Abstract
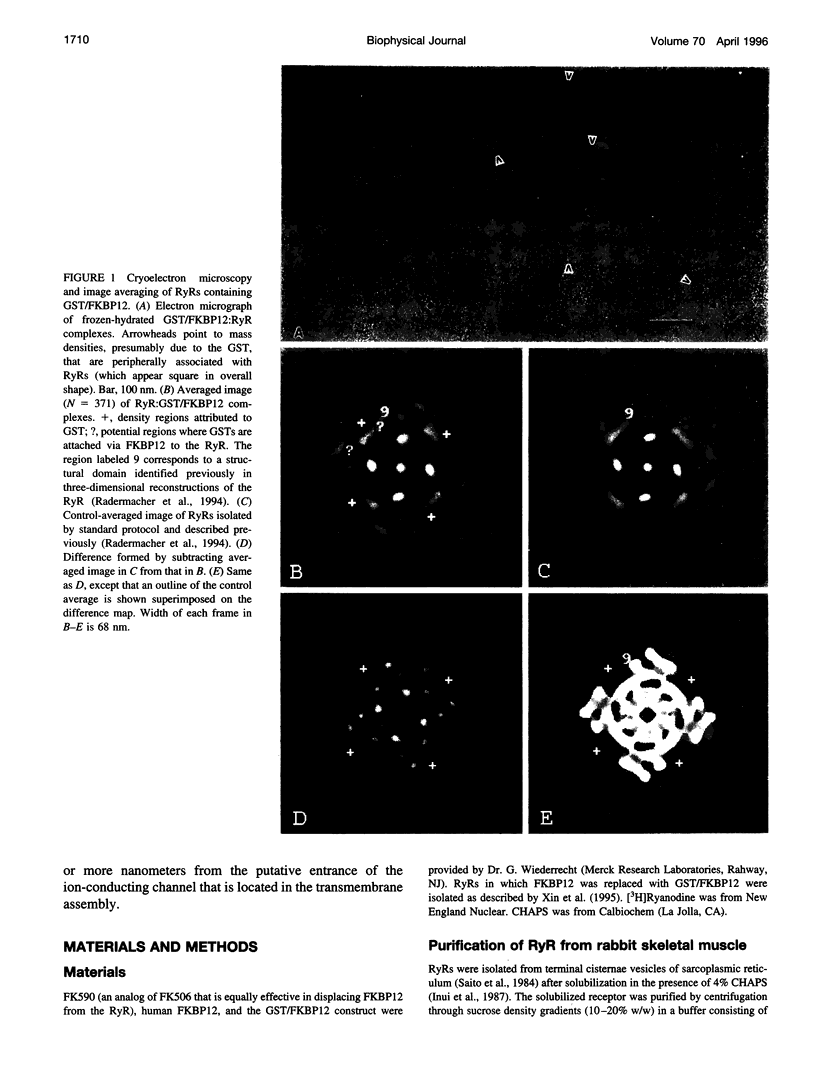
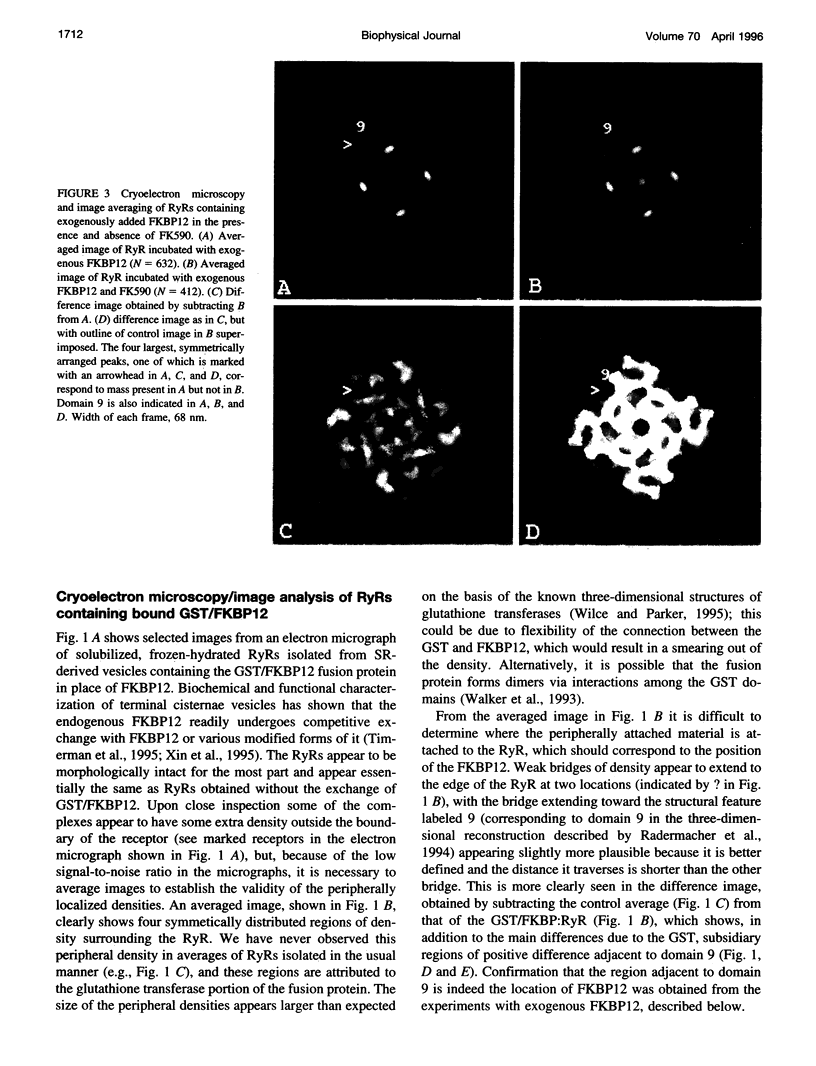
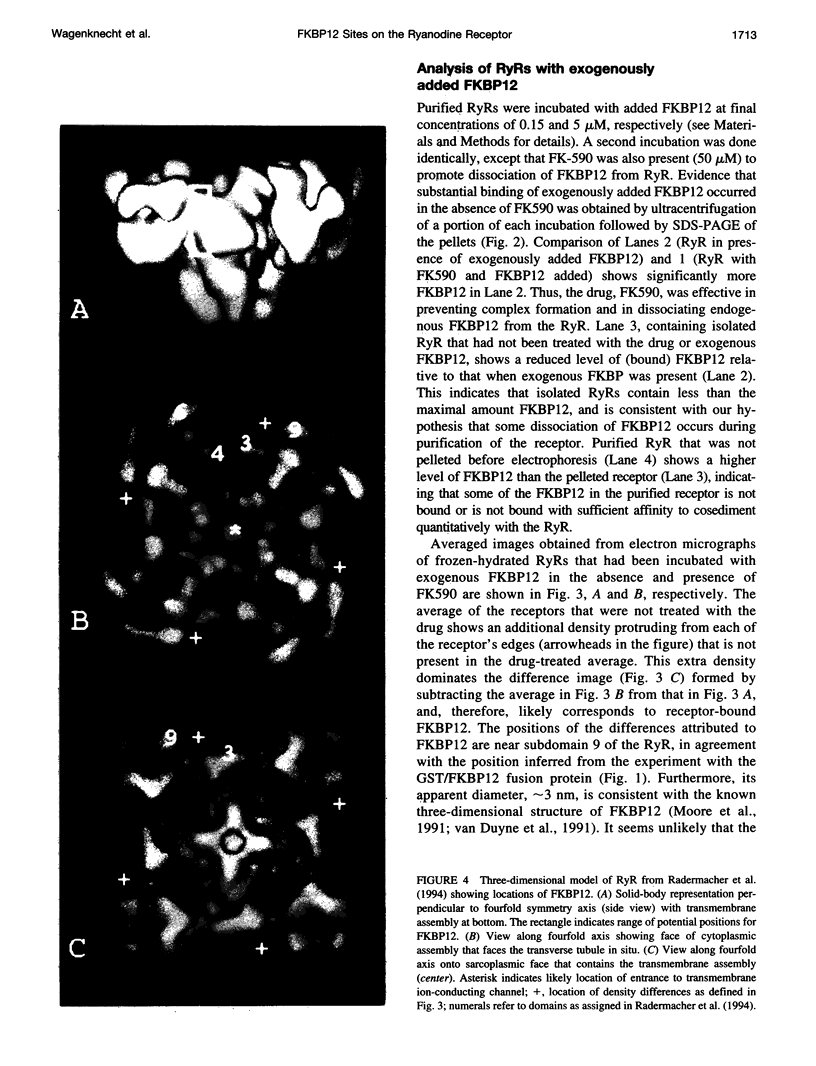
A 12-kDa immunophilin (FKBP12) is an integral component of the skeletal muscle ryanodine receptor (RyR). The RyR is a hetero-oligomeric complex with structural formula (FKBP)4(Ryr1)4, where Ryr1 is the 565-kDa product of the Ryr1 gene. To aid in the detection of the immunophilin's location in the receptor, we exchanged the FKBP12 present in RyR-enriched vesicles derived from sarcoplasmic reticulum with an engineered construct of FKBP12 fused to glutathione S-transferase and then isolated the complexes. Cryoelectron microscopy and image averaging of the complexes (in an orientation displaying the RyR's fourfold symmetry) revealed four symmetrically distributed, diffuse density regions that were located just outside the boundary defining the cytoplasmic assembly of the RyR. These regions are attributed to the glutathione transferase portion of the fusion protein because they are absent from receptors lacking the fusion protein. To more precisely define the location of FKBP12, we similarly analyzed complexes of RyR containing FKBP12 itself. Apparently some FKBP is lost during the purification or storage of the RyR because, to detect the receptor-bound immunophilin, it was necessary to add FKBP12 to the purified receptor before electron microscopy. Averaged images of these complexes showed a region of density that had not been observed previously in images of isolated receptors, and its position, along the edges of the transmembrane assembly, agreed with the position of the FKBP12 deduced from the experiments with the fusion protein. The proposed locations for FKBP12 are about 10 nm from the transmembrane baseplate assembly that contains the ion channel of the RyR.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brillantes A. B., Ondrias K., Scott A., Kobrinsky E., Ondriasová E., Moschella M. C., Jayaraman T., Landers M., Ehrlich B. E., Marks A. R. Stabilization of calcium release channel (ryanodine receptor) function by FK506-binding protein. Cell. 1994 May 20;77(4):513–523. doi: 10.1016/0092-8674(94)90214-3. [DOI] [PubMed] [Google Scholar]
