Abstract
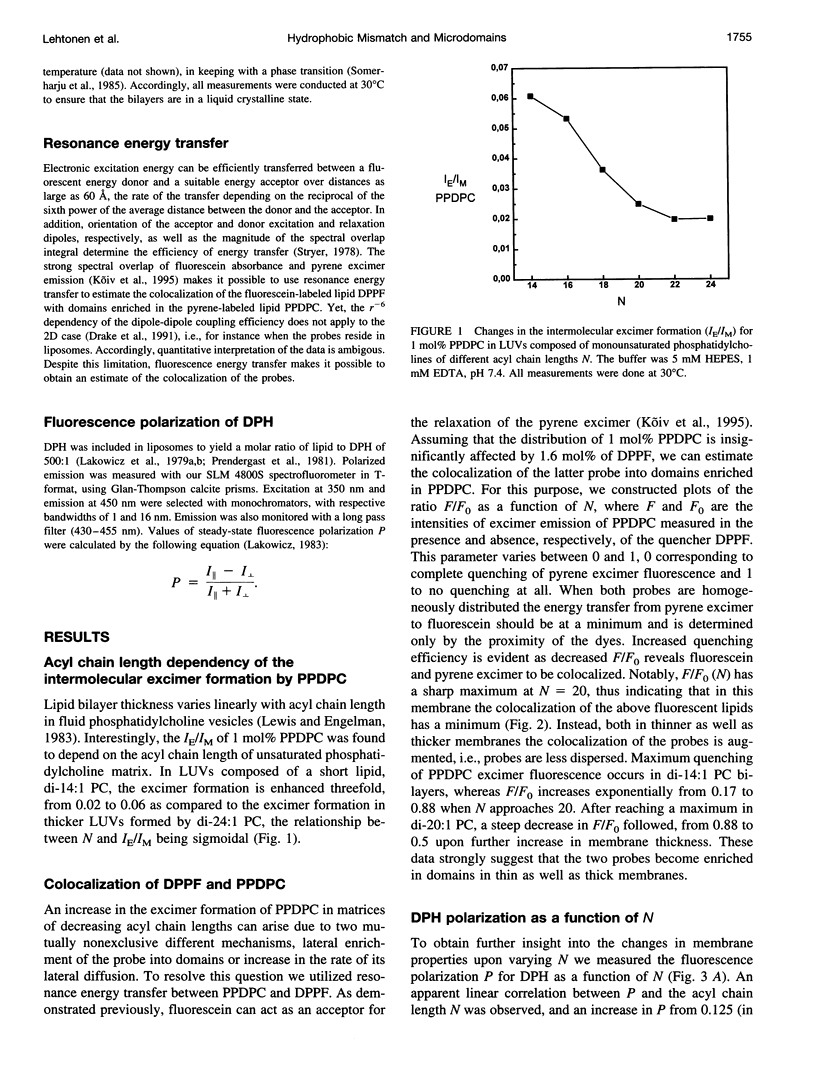
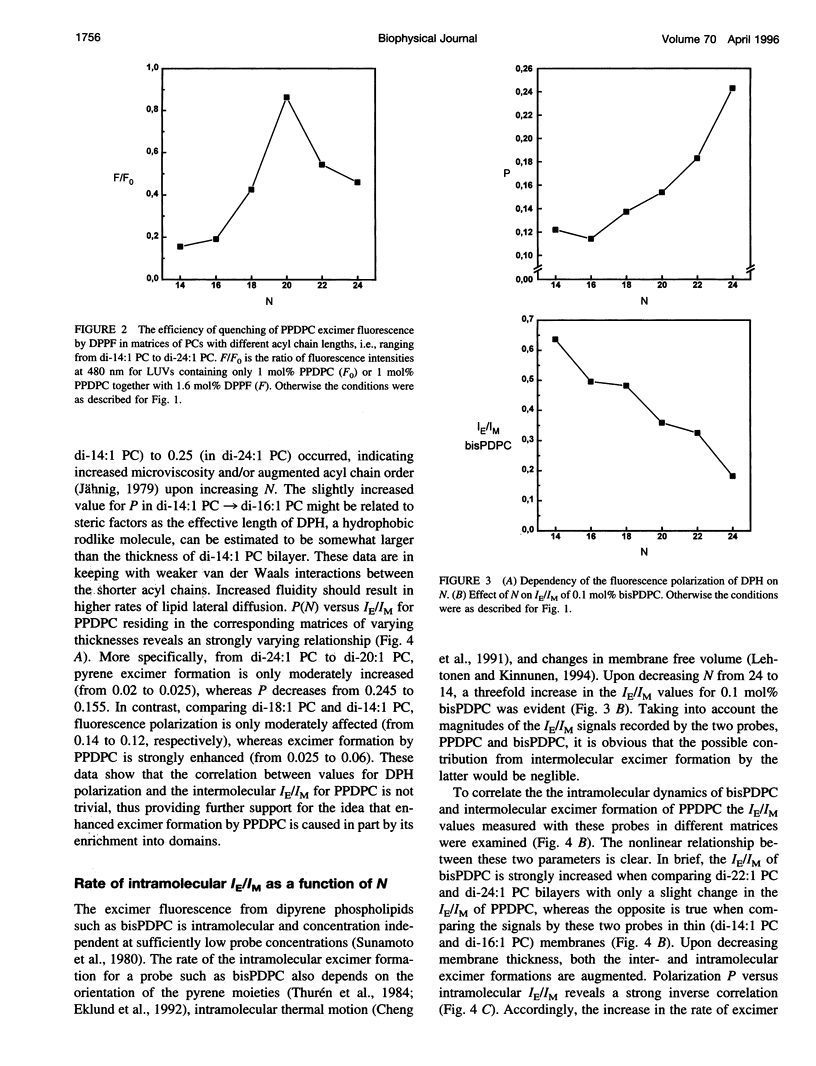
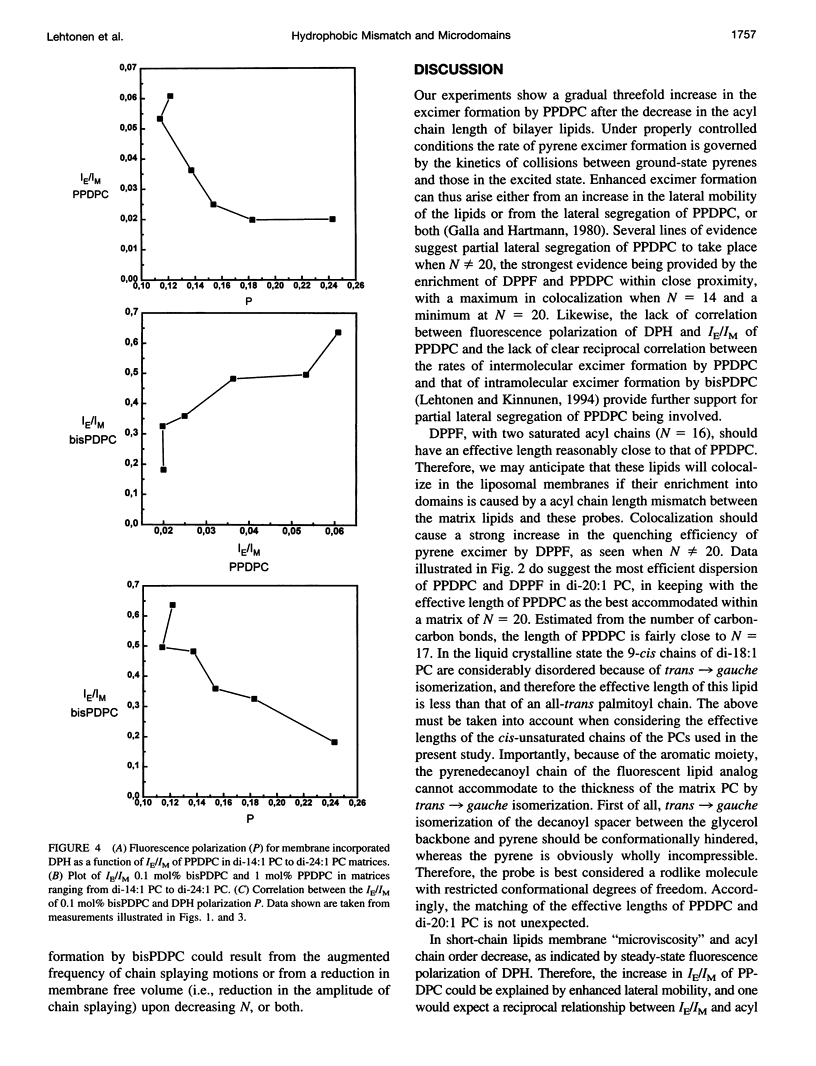
The excimer-to-monomer fluorescence emission intensity ratio (IE/IM) of the fluorescent probe 1-palmitoyl-2-[(pyren-1-yl)]decanoyl-sn-glycero-3-phosphocholine (PPDPC, 1 mol%) was measured at 30 degrees C as a function of the thickness of fluid liposomal membranes composed of phosphatidylcholines (PCs) with homologous monounsaturated acyl chains of varying lengths N (= number of carbon atoms). Upon decreasing N from di-24:1 PC to di-14:1 PC, the rate of excimer formation was sigmoidally augmented from 0.02 to 0.06. This increase in IE/IM can arise either from enhanced lateral mobility or from the lateral enrichment of PPDPC into domains, or both. Direct evidence for partial lateral segregation of PPDPC being involved is provided by experiments where 1.6 mol% of 1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamino-N- (5-fluoresceinthiocarbamoyl) (DPPF) was included together with PPDPC into the bilayers. Notably, because of spectral overlap DPPF can function as a resonance energy transfer acceptor for pyrene excimer. Fluorescence intensity ratio (F/Fo) measured at 480 nm for PPDPC/DPPF (yielding F) and PPDPC (yielding Fo) containing membranes as a function of N reveals a sharp maximum for di-20:1 PC, i.e., the quenching of pyrene excimer fluorescence by DPPF is least efficient in this lipid and is enhanced upon either decrease or increase in N. This is compatible with colocalization of DPPF in PPDPC enriched domains when N not equal to 20, whereas in di-20:1 PC these probes appear to be effectively dispersed. The driving force for the enrichment of PPDPC in thin (N < 20) and thick (N > 20) PC matrices is likely to be hydrophobic mismatch of the effective ¿lengths of the matrix phospholipids and the fluorescent probes. We also measured fluorescence polarization (P) for 1,6-diphenyl-1,3,5-hexatriene (DPH) as well as the IE/IM for the intramolecular excimer forming probe 1,2-bis[(pyren-1-yl)]decanoyl-sn-glycero-3-phosphocholine (bisPDPC) as a function of N. In brief, neither the fluorescence polarization data and nor the measurements of intramolecular chain dynamics using bisPDPC concur with enhanced lateral diffusion as the sole cause for the increase in the IE/IM for PPDPC in thin membranes. Our findings suggest hydrophobic mismatch as the cause of microdomain formation of lipids in fluid, liquid crystalline bilayers, while simultaneously allowing for a high rates of lateral diffusion. Such hydrophobic mismatch-induced compositional fluctuations would also offer one plausible explanation for the chain length diversity observed for biological membranes.