Abstract
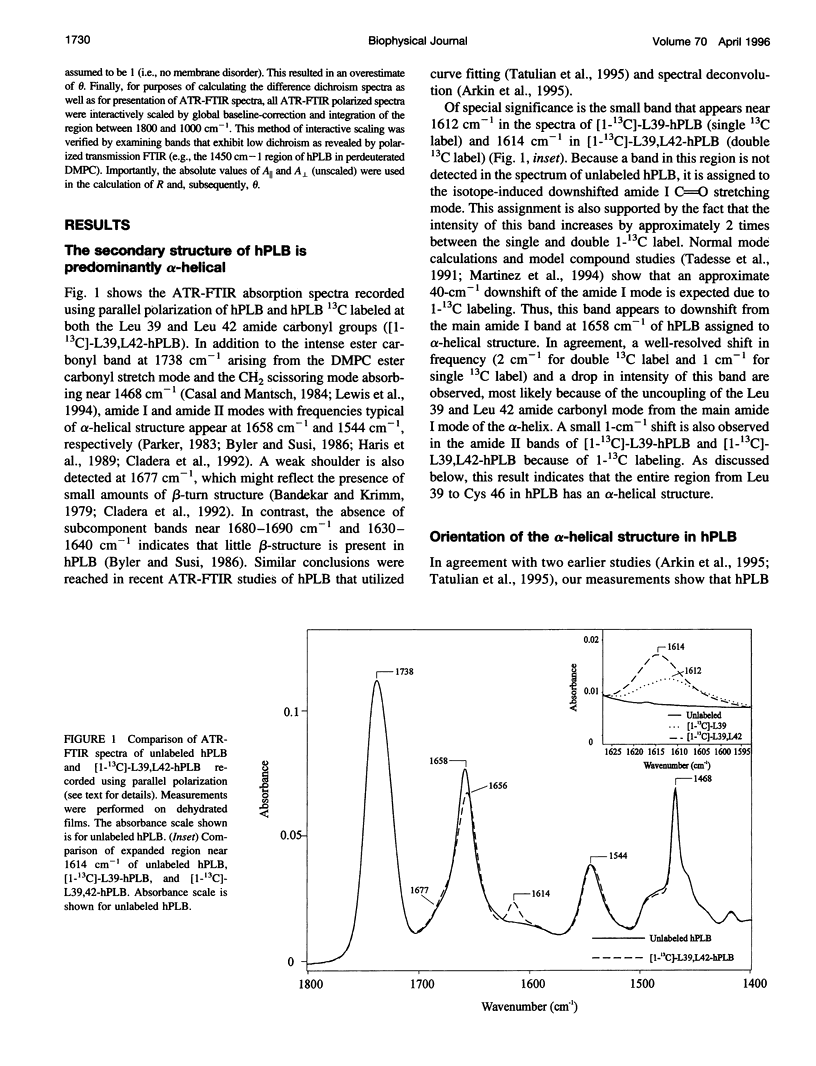
Phospholamban is a 52-amino acid residue membrane protein that regulates Ca(2+)-ATPase activity in the sarcoplasmic reticulum of cardiac muscle cells. The hydrophobic C-terminal 28 amino acid fragment of phospholamban (hPLB) anchors the protein in the membrane and may form part of a Ca(2+)-selective ion channel. We have used polarized attenuated total reflection-Fourier transform infrared (ATR-FTIR) spectroscopy along with site-directed isotope labeling to probe the local structure of hPLB. The frequency and dichroism of the amide I and II bands appearing at 1658 cm-1 and 1544 cm-1, respectively, show that dehydrated and hydrated hPLB reconstituted into dimyristoylphosphatidycholine bilayer membranes is predominantly alpha-helical and has a net transmembrane orientation. Specific local secondary structure of hPLB was probed by incorporating 13C at two positions in the protein backbone. A small band seen near 1614 cm-1 is assigned to the amide I mode of the 13C-labeled amide carbonyl group(s). The frequency and dichroism of this band indicate that residues 39 and 46 are alpha-helical, with an axial orientation that is approximately 30 degrees relative to the membrane normal. Upon exposure to 2H2O (D2O), 30% of the peptide amide groups in hPLB undergo a slow deuterium/hydrogen exchange. The remainder of the protein, including the peptide groups of Leu-39 and Leu-42, appear inaccessible to exchange, indicating that most of the hPLB fragment is embedded in the lipid bilayer. By extending spectroscopic characterization of PLB to include hydrated, deuterated as well as site-directed isotope-labeled hPLB films, our results strongly support models of PLB that predict the existence of an alpha-helical hydrophobic region spanning the membrane domain.