- Cameron A. M., Steiner J. P., Sabatini D. M., Kaplin A. I., Walensky L. D., Snyder S. H. Immunophilin FK506 binding protein associated with inositol 1,4,5-trisphosphate receptor modulates calcium flux. Proc Natl Acad Sci U S A. 1995 Feb 28;92(5):1784–1788. doi: 10.1073/pnas.92.5.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S. R., Zhang L., MacLennan D. H. Asymmetrical blockade of the Ca2+ release channel (ryanodine receptor) by 12-kDa FK506 binding protein. Proc Natl Acad Sci U S A. 1994 Dec 6;91(25):11953–11957. doi: 10.1073/pnas.91.25.11953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins J. H. Sequence analysis of the ryanodine receptor: possible association with a 12K, FK506-binding immunophilin/protein kinase C inhibitor. Biochem Biophys Res Commun. 1991 Aug 15;178(3):1288–1290. doi: 10.1016/0006-291x(91)91033-9. [DOI] [PubMed] [Google Scholar]
- Coronado R., Morrissette J., Sukhareva M., Vaughan D. M. Structure and function of ryanodine receptors. Am J Physiol. 1994 Jun;266(6 Pt 1):C1485–C1504. doi: 10.1152/ajpcell.1994.266.6.C1485. [DOI] [PubMed] [Google Scholar]
- Fleischer S., Inui M. Biochemistry and biophysics of excitation-contraction coupling. Annu Rev Biophys Biophys Chem. 1989;18:333–364. doi: 10.1146/annurev.bb.18.060189.002001. [DOI] [PubMed] [Google Scholar]
- Frank J., Verschoor A., Boublik M. Computer averaging of electron micrographs of 40S ribosomal subunits. Science. 1981 Dec 18;214(4527):1353–1355. doi: 10.1126/science.7313694. [DOI] [PubMed] [Google Scholar]
- Fruman D. A., Burakoff S. J., Bierer B. E. Immunophilins in protein folding and immunosuppression. FASEB J. 1994 Apr 1;8(6):391–400. doi: 10.1096/fasebj.8.6.7513288. [DOI] [PubMed] [Google Scholar]
- Inui M., Saito A., Fleischer S. Purification of the ryanodine receptor and identity with feet structures of junctional terminal cisternae of sarcoplasmic reticulum from fast skeletal muscle. J Biol Chem. 1987 Feb 5;262(4):1740–1747. [PubMed] [Google Scholar]