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashley R. H., Brammer M. J. A fluorescence polarization study of calcium and phase behaviour in synaptosomal lipids. Biochim Biophys Acta. 1984 Jan 25;769(2):363–369. doi: 10.1016/0005-2736(84)90318-3. [DOI] [PubMed] [Google Scholar]
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Burack W. R., Biltonen R. L. Lipid bilayer heterogeneities and modulation of phospholipase A2 activity. Chem Phys Lipids. 1994 Sep 6;73(1-2):209–222. doi: 10.1016/0009-3084(94)90182-1. [DOI] [PubMed] [Google Scholar]
- Cheng K. H., Chen S. Y., Butko P., Van der Meer B. W., Somerharju P. Intramolecular excimer formation of pyrene-labeled lipids in lamellar and inverted hexagonal phases of lipid mixtures containing unsaturated phosphatidylethanolamine. Biophys Chem. 1991 Feb;39(2):137–144. [PubMed] [Google Scholar]
- Chong P. L., Tang D., Sugar I. P. Exploration of physical principles underlying lipid regular distribution: effects of pressure, temperature, and radius of curvature on E/M dips in pyrene-labeled PC/DMPC binary mixtures. Biophys J. 1994 Jun;66(6):2029–2038. doi: 10.1016/S0006-3495(94)80996-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornea R. L., Thomas D. D. Effects of membrane thickness on the molecular dynamics and enzymatic activity of reconstituted Ca-ATPase. Biochemistry. 1994 Mar 15;33(10):2912–2920. doi: 10.1021/bi00176a022. [DOI] [PubMed] [Google Scholar]
- Drake J. M., Klafter J., Levitz P. Chemical and biological microstructures as probed by dynamic processes. Science. 1991 Mar 29;251(5001):1574–1579. doi: 10.1126/science.2011737. [DOI] [PubMed] [Google Scholar]
- Eklund K. K., Kinnunen P. K. Effects of polyamines on the thermotropic behaviour of dipalmitoylphosphatidylglycerol. Chem Phys Lipids. 1986 Jan;39(1-2):109–117. doi: 10.1016/0009-3084(86)90104-0. [DOI] [PubMed] [Google Scholar]
- Eklund K. K., Virtanen J. A., Kinnunen P. K., Kasurinen J., Somerharju P. J. Conformation of phosphatidylcholine in neat and cholesterol-containing liquid-crystalline bilayers. Application of a novel method. Biochemistry. 1992 Sep 15;31(36):8560–8565. doi: 10.1021/bi00151a025. [DOI] [PubMed] [Google Scholar]
- Eklund K. K., Vuorinen J., Mikkola J., Virtanen J. A., Kinnunen P. K. Ca2+-induced lateral phase separation in phosphatidic acid/phosphatidylcholine monolayers as revealed by fluorescence microscopy. Biochemistry. 1988 May 3;27(9):3433–3437. doi: 10.1021/bi00409a046. [DOI] [PubMed] [Google Scholar]