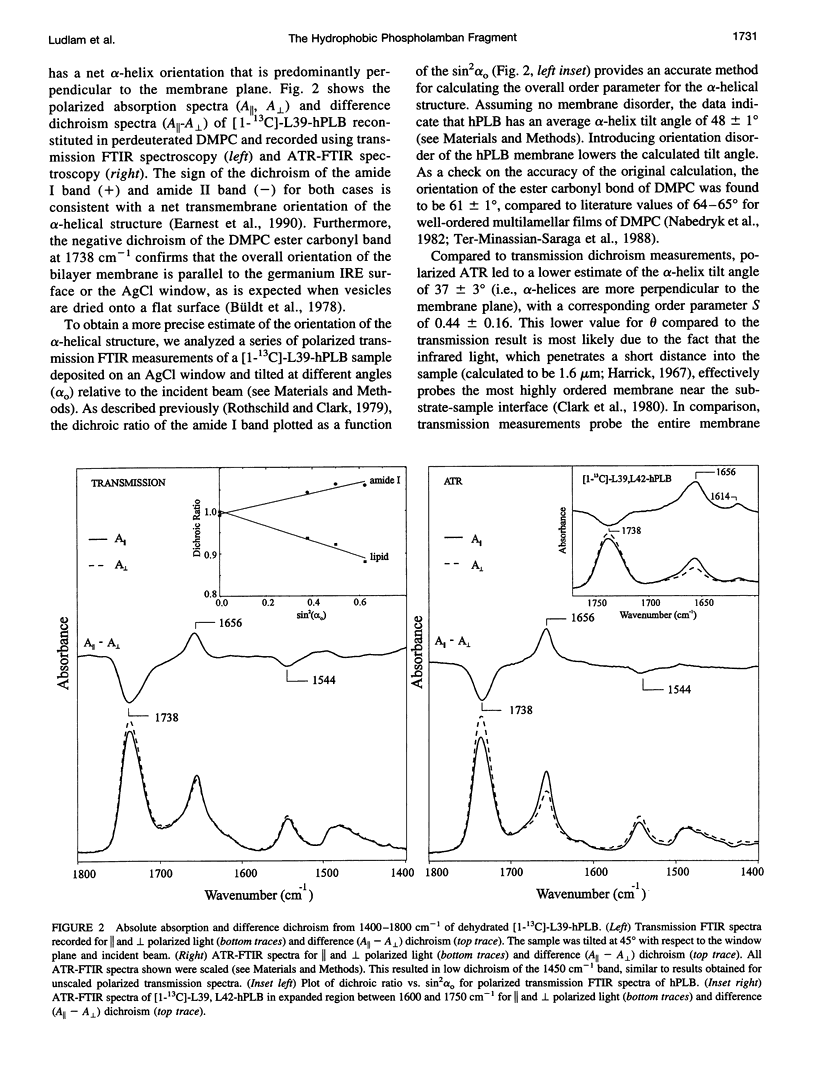
Full text
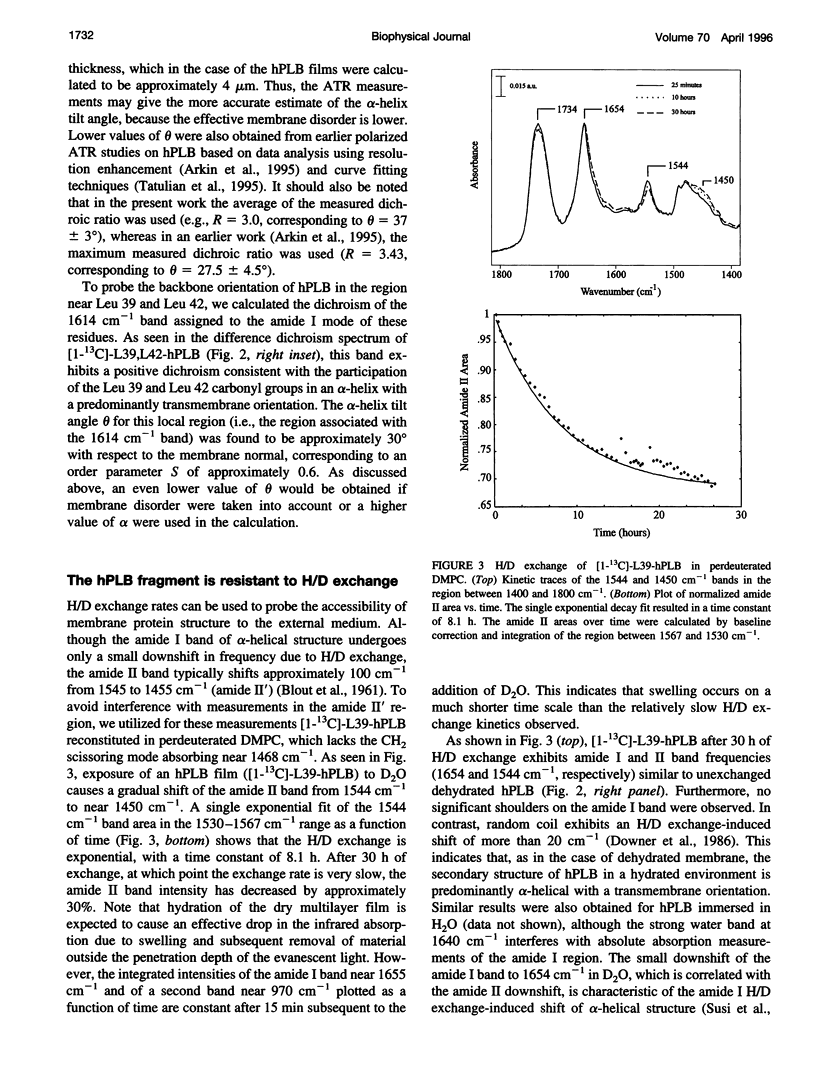
PDF

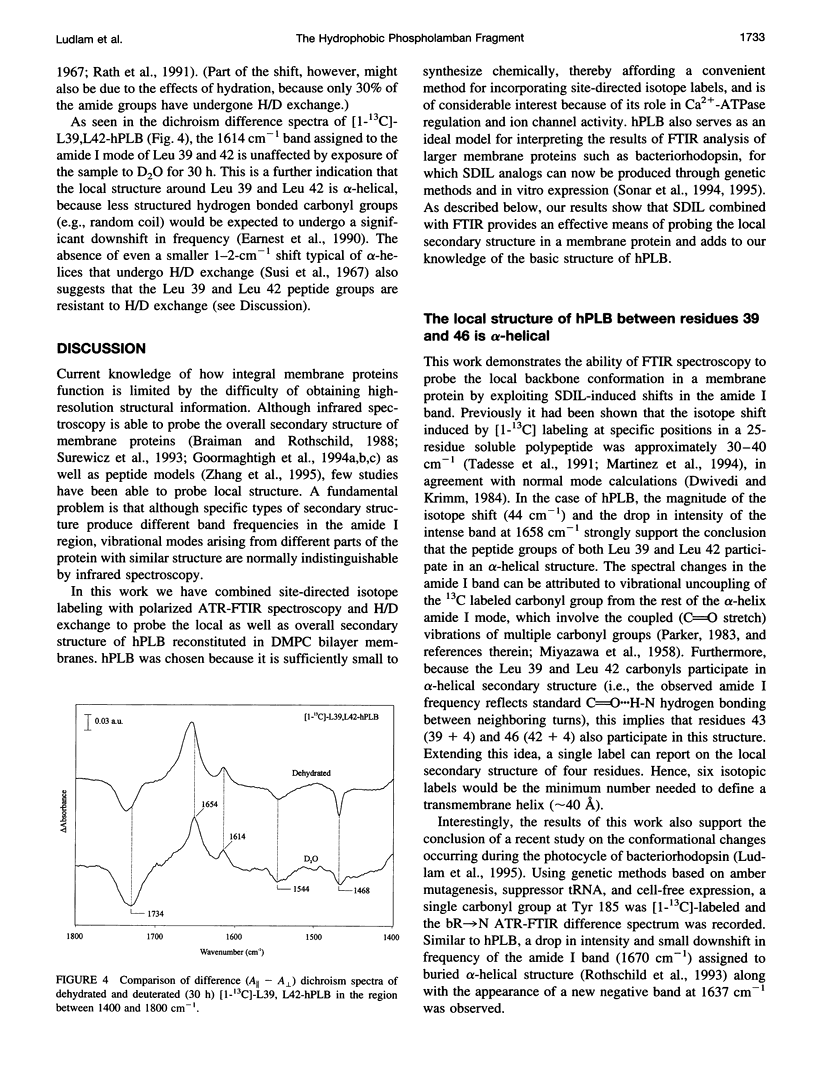



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. D., Arkin I. T., Engelman D. M., Brünger A. T. Computational searching and mutagenesis suggest a structure for the pentameric transmembrane domain of phospholamban. Nat Struct Biol. 1995 Feb;2(2):154–162. doi: 10.1038/nsb0295-154. [DOI] [PubMed] [Google Scholar]
- Arkin I. T., Adams P. D., MacKenzie K. R., Lemmon M. A., Brünger A. T., Engelman D. M. Structural organization of the pentameric transmembrane alpha-helices of phospholamban, a cardiac ion channel. EMBO J. 1994 Oct 17;13(20):4757–4764. doi: 10.1002/j.1460-2075.1994.tb06801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arkin I. T., Rothman M., Ludlam C. F., Aimoto S., Engelman D. M., Rothschild K. J., Smith S. O. Structural model of the phospholamban ion channel complex in phospholipid membranes. J Mol Biol. 1995 May 12;248(4):824–834. doi: 10.1006/jmbi.1995.0263. [DOI] [PubMed] [Google Scholar]
- BRADBURY E. M., BROWN L., DOWNIE A. R., ELLIOTT A., FRASER R. D., HANBY W. E. The structure of the omegaform of poly-Beta-benzyl-L-aspartate. J Mol Biol. 1962 Aug;5:230–247. doi: 10.1016/s0022-2836(62)80086-2. [DOI] [PubMed] [Google Scholar]
- Bandekar J., Krimm S. Vibrational analysis of peptides, polypeptides, and proteins: Characteristic amide bands of beta-turns. Proc Natl Acad Sci U S A. 1979 Feb;76(2):774–777. doi: 10.1073/pnas.76.2.774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braiman M. S., Rothschild K. J. Fourier transform infrared techniques for probing membrane protein structure. Annu Rev Biophys Biophys Chem. 1988;17:541–570. doi: 10.1146/annurev.bb.17.060188.002545. [DOI] [PubMed] [Google Scholar]
- Byler D. M., Susi H. Examination of the secondary structure of proteins by deconvolved FTIR spectra. Biopolymers. 1986 Mar;25(3):469–487. doi: 10.1002/bip.360250307. [DOI] [PubMed] [Google Scholar]