- Jayaraman T., Brillantes A. M., Timerman A. P., Fleischer S., Erdjument-Bromage H., Tempst P., Marks A. R. FK506 binding protein associated with the calcium release channel (ryanodine receptor). J Biol Chem. 1992 May 15;267(14):9474–9477. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lai F. A., Erickson H. P., Rousseau E., Liu Q. Y., Meissner G. Purification and reconstitution of the calcium release channel from skeletal muscle. Nature. 1988 Jan 28;331(6154):315–319. doi: 10.1038/331315a0. [DOI] [PubMed] [Google Scholar]
- Ma J., Bhat M. B., Zhao J. Rectification of skeletal muscle ryanodine receptor mediated by FK506 binding protein. Biophys J. 1995 Dec;69(6):2398–2404. doi: 10.1016/S0006-3495(95)80109-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayrleitner M., Timerman A. P., Wiederrecht G., Fleischer S. The calcium release channel of sarcoplasmic reticulum is modulated by FK-506 binding protein: effect of FKBP-12 on single channel activity of the skeletal muscle ryanodine receptor. Cell Calcium. 1994 Feb;15(2):99–108. doi: 10.1016/0143-4160(94)90048-5. [DOI] [PubMed] [Google Scholar]
- Meissner G. Ryanodine receptor/Ca2+ release channels and their regulation by endogenous effectors. Annu Rev Physiol. 1994;56:485–508. doi: 10.1146/annurev.ph.56.030194.002413. [DOI] [PubMed] [Google Scholar]
- Moore J. M., Peattie D. A., Fitzgibbon M. J., Thomson J. A. Solution structure of the major binding protein for the immunosuppressant FK506. Nature. 1991 May 16;351(6323):248–250. doi: 10.1038/351248a0. [DOI] [PubMed] [Google Scholar]
- Ogawa Y. Role of ryanodine receptors. Crit Rev Biochem Mol Biol. 1994;29(4):229–274. doi: 10.3109/10409239409083482. [DOI] [PubMed] [Google Scholar]
- Radermacher M., Rao V., Grassucci R., Frank J., Timerman A. P., Fleischer S., Wagenknecht T. Cryo-electron microscopy and three-dimensional reconstruction of the calcium release channel/ryanodine receptor from skeletal muscle. J Cell Biol. 1994 Oct;127(2):411–423. doi: 10.1083/jcb.127.2.