Abstract
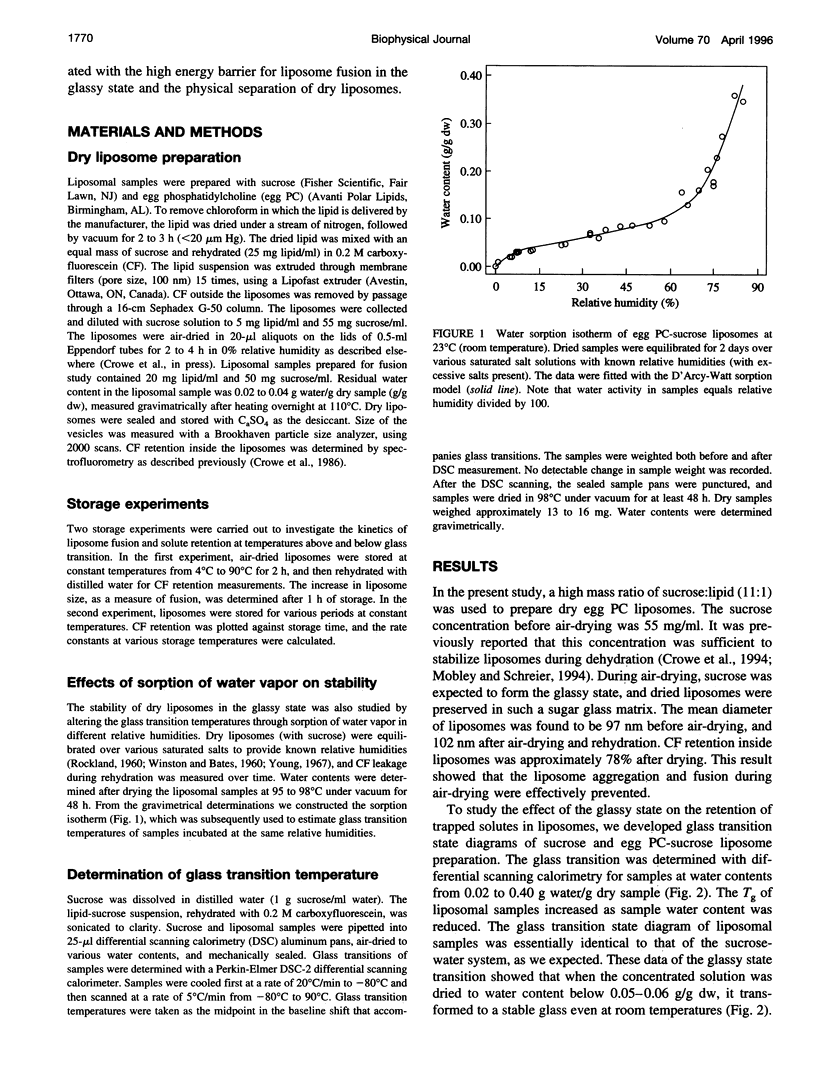