- Galla H. J., Hartmann W. Excimer-forming lipids in membrane research. Chem Phys Lipids. 1980 Oct;27(3):199–219. doi: 10.1016/0009-3084(80)90036-5. [DOI] [PubMed] [Google Scholar]
- Galla H. J., Hartmann W., Theilen U., Sackmann E. On two-dimensional passive random walk in lipid bilayers and fluid pathways in biomembranes. J Membr Biol. 1979 Jul 31;48(3):215–236. doi: 10.1007/BF01872892. [DOI] [PubMed] [Google Scholar]
- Galla H. J., Sackmann E. Lateral diffusion in the hydrophobic region of membranes: use of pyrene excimers as optical probes. Biochim Biophys Acta. 1974 Feb 26;339(1):103–115. doi: 10.1016/0005-2736(74)90336-8. [DOI] [PubMed] [Google Scholar]
- Gascard P., Sauvage M., Sulpice J. C., Giraud F. Characterization of structural and functional phosphoinositide domains in human erythrocyte membranes. Biochemistry. 1993 Jun 15;32(23):5941–5948. doi: 10.1021/bi00074a004. [DOI] [PubMed] [Google Scholar]
- Gordon-Kamm W. J., Steponkus P. L. Lamellar-to-hexagonalII phase transitions in the plasma membrane of isolated protoplasts after freeze-induced dehydration. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6373–6377. doi: 10.1073/pnas.81.20.6373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haverstick D. M., Glaser M. Visualization of domain formation in the inner and outer leaflets of a phospholipid bilayer. J Cell Biol. 1988 Jun;106(6):1885–1892. doi: 10.1083/jcb.106.6.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He N. B., Hui S. W. Electron microscopic observation of domain movement in reconstituted erythrocyte membranes. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7304–7308. doi: 10.1073/pnas.82.21.7304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heijne G. The distribution of positively charged residues in bacterial inner membrane proteins correlates with the trans-membrane topology. EMBO J. 1986 Nov;5(11):3021–3027. doi: 10.1002/j.1460-2075.1986.tb04601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinderliter A. K., Huang J., Feigenson G. W. Detection of phase separation in fluid phosphatidylserine/phosphatidylcholine mixtures. Biophys J. 1994 Nov;67(5):1906–1911. doi: 10.1016/S0006-3495(94)80673-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horváth L. I., Brophy P. J., Marsh D. Influence of polar residue deletions on lipid-protein interactions with the myelin proteolipid protein. Spin-label ESR studies with DM-20/lipid recombinants. Biochemistry. 1990 Mar 20;29(11):2635–2638. doi: 10.1021/bi00463a002. [DOI] [PubMed] [Google Scholar]
