Abstract
Lidocaine is a widely used local anesthetic and antiarrhythmic drug that is believed to exert its clinically important action by blocking voltage-gated Na+ channels. Studies of Na+ channels from different species and tissues and the complexity of the drug-channel interaction create difficulty in understanding whether there are Na+ channel isoform specific differences in the affinity for lidocaine. Clinical usage suggests that lidocaine selectively targets cardiac Na+ channels because it is effective for the treatment of arrhythmias with few side effects on muscle or neuronal channels except at higher concentrations. One possibility for this selectivity is an intrinsically higher drug-binding affinity of the cardiac isoform. Alternatively, lidocaine may appear cardioselective because of preferential interactions with the inactivated state of the Na+ channel, which is occupied much longer in cardiac cells. Recombinant skeletal muscle (hSkM1) and cardiac sodium channels (hH1) were studied under identical conditions, with a whole-cell voltage clamp used to distinguish the mechanisms of lidocaine block. Tonic block at high concentrations of lidocaine (0.1 mM) was greater in hH1 than in hSkM1. This was also true for use-dependent block, for which 25-microM lidocaine produced an inhibition in hH1 equivalent to 0.1 mM in the skeletal muscle isoform. Pulse protocols optimized to explore inactivated-state block revealed that hSkM1 was five to eight times less sensitive to block by lidocaine than was hH1. The results also indicate that relatively more open-state block occurs in hSkM1. Thus, the cardiac sodium channel is intrinsically more sensitive to inhibition by lidocaine.
Full text
PDF
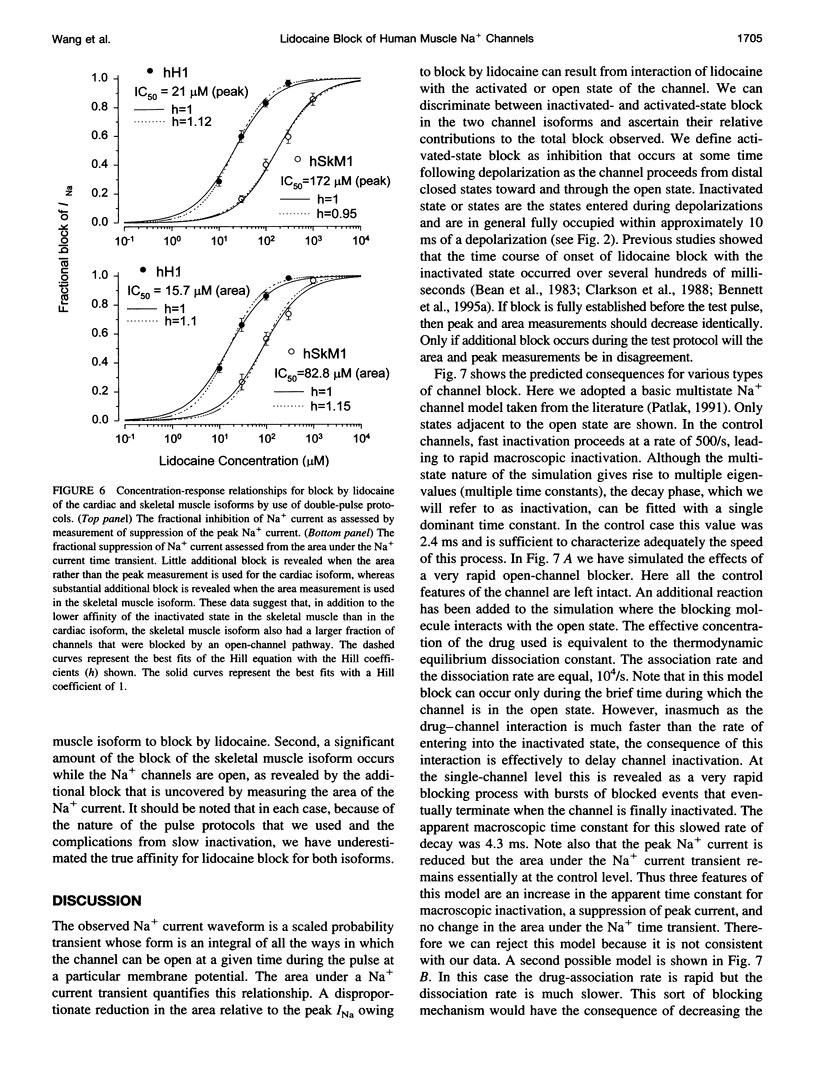
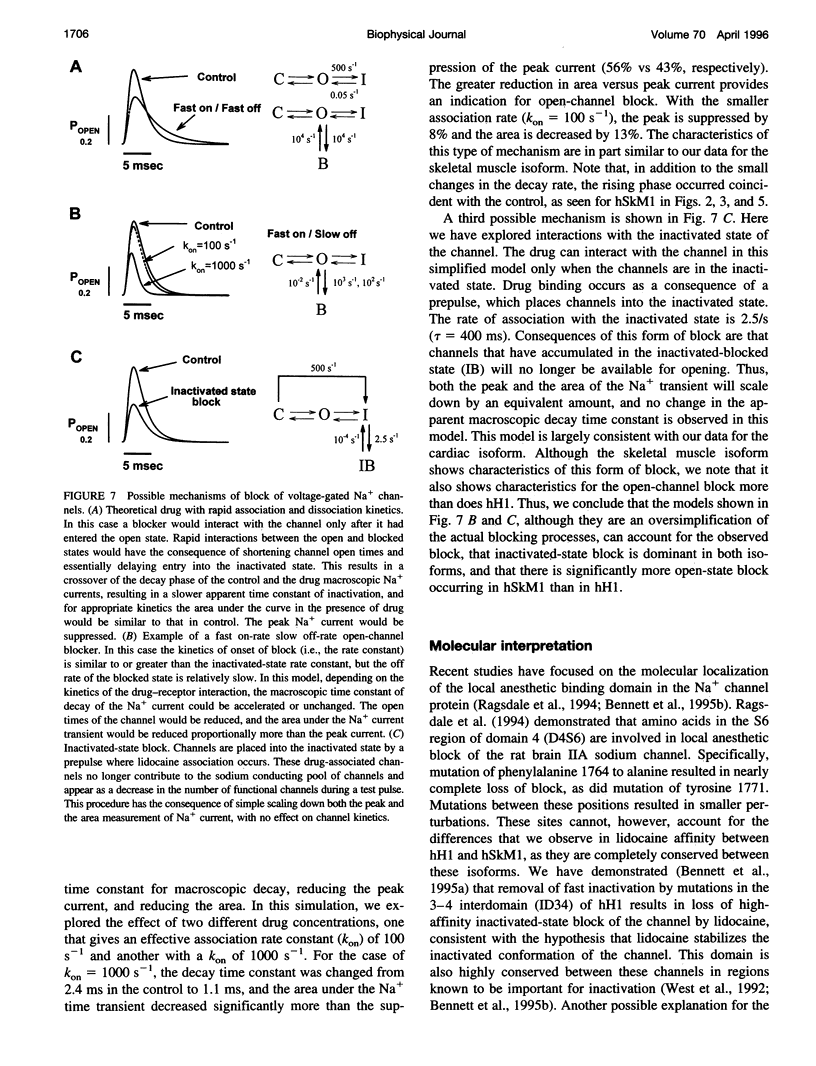


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldrich R. W., Corey D. P., Stevens C. F. A reinterpretation of mammalian sodium channel gating based on single channel recording. Nature. 1983 Dec 1;306(5942):436–441. doi: 10.1038/306436a0. [DOI] [PubMed] [Google Scholar]
- Alpert L. A., Fozzard H. A., Hanck D. A., Makielski J. C. Is there a second external lidocaine binding site on mammalian cardiac cells? Am J Physiol. 1989 Jul;257(1 Pt 2):H79–H84. doi: 10.1152/ajpheart.1989.257.1.H79. [DOI] [PubMed] [Google Scholar]
