 1989 Dec 26;28(26):10072–10078. doi: 10.1021/bi00452a029. [DOI] [PubMed] [Google Scholar]
- Diekmann S., Hillen W., Jung M., Wells R. D., Pörschke D. Electric properties and structure of DNA-restriction fragments from measurements of the electric dichroism. Biophys Chem. 1982 May;15(2):157–167. doi: 10.1016/0301-4622(82)80028-8. [DOI] [PubMed] [Google Scholar]
- Dudai Y., Silman I. Affinity chromatography of acetylcholinesterase. Methods Enzymol. 1974;34:571–580. doi: 10.1016/s0076-6879(74)34076-1. [DOI] [PubMed] [Google Scholar]
- ELLMAN G. L., COURTNEY K. D., ANDRES V., Jr, FEATHER-STONE R. M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961 Jul;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- Massoulié J., Bon S. Affinity chromatography of acetylcholinesterase. The importance of hydrophobic interactions. Eur J Biochem. 1976 Sep 15;68(2):531–539. doi: 10.1111/j.1432-1033.1976.tb10841.x. [DOI] [PubMed] [Google Scholar]
- Nolte H. J., Rosenberry T. L., Neumann E. Effective charge on acetylcholinesterase active sites determined from the ionic strength dependence of association rate constants with cationic ligands. Biochemistry. 1980 Aug 5;19(16):3705–3711. doi: 10.1021/bi00557a011. [DOI] [PubMed] [Google Scholar]
- Porschke D., Jung M. The conformation of single stranded oligonucleotides and of oligonucleotide-oligopeptide complexes from their rotation relaxation in the nanosecond time range. J Biomol Struct Dyn. 1985 Jun;2(6):1173–1184. doi: 10.1080/07391102.1985.10507631. [DOI] [PubMed] [Google Scholar]
- Porschke D. The mechanism of ion polarisation along DNA double helices. Biophys Chem. 1985 Aug;22(3):237–247. doi: 10.1016/0301-4622(85)80046-6. [DOI] [PubMed] [Google Scholar]
- Pörschke D. Electric, optical and hydrodynamic parameters of lac repressor from measurements of the electric dichroism. High permanent dipole moment associated with the protein. Biophys Chem. 1987 Nov;28(2):137–147. doi: 10.1016/0301-4622(87)80083-2. [DOI] [PubMed] [Google Scholar]
- Pörschke D. Structure and dynamics of a tryptophanepeptide-polynucleotide complex. Nucleic Acids Res. 1980 Apr 11;8(7):1591–1612. doi: 10.1093/nar/8.7.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripoll D. R., Faerman C. H., Axelsen P. H., Silman I., Sussman J. L. An electrostatic mechanism for substrate guidance down the aromatic gorge of acetylcholinesterase. Proc Natl Acad Sci U S A. 1993 Jun 1;90(11):5128–5132. doi: 10.1073/pnas.90.11.5128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafferman A., Ordentlich A., Barak D., Kronman C., Ber R., Bino T., Ariel N., Osman R., Velan B. Electrostatic attraction by surface charge does not contribute to the catalytic efficiency of acetylcholinesterase. EMBO J. 1994 Aug 1;13(15):3448–3455. doi: 10.1002/j.1460-2075.1994.tb06650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussman J. L., Harel M., Frolow F., Oefner C., Goldman A., Toker L., Silman I. Atomic structure of acetylcholinesterase from Torpedo californica: a prototypic acetylcholine-binding protein. Science. 1991 Aug 23;253(5022):872–879. doi: 10.1126/science.1678899. [DOI] [PubMed] [Google Scholar]
- Tan R. C., Truong T. N., McCammon J. A., Sussman J. L. Acetylcholinesterase: electrostatic steering increases the rate of ligand binding. Biochemistry. 1993 Jan 19;32(2):401–403. doi: 10.1021/bi00053a003. [DOI] [PubMed] [Google Scholar]