- Hresko R. C., Sugár I. P., Barenholz Y., Thompson T. E. Lateral distribution of a pyrene-labeled phosphatidylcholine in phosphatidylcholine bilayers: fluorescence phase and modulation study. Biochemistry. 1986 Jul 1;25(13):3813–3823. doi: 10.1021/bi00361a012. [DOI] [PubMed] [Google Scholar]
- Hui S. W. Geometry of phase-separated domains in phospholipid bilayers by diffraction-contrast electron microscopy. Biophys J. 1981 Jun;34(3):383–395. doi: 10.1016/S0006-3495(81)84857-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda T., Yamaguchi H., Tazuke S. Phase separation in phospholipid bilayers induced by biologically active polycations. Biochim Biophys Acta. 1990 Jul 9;1026(1):105–112. doi: 10.1016/0005-2736(90)90339-p. [DOI] [PubMed] [Google Scholar]
- Jacobson K., Papahadjopoulos D. Phase transitions and phase separations in phospholipid membranes induced by changes in temperature, pH, and concentration of bivalent cations. Biochemistry. 1975 Jan 14;14(1):152–161. doi: 10.1021/bi00672a026. [DOI] [PubMed] [Google Scholar]
- Johannsson A., Smith G. A., Metcalfe J. C. The effect of bilayer thickness on the activity of (Na+ + K+)-ATPase. Biochim Biophys Acta. 1981 Mar 6;641(2):416–421. doi: 10.1016/0005-2736(81)90498-3. [DOI] [PubMed] [Google Scholar]
- Junker M., Creutz C. E. Endonexin (annexin IV)-mediated lateral segregation of phosphatidylglycerol in phosphatidylglycerol/phosphatidylcholine membranes. Biochemistry. 1993 Sep 28;32(38):9968–9974. doi: 10.1021/bi00089a012. [DOI] [PubMed] [Google Scholar]
- Jähnig F. Structural order of lipids and proteins in membranes: evaluation of fluorescence anisotropy data. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6361–6365. doi: 10.1073/pnas.76.12.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnunen P. K., Kõiv A., Lehtonen J. Y., Rytömaa M., Mustonen P. Lipid dynamics and peripheral interactions of proteins with membrane surfaces. Chem Phys Lipids. 1994 Sep 6;73(1-2):181–207. doi: 10.1016/0009-3084(94)90181-3. [DOI] [PubMed] [Google Scholar]
- Kinnunen P. K. On the principles of functional ordering in biological membranes. Chem Phys Lipids. 1991 Mar;57(2-3):375–399. doi: 10.1016/0009-3084(91)90087-r. [DOI] [PubMed] [Google Scholar]
- Knoll W., Schmidt G., Rötzer H., Henkel T., Pfeiffer W., Sackmann E., Mittler-Neher S., Spinke J. Lateral order in binary lipid alloys and its coupling to membrane functions. Chem Phys Lipids. 1991 Mar;57(2-3):363–374. doi: 10.1016/0009-3084(91)90086-q. [DOI] [PubMed] [Google Scholar]
- Kõiv A., Palvimo J., Kinnunen P. K. Evidence for ternary complex formation by histone H1, DNA, and liposomes. Biochemistry. 1995 Jun 27;34(25):8018–8027. doi: 10.1021/bi00025a007. [DOI] [PubMed] [Google Scholar]
- Lakowicz J. R., Prendergast F. G., Hogen D. Differential polarized phase fluorometric investigations of diphenylhexatriene in lipid bilayers. Quantitation of hindered depolarizing rotations. Biochemistry. 1979 Feb 6;18(3):508–519. doi: 10.1021/bi00570a021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakowicz J. R., Prendergast F. G., Hogen D. Fluorescence anisotropy measurements under oxygen quenching conditions as a method to quantify the depolarizing rotations of fluorophores. Application to diphenylhexatriene in isotropic solvents and in lipid bilayers. Biochemistry. 1979 Feb 6;18(3):520–527. doi: 10.1021/bi00570a022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehtonen J. Y., Kinnunen P. K. Changes in the lipid dynamics of liposomal membranes induced by poly(ethylene glycol): free volume alterations revealed by inter- and intramolecular excimer-forming phospholipid analogs. Biophys J. 1994 Jun;66(6):1981–1990. doi: 10.1016/S0006-3495(94)80991-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehtonen J. Y., Kinnunen P. K. Poly(ethylene glycol)-induced and temperature-dependent phase separation in fluid binary phospholipid membranes. Biophys J. 1995 Feb;68(2):525–535. doi: 10.1016/S0006-3495(95)80214-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis B. A., Engelman D. M. Lipid bilayer thickness varies linearly with acyl chain length in fluid phosphatidylcholine vesicles. J Mol Biol. 1983 May 15;166(2):211–217. doi: 10.1016/s0022-2836(83)80007-2. [DOI] [PubMed] [Google Scholar]
- Litman B. J., Lewis E. N., Levin I. W. Packing characteristics of highly unsaturated bilayer lipids: Raman spectroscopic studies of multilamellar phosphatidylcholine dispersions. Biochemistry. 1991 Jan 15;30(2):313–319. doi: 10.1021/bi00216a001. [DOI] [PubMed] [Google Scholar]
- Luan P., Glaser M. Formation of membrane domains by the envelope proteins of vesicular stomatitis virus. Biochemistry. 1994 Apr 19;33(15):4483–4489. doi: 10.1021/bi00181a007. [DOI] [PubMed] [Google Scholar]
- MacDonald R. C., MacDonald R. I., Menco B. P., Takeshita K., Subbarao N. K., Hu L. R. Small-volume extrusion apparatus for preparation of large, unilamellar vesicles. Biochim Biophys Acta. 1991 Jan 30;1061(2):297–303. doi: 10.1016/0005-2736(91)90295-j. [DOI] [PubMed] [Google Scholar]
- Mayer L. D., Nelsestuen G. L. Calcium and prothrombin-induced lateral phase separation in membranes. Biochemistry. 1981 Apr 28;20(9):2457–2463. doi: 10.1021/bi00512a015. [DOI] [PubMed] [Google Scholar]
- McMullen T. P., Lewis R. N., McElhaney R. N. Comparative differential scanning calorimetric and FTIR and 31P-NMR spectroscopic studies of the effects of cholesterol and androstenol on the thermotropic phase behavior and organization of phosphatidylcholine bilayers. Biophys J. 1994 Mar;66(3 Pt 1):741–752. doi: 10.1016/s0006-3495(94)80850-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMullen T. P., Lewis R. N., McElhaney R. N. Differential scanning calorimetric study of the effect of cholesterol on the thermotropic phase behavior of a homologous series of linear saturated phosphatidylcholines. Biochemistry. 1993 Jan 19;32(2):516–522. doi: 10.1021/bi00053a016. [DOI] [PubMed] [Google Scholar]
- Melchior D. L. Lipid domains in fluid membranes: a quick-freeze differential scanning calorimetry study. Science. 1986 Dec 19;234(4783):1577–1580. doi: 10.1126/science.3787264. [DOI] [PubMed] [Google Scholar]
- Momsen W. E., Momsen M. M., Brockman H. L. Lipid structural reorganization induced by the pancreatic lipase cofactor, procolipase. Biochemistry. 1995 May 30;34(21):7271–7281. doi: 10.1021/bi00021a044. [DOI] [PubMed] [Google Scholar]
- Morrow M. R., Huschilt J. C., Davis J. H. Simultaneous modeling of phase and calorimetric behavior in an amphiphilic peptide/phospholipid model membrane. Biochemistry. 1985 Sep 24;24(20):5396–5406. doi: 10.1021/bi00341a018. [DOI] [PubMed] [Google Scholar]
- Mouritsen O. G., Bloom M. Mattress model of lipid-protein interactions in membranes. Biophys J. 1984 Aug;46(2):141–153. doi: 10.1016/S0006-3495(84)84007-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouritsen O. G., Jørgensen K. Dynamical order and disorder in lipid bilayers. Chem Phys Lipids. 1994 Sep 6;73(1-2):3–25. doi: 10.1016/0009-3084(94)90171-6. [DOI] [PubMed] [Google Scholar]
- Mustonen P., Lehtonen J., Kõiv A., Kinnunen P. K. Effects of sphingosine on peripheral membrane interactions: comparison of adriamycin, cytochrome c, and phospholipase A2. Biochemistry. 1993 May 25;32(20):5373–5380. doi: 10.1021/bi00071a012. [DOI] [PubMed] [Google Scholar]
- Mustonen P., Virtanen J. A., Somerharju P. J., Kinnunen P. K. Binding of cytochrome c to liposomes as revealed by the quenching of fluorescence from pyrene-labeled phospholipids. Biochemistry. 1987 Jun 2;26(11):2991–2997. doi: 10.1021/bi00385a006. [DOI] [PubMed] [Google Scholar]
- Neubig R. R. Membrane organization in G-protein mechanisms. FASEB J. 1994 Sep;8(12):939–946. doi: 10.1096/fasebj.8.12.8088459. [DOI] [PubMed] [Google Scholar]
- Olson F., Hunt C. A., Szoka F. C., Vail W. J., Papahadjopoulos D. Preparation of liposomes of defined size distribution by extrusion through polycarbonate membranes. Biochim Biophys Acta. 1979 Oct 19;557(1):9–23. doi: 10.1016/0005-2736(79)90085-3. [DOI] [PubMed] [Google Scholar]
- Orci L., Thorens B., Ravazzola M., Lodish H. F. Localization of the pancreatic beta cell glucose transporter to specific plasma membrane domains. Science. 1989 Jul 21;245(4915):295–297. doi: 10.1126/science.2665080. [DOI] [PubMed] [Google Scholar]
- Ortiz A., Villalaín J., Gómez-Fernández J. C. Interaction of diacylglycerols with phosphatidylcholine vesicles as studied by differential scanning calorimetry and fluorescence probe depolarization. Biochemistry. 1988 Dec 13;27(25):9030–9036. doi: 10.1021/bi00425a022. [DOI] [PubMed] [Google Scholar]
- Pagano R. E., Cherry R. J., Chapman D. Phase transitions and heterogeneity in lipid bilayers. Science. 1973 Aug 10;181(4099):557–559. doi: 10.1126/science.181.4099.557. [DOI] [PubMed] [Google Scholar]
- Parente R. A., Lentz B. R. Fusion and phase separation monitored by lifetime changes of a fluorescent phospholipid probe. Biochemistry. 1986 Mar 11;25(5):1021–1026. doi: 10.1021/bi00353a011. [DOI] [PubMed] [Google Scholar]
- Prendergast F. G., Haugland R. P., Callahan P. J. 1-[4-(Trimethylamino)phenyl]-6-phenylhexa-1,3,5-triene: synthesis, fluorescence properties, and use as a fluorescence probe of lipid bilayers. Biochemistry. 1981 Dec 22;20(26):7333–7338. doi: 10.1021/bi00529a002. [DOI] [PubMed] [Google Scholar]
- Rodgers W., Glaser M. Characterization of lipid domains in erythrocyte membranes. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1364–1368. doi: 10.1073/pnas.88.4.1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe E. S. Induction of lateral phase separations in binary lipid mixtures by alcohol. Biochemistry. 1987 Jan 13;26(1):46–51. doi: 10.1021/bi00375a007. [DOI] [PubMed] [Google Scholar]
- Selinsky B. S., Yeagle P. L. Effects of potassium on lipid-protein interactions in light sarcoplasmic reticulum. Biochemistry. 1990 Jan 16;29(2):415–421. doi: 10.1021/bi00454a016. [DOI] [PubMed] [Google Scholar]
- Shimshick E. J., McConnell H. M. Lateral phase separation in phospholipid membranes. Biochemistry. 1973 Jun 5;12(12):2351–2360. doi: 10.1021/bi00736a026. [DOI] [PubMed] [Google Scholar]
- Silvius J. R. Calcium-induced lipid phase separations and interactions of phosphatidylcholine/anionic phospholipid vesicles. Fluorescence studies using carbazole-labeled and brominated phospholipids. Biochemistry. 1990 Mar 27;29(12):2930–2938. doi: 10.1021/bi00464a007. [DOI] [PubMed] [Google Scholar]
- Somerharju P. J., Virtanen J. A., Eklund K. K., Vainio P., Kinnunen P. K. 1-Palmitoyl-2-pyrenedecanoyl glycerophospholipids as membrane probes: evidence for regular distribution in liquid-crystalline phosphatidylcholine bilayers. Biochemistry. 1985 May 21;24(11):2773–2781. doi: 10.1021/bi00332a027. [DOI] [PubMed] [Google Scholar]
- Stryer L. Fluorescence energy transfer as a spectroscopic ruler. Annu Rev Biochem. 1978;47:819–846. doi: 10.1146/annurev.bi.47.070178.004131. [DOI] [PubMed] [Google Scholar]
- Tang D., Chong P. L. E/M dips. Evidence for lipids regularly distributed into hexagonal super-lattices in pyrene-PC/DMPC binary mixtures at specific concentrations. Biophys J. 1992 Oct;63(4):903–910. doi: 10.1016/S0006-3495(92)81672-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang D., Wieb van der Meer B., Chen S. Y. Evidence for a regular distribution of cholesterol in phospholipid bilayers from diphenylhexatriene fluorescence. Biophys J. 1995 May;68(5):1944–1951. doi: 10.1016/S0006-3495(95)80371-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurmond R. L., Niemi A. R., Lindblom G., Wieslander A., Rilfors L. Membrane thickness and molecular ordering in Acholeplasma laidlawii strain A studied by 2H NMR spectroscopy. Biochemistry. 1994 Nov 15;33(45):13178–13188. doi: 10.1021/bi00249a004. [DOI] [PubMed] [Google Scholar]
- Tilcock C. P., Cullis P. R. The polymorphic phase behaviour of mixed phosphatidylserine-phosphatidylethanolamine model systems as detected by 31P-NMR. Biochim Biophys Acta. 1981 Feb 20;641(1):189–201. doi: 10.1016/0005-2736(81)90583-6. [DOI] [PubMed] [Google Scholar]
- Vaz W. L. Percolation properties of two-component, two-phase phospholipid bilayers. Mol Membr Biol. 1995 Jan-Mar;12(1):39–43. doi: 10.3109/09687689509038493. [DOI] [PubMed] [Google Scholar]
- Webb M. S., Hui S. W., Steponkus P. L. Dehydration-induced lamellar-to-hexagonal-II phase transitions in DOPE/DOPC mixtures. Biochim Biophys Acta. 1993 Jan 18;1145(1):93–104. doi: 10.1016/0005-2736(93)90385-d. [DOI] [PubMed] [Google Scholar]
- Yang L., Glaser M. Membrane domains containing phosphatidylserine and substrate can be important for the activation of protein kinase C. Biochemistry. 1995 Feb 7;34(5):1500–1506. doi: 10.1021/bi00005a005. [DOI] [PubMed] [Google Scholar]
- Zhang Y. P., Lewis R. N., Hodges R. S., McElhaney R. N. Interaction of a peptide model of a hydrophobic transmembrane alpha-helical segment of a membrane protein with phosphatidylcholine bilayers: differential scanning calorimetric and FTIR spectroscopic studies. Biochemistry. 1992 Nov 24;31(46):11579–11588. doi: 10.1021/bi00161a042. [DOI] [PubMed] [Google Scholar]