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radermacher M., Wagenknecht T., Grassucci R., Frank J., Inui M., Chadwick C., Fleischer S. Cryo-EM of the native structure of the calcium release channel/ryanodine receptor from sarcoplasmic reticulum. Biophys J. 1992 Apr;61(4):936–940. doi: 10.1016/S0006-3495(92)81900-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford S. L., Zuker C. S. Protein folding and the regulation of signaling pathways. Cell. 1994 Dec 30;79(7):1129–1132. doi: 10.1016/0092-8674(94)90003-5. [DOI] [PubMed] [Google Scholar]
- Saito A., Seiler S., Chu A., Fleischer S. Preparation and morphology of sarcoplasmic reticulum terminal cisternae from rabbit skeletal muscle. J Cell Biol. 1984 Sep;99(3):875–885. doi: 10.1083/jcb.99.3.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timerman A. P., Jayaraman T., Wiederrecht G., Onoue H., Marks A. R., Fleischer S. The ryanodine receptor from canine heart sarcoplasmic reticulum is associated with a novel FK-506 binding protein. Biochem Biophys Res Commun. 1994 Jan 28;198(2):701–706. doi: 10.1006/bbrc.1994.1101. [DOI] [PubMed] [Google Scholar]
- Timerman A. P., Ogunbumni E., Freund E., Wiederrecht G., Marks A. R., Fleischer S. The calcium release channel of sarcoplasmic reticulum is modulated by FK-506-binding protein. Dissociation and reconstitution of FKBP-12 to the calcium release channel of skeletal muscle sarcoplasmic reticulum. J Biol Chem. 1993 Nov 5;268(31):22992–22999. [PubMed] [Google Scholar]
- Timerman A. P., Wiederrecht G., Marcy A., Fleischer S. Characterization of an exchange reaction between soluble FKBP-12 and the FKBP.ryanodine receptor complex. Modulation by FKBP mutants deficient in peptidyl-prolyl isomerase activity. J Biol Chem. 1995 Feb 10;270(6):2451–2459. doi: 10.1074/jbc.270.6.2451. [DOI] [PubMed] [Google Scholar]
- Tripathy A., Xu L., Mann G., Meissner G. Calmodulin activation and inhibition of skeletal muscle Ca2+ release channel (ryanodine receptor). Biophys J. 1995 Jul;69(1):106–119. doi: 10.1016/S0006-3495(95)79880-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Duyne G. D., Standaert R. F., Karplus P. A., Schreiber S. L., Clardy J. Atomic structure of FKBP-FK506, an immunophilin-immunosuppressant complex. Science. 1991 May 10;252(5007):839–842. doi: 10.1126/science.1709302. [DOI] [PubMed] [Google Scholar]