- Büldt G., Gally H. U., Seelig A., Seelig J., Zaccai G. Neutron diffraction studies on selectively deuterated phospholipid bilayers. Nature. 1978 Jan 12;271(5641):182–184. doi: 10.1038/271182a0. [DOI] [PubMed] [Google Scholar]
- Casal H. L., Mantsch H. H. Polymorphic phase behaviour of phospholipid membranes studied by infrared spectroscopy. Biochim Biophys Acta. 1984 Dec 4;779(4):381–401. doi: 10.1016/0304-4157(84)90017-0. [DOI] [PubMed] [Google Scholar]
- Cladera J., Sabés M., Padrós E. Fourier transform infrared analysis of bacteriorhodopsin secondary structure. Biochemistry. 1992 Dec 15;31(49):12363–12368. doi: 10.1021/bi00164a010. [DOI] [PubMed] [Google Scholar]
- Clark N. A., Rothschild K. J., Luippold D. A., Simon B. A. Surface-induced lamellar orientation of multilayer membrane arrays. Theoretical analysis and a new method with application to purple membrane fragments. Biophys J. 1980 Jul;31(1):65–96. doi: 10.1016/S0006-3495(80)85041-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downer N. W., Bruchman T. J., Hazzard J. H. Infrared spectroscopic study of photoreceptor membrane and purple membrane. Protein secondary structure and hydrogen deuterium exchange. J Biol Chem. 1986 Mar 15;261(8):3640–3647. [PubMed] [Google Scholar]
- Dwivedi A. M., Krimm S. Vibrational analysis of peptides, polypeptides, and proteins. XVIII. Conformational sensitivity of the alpha-helix spectrum: alpha I- and alpha II-poly(L-alanine). Biopolymers. 1984 May;23(5):923–943. doi: 10.1002/bip.360230509. [DOI] [PubMed] [Google Scholar]
- Earnest T. N., Herzfeld J., Rothschild K. J. Polarized Fourier transform infrared spectroscopy of bacteriorhodopsin. Transmembrane alpha helices are resistant to hydrogen/deuterium exchange. Biophys J. 1990 Dec;58(6):1539–1546. doi: 10.1016/S0006-3495(90)82498-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey S., Tamm L. K. Orientation of melittin in phospholipid bilayers. A polarized attenuated total reflection infrared study. Biophys J. 1991 Oct;60(4):922–930. doi: 