- Bean B. P., Cohen C. J., Tsien R. W. Lidocaine block of cardiac sodium channels. J Gen Physiol. 1983 May;81(5):613–642. doi: 10.1085/jgp.81.5.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett P. B., Valenzuela C., Chen L. Q., Kallen R. G. On the molecular nature of the lidocaine receptor of cardiac Na+ channels. Modification of block by alterations in the alpha-subunit III-IV interdomain. Circ Res. 1995 Sep;77(3):584–592. doi: 10.1161/01.res.77.3.584. [DOI] [PubMed] [Google Scholar]
- Bennett P. B., Woosley R. L., Hondeghem L. M. Competition between lidocaine and one of its metabolites, glycylxylidide, for cardiac sodium channels. Circulation. 1988 Sep;78(3):692–700. doi: 10.1161/01.cir.78.3.692. [DOI] [PubMed] [Google Scholar]
- Bennett P. B., Yazawa K., Makita N., George A. L., Jr Molecular mechanism for an inherited cardiac arrhythmia. Nature. 1995 Aug 24;376(6542):683–685. doi: 10.1038/376683a0. [DOI] [PubMed] [Google Scholar]
- Cahalan M. D., Almers W. Interactions between quaternary lidocaine, the sodium channel gates, and tetrodotoxin. Biophys J. 1979 Jul;27(1):39–55. doi: 10.1016/S0006-3495(79)85201-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chahine M., Bennett P. B., George A. L., Jr, Horn R. Functional expression and properties of the human skeletal muscle sodium channel. Pflugers Arch. 1994 May;427(1-2):136–142. doi: 10.1007/BF00585952. [DOI] [PubMed] [Google Scholar]
- Clarkson C. W., Follmer C. H., Ten Eick R. E., Hondeghem L. M., Yeh J. Z. Evidence for two components of sodium channel block by lidocaine in isolated cardiac myocytes. Circ Res. 1988 Nov;63(5):869–878. doi: 10.1161/01.res.63.5.869. [DOI] [PubMed] [Google Scholar]
- Gellens M. E., George A. L., Jr, Chen L. Q., Chahine M., Horn R., Barchi R. L., Kallen R. G. Primary structure and functional expression of the human cardiac tetrodotoxin-insensitive voltage-dependent sodium channel. Proc Natl Acad Sci U S A. 1992 Jan 15;89(2):554–558. doi: 10.1073/pnas.89.2.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George A. L., Jr, Komisarof J., Kallen R. G., Barchi R. L. Primary structure of the adult human skeletal muscle voltage-dependent sodium channel. Ann Neurol. 1992 Feb;31(2):131–137. doi: 10.1002/ana.410310203. [DOI] [PubMed] [Google Scholar]
- George A. L., Jr, Varkony T. A., Drabkin H. A., Han J., Knops J. F., Finley W. H., Brown G. B., Ward D. C., Haas M. Assignment of the human heart tetrodotoxin-resistant voltage-gated Na+ channel alpha-subunit gene (SCN5A) to band 3p21. Cytogenet Cell Genet. 1995;68(1-2):67–70. doi: 10.1159/000133892. [DOI] [PubMed] [Google Scholar]
- Grant A. O., Dietz M. A., Gilliam F. R., 3rd, Starmer C. F. Blockade of cardiac sodium channels by lidocaine. Single-channel analysis. Circ Res. 1989 Nov;65(5):1247–1262. doi: 10.1161/01.res.65.5.1247. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hille B. Local anesthetics: hydrophilic and hydrophobic pathways for the drug-receptor reaction. J Gen Physiol. 1977 Apr;69(4):497–515. doi: 10.1085/jgp.69.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hondeghem L. M., Katzung B. G. Time- and voltage-dependent interactions of antiarrhythmic drugs with cardiac sodium channels. Biochim Biophys Acta. 1977 Nov 14;472(3-4):373–398. doi: 10.1016/0304-4157(77)90003-x. [DOI] [PubMed] [Google Scholar]
- Kayano T., Noda M., Flockerzi V., Takahashi H., Numa S. Primary structure of rat brain sodium channel III deduced from the cDNA sequence. FEBS Lett. 1988 Feb 8;228(1):187–194. doi: 10.1016/0014-5793(88)80614-8. [DOI] [PubMed] [Google Scholar]
- Kuo C. C., Bean B. P. Na+ channels must deactivate to recover from inactivation. Neuron. 1994 Apr;12(4):819–829. doi: 10.1016/0896-6273(94)90335-2. [DOI] [PubMed] [Google Scholar]
- McDonald T. V., Courtney K. R., Clusin W. T. Use-dependent block of single sodium channels by lidocaine in guinea pig ventricular myocytes. Biophys J. 1989 Jun;55(6):1261–1266. doi: 10.1016/S0006-3495(89)82921-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda M., Ikeda T., Suzuki H., Takeshima H., Takahashi T., Kuno M., Numa S. Expression of functional sodium channels from cloned cDNA. 1986 Aug 28-Sep 3Nature. 322(6082):826–828. doi: 10.1038/322826a0. [DOI] [PubMed] [Google Scholar]
- Noda M., Shimizu S., Tanabe T., Takai T., Kayano T., Ikeda T., Takahashi H., Nakayama H., Kanaoka Y., Minamino N. Primary structure of Electrophorus electricus sodium channel deduced from cDNA sequence. Nature. 1984 Nov 8;312(5990):121–127. doi: 10.1038/312121a0. [DOI] [PubMed] [Google Scholar]
- Patlak J. Molecular kinetics of voltage-dependent Na+ channels. Physiol Rev. 1991 Oct;71(4):1047–1080. doi: 10.1152/physrev.1991.71.4.1047. [DOI] [PubMed] [Google Scholar]
- Ragsdale D. S., McPhee J. C., Scheuer T., Catterall W. A. Molecular determinants of state-dependent block of Na+ channels by local anesthetics. Science. 1994 Sep 16;265(5179):1724–1728. doi: 10.1126/science.8085162. [DOI] [PubMed] [Google Scholar]
- Rogart R. B., Cribbs L. L., Muglia L. K., Kephart D. D., Kaiser M. W. Molecular cloning of a putative tetrodotoxin-resistant rat heart Na+ channel isoform. Proc Natl Acad Sci U S A. 1989 Oct;86(20):8170–8174. doi: 10.1073/pnas.86.20.8170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Chapula J., Tsuda Y., Josephson I. R. Voltage- and use-dependent effects of lidocaine on sodium current in rat single ventricular cells. Circ Res. 1983 May;52(5):557–565. doi: 10.1161/01.res.52.5.557. [DOI] [PubMed] [Google Scholar]
- Schwarz W., Palade P. T., Hille B. Local anesthetics. Effect of pH on use-dependent block of sodium channels in frog muscle. Biophys J. 1977 Dec;20(3):343–368. doi: 10.1016/S0006-3495(77)85554-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starmer C. F., Grant A. O., Strauss H. C. Mechanisms of use-dependent block of sodium channels in excitable membranes by local anesthetics. Biophys J. 1984 Jul;46(1):15–27. doi: 10.1016/S0006-3495(84)83994-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strichartz G. R. The inhibition of sodium currents in myelinated nerve by quaternary derivatives of lidocaine. J Gen Physiol. 1973 Jul;62(1):37–57. doi: 10.1085/jgp.62.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimmer J. S., Cooperman S. S., Tomiko S. A., Zhou J. Y., Crean S. M., Boyle M. B., Kallen R. G., Sheng Z. H., Barchi R. L., Sigworth F. J. Primary structure and functional expression of a mammalian skeletal muscle sodium channel. Neuron. 1989 Jul;3(1):33–49. doi: 10.1016/0896-6273(89)90113-x. [DOI] [PubMed] [Google Scholar]
- Valenzuela C., Bennett P. B., Jr Gating of cardiac Na+ channels in excised membrane patches after modification by alpha-chymotrypsin. Biophys J. 1994 Jul;67(1):161–171. doi: 10.1016/S0006-3495(94)80465-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D. W., George A. L., Jr, Bennett P. B. Comparison of heterologously expressed human cardiac and skeletal muscle sodium channels. Biophys J. 1996 Jan;70(1):238–245. doi: 10.1016/S0006-3495(96)79566-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West J. W., Patton D. E., Scheuer T., Wang Y., Goldin A. L., Catterall W. A. A cluster of hydrophobic amino acid residues required for fast Na(+)-channel inactivation. Proc Natl Acad Sci U S A. 1992 Nov 15;89(22):10910–10914. doi: 10.1073/pnas.89.22.10910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue D. T., Lawrence J. H., Marban E. Two molecular transitions influence cardiac sodium channel gating. Science. 1989 Apr 21;244(4902):349–352. doi: 10.1126/science.2540529. [DOI] [PubMed] [Google Scholar]