- Wagenknecht T., Berkowitz J., Grassucci R., Timerman A. P., Fleischer S. Localization of calmodulin binding sites on the ryanodine receptor from skeletal muscle by electron microscopy. Biophys J. 1994 Dec;67(6):2286–2295. doi: 10.1016/S0006-3495(94)80714-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagenknecht T., Grassucci R., Frank J., Saito A., Inui M., Fleischer S. Three-dimensional architecture of the calcium channel/foot structure of sarcoplasmic reticulum. Nature. 1989 Mar 9;338(6211):167–170. doi: 10.1038/338167a0. [DOI] [PubMed] [Google Scholar]
- Wagenknecht T., Radermacher M. Three-dimensional architecture of the skeletal muscle ryanodine receptor. FEBS Lett. 1995 Aug 1;369(1):43–46. doi: 10.1016/0014-5793(95)00581-s. [DOI] [PubMed] [Google Scholar]
- Walker J., Crowley P., Moreman A. D., Barrett J. Biochemical properties of cloned glutathione S-transferases from Schistosoma mansoni and Schistosoma japonicum. Mol Biochem Parasitol. 1993 Oct;61(2):255–264. doi: 10.1016/0166-6851(93)90071-5. [DOI] [PubMed] [Google Scholar]
- Wilce M. C., Parker M. W. Structure and function of glutathione S-transferases. Biochim Biophys Acta. 1994 Mar 16;1205(1):1–18. doi: 10.1016/0167-4838(94)90086-8. [DOI] [PubMed] [Google Scholar]
- Xin H. B., Timerman A. P., Onoue H., Wiederrecht G. J., Fleischer S. Affinity purification of the ryanodine receptor/calcium release channel from fast twitch skeletal muscle based on its tight association with FKBP12. Biochem Biophys Res Commun. 1995 Sep 5;214(1):263–270. doi: 10.1006/bbrc.1995.2283. [DOI] [PubMed] [Google Scholar]
- Yang H. C., Reedy M. M., Burke C. L., Strasburg G. M. Calmodulin interaction with the skeletal muscle sarcoplasmic reticulum calcium channel protein. Biochemistry. 1994 Jan 18;33(2):518–525. doi: 10.1021/bi00168a017. [DOI] [PubMed] [Google Scholar]