10.1016/S0006-3495(91)82126-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fringeli U. P., Apell H. J., Fringeli M., Läuger P. Polarized infrared absorption of Na+/K+-ATPase studied by attenuated total reflection spectroscopy. Biochim Biophys Acta. 1989 Sep 18;984(3):301–312. doi: 10.1016/0005-2736(89)90297-6. [DOI] [PubMed] [Google Scholar]
- Goormaghtigh E., Cabiaux V., Ruysschaert J. M. Determination of soluble and membrane protein structure by Fourier transform infrared spectroscopy. I. Assignments and model compounds. Subcell Biochem. 1994;23:329–362. doi: 10.1007/978-1-4615-1863-1_8. [DOI] [PubMed] [Google Scholar]
- Goormaghtigh E., Cabiaux V., Ruysschaert J. M. Determination of soluble and membrane protein structure by Fourier transform infrared spectroscopy. II. Experimental aspects, side chain structure, and H/D exchange. Subcell Biochem. 1994;23:363–403. doi: 10.1007/978-1-4615-1863-1_9. [DOI] [PubMed] [Google Scholar]
- Goormaghtigh E., Cabiaux V., Ruysschaert J. M. Determination of soluble and membrane protein structure by Fourier transform infrared spectroscopy. III. Secondary structures. Subcell Biochem. 1994;23:405–450. doi: 10.1007/978-1-4615-1863-1_10. [DOI] [PubMed] [Google Scholar]
- Haris P. I., Coke M., Chapman D. Fourier transform infrared spectroscopic investigation of rhodopsin structure and its comparison with bacteriorhodopsin. Biochim Biophys Acta. 1989 Apr 6;995(2):160–167. doi: 10.1016/0167-4838(89)90075-7. [DOI] [PubMed] [Google Scholar]
- Kovacs R. J., Nelson M. T., Simmerman H. K., Jones L. R. Phospholamban forms Ca2+-selective channels in lipid bilayers. J Biol Chem. 1988 Dec 5;263(34):18364–18368. [PubMed] [Google Scholar]
- Lewis R. N., McElhaney R. N., Pohle W., Mantsch H. H. Components of the carbonyl stretching band in the infrared spectra of hydrated 1,2-diacylglycerolipid bilayers: a reevaluation. Biophys J. 1994 Dec;67(6):2367–2375. doi: 10.1016/S0006-3495(94)80723-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludlam C. F., Sonar S., Lee C. P., Coleman M., Herzfeld J., RajBhandary U. L., Rothschild K. J. Site-directed isotope labeling and ATR-FTIR difference spectroscopy of bacteriorhodopsin: the peptide carbonyl group of Tyr 185 is structurally active during the bR-->N transition. Biochemistry. 1995 Jan 10;34(1):2–6. doi: 10.1021/bi00001a001. [DOI] [PubMed] [Google Scholar]
- Marrero H., Rothschild K. J. Conformational changes in bacteriorhodopsin studied by infrared attenuated total reflection. Biophys J. 1987 Oct;52(4):629–635. doi: 10.1016/S0006-3495(87)83254-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Méthot N., McCarthy M. P., Baenziger J. E. Secondary structure of the nicotinic acetylcholine receptor: implications for structural models of a ligand-gated ion channel. Biochemistry. 1994 Jun 21;33(24):7709–7717. doi: 10.1021/bi00190a026. [DOI] [PubMed] [Google Scholar]
- Nabedryk E., Bardin A. M., Breton J. Further characterization of protein secondary structures in purple membrane by circular dichroism and polarized infrared spectroscopies. Biophys J. 1985 Dec;48(6):873–876. doi: 10.1016/S0006-3495(85)83848-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabedryk E., Gingold M. P., Breton J. Orientation of gramicidin A transmembrane channel. Infrared dichroism study of gramicidin in vesicles. Biophys J. 1982 Jun;38(3):243–249. doi: 10.1016/S0006-3495(82)84555-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rath P., Bousché O., Merrill A. R., Cramer W. A., Rothschild K. J. Fourier transform infrared evidence for a predominantly alpha-helical structure of the membrane bound channel forming COOH-terminal peptide of colicin E1. Biophys J. 1991 Mar;59(3):516–522. doi: 10.1016/S0006-3495(91)82268-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothschild K. J., Clark N. A. Polarized infrared spectroscopy of oriented purple membrane. Biophys J. 1979 Mar;25(3):473–487. doi: 10.1016/S0006-3495(79)85317-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothschild K. J., Marti T., Sonar S., He Y. W., Rath P., Fischer W., Khorana H. G. Asp96 deprotonation and transmembrane alpha-helical structural changes in bacteriorhodopsin. J Biol Chem. 1993 Dec 25;268(36):27046–27052. [PubMed] [Google Scholar]
- Rothschild K. J., Sanches R., Hsiao T. L., Clark N. A. A spectroscopic study of rhodopsin alpha-helix orientation. Biophys J. 1980 Jul;31(1):53–64. doi: 10.1016/S0006-3495(80)85040-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmerman H. K., Lovelace D. E., Jones L. R. Secondary structure of detergent-solubilized phospholamban, a phosphorylatable, oligomeric protein of cardiac sarcoplasmic reticulum. Biochim Biophys Acta. 1989 Aug 31;997(3):322–329. doi: 10.1016/0167-4838(89)90203-3. [DOI] [PubMed] [Google Scholar]
- Sonar S., Lee C. P., Coleman M., Patel N., Liu X., Marti T., Khorana H. G., RajBhandary U. L., Rothschild K. J. Site-directed isotope labelling and FTIR spectroscopy of bacteriorhodopsin. Nat Struct Biol. 1994 Aug;1(8):512–517. doi: 10.1038/nsb0894-512. [DOI] [PubMed] [Google Scholar]
- Surewicz W. K., Mantsch H. H., Chapman D. Determination of protein secondary structure by Fourier transform infrared spectroscopy: a critical assessment. Biochemistry. 1993 Jan 19;32(2):389–394. doi: 10.1021/bi00053a001. [DOI] [PubMed] [Google Scholar]
- Susi H., Timasheff S. N., Stevens L. Infrared spectra and protein conformations in aqueous solutions. I. The amide I band in H2O and D2O solutions. J Biol Chem. 1967 Dec 10;242(23):5460–5466. [PubMed] [Google Scholar]
- Tada M., Kadoma M. Regulation of the Ca2+ pump ATPase by cAMP-dependent phosphorylation of phospholamban. Bioessays. 1989 May;10(5):157–163. doi: 10.1002/bies.950100505. [DOI] [PubMed] [Google Scholar]
- Tada M. Molecular structure and function of phospholamban in regulating the calcium pump from sarcoplasmic reticulum. Ann N Y Acad Sci. 1992 Nov 30;671:92–103. doi: 10.1111/j.1749-6632.1992.tb43787.x. [DOI] [PubMed] [Google Scholar]
- Tamm L. K., Tatulian S. A. Orientation of functional and nonfunctional PTS permease signal sequences in lipid bilayers. A polarized attenuated total reflection infrared study. Biochemistry. 1993 Aug 3;32(30):7720–7726. doi: 10.1021/bi00081a017. [DOI] [PubMed] [Google Scholar]
- Tatulian S. A., Jones L. R., Reddy L. G., Stokes D. L., Tamm L. K. Secondary structure and orientation of phospholamban reconstituted in supported bilayers from polarized attenuated total reflection FTIR spectroscopy. Biochemistry. 1995 Apr 4;34(13):4448–4456. doi: 10.1021/bi00013a038. [DOI] [PubMed] [Google Scholar]
- Ter-Minassian-Saraga L., Okamura E., Umemura J., Takenaka T. Fourier transform infrared-attenuated total reflection spectroscopy of hydration of dimyristoylphosphatidylcholine multibilayers. Biochim Biophys Acta. 1988 Dec 22;946(2):417–423. doi: 10.1016/0005-2736(88)90417-8. [DOI] [PubMed] [Google Scholar]
- Toyofuku T., Kurzydlowski K., Tada M., MacLennan D. H. Amino acids Glu2 to Ile18 in the cytoplasmic domain of phospholamban are essential for functional association with the Ca(2+)-ATPase of sarcoplasmic reticulum. J Biol Chem. 1994 Jan 28;269(4):3088–3094. [PubMed] [Google Scholar]
- Wegener A. D., Simmerman H. K., Lindemann J. P., Jones L. R. Phospholamban phosphorylation in intact ventricles. Phosphorylation of serine 16 and threonine 17 in response to beta-adrenergic stimulation. J Biol Chem. 1989 Jul 5;264(19):11468–11474. [PubMed] [Google Scholar]
- Zhang Y. P., Lewis R. N., Hodges R. S., McElhaney R. N. FTIR spectroscopic studies of the conformation and amide hydrogen exchange of a peptide model of the hydrophobic transmembrane alpha-helices of membrane proteins. Biochemistry. 1992 Nov 24;31(46):11572–11578. doi: 10.1021/bi00161a041. [DOI] [PubMed] [Google Scholar]
- Zhang Y. P., Lewis R. N., Hodges R. S., McElhaney R. N. Peptide models of helical hydrophobic transmembrane segments of membrane proteins. 2. Differential scanning calorimetric and FTIR spectroscopic studies of the interaction of Ac-K2-(LA)12-K2-amide with phosphatidylcholine bilayers. Biochemistry. 1995 Feb 21;34(7):2362–2371. doi: 10.1021/bi00007a032. [DOI] [PubMed] [Google Scholar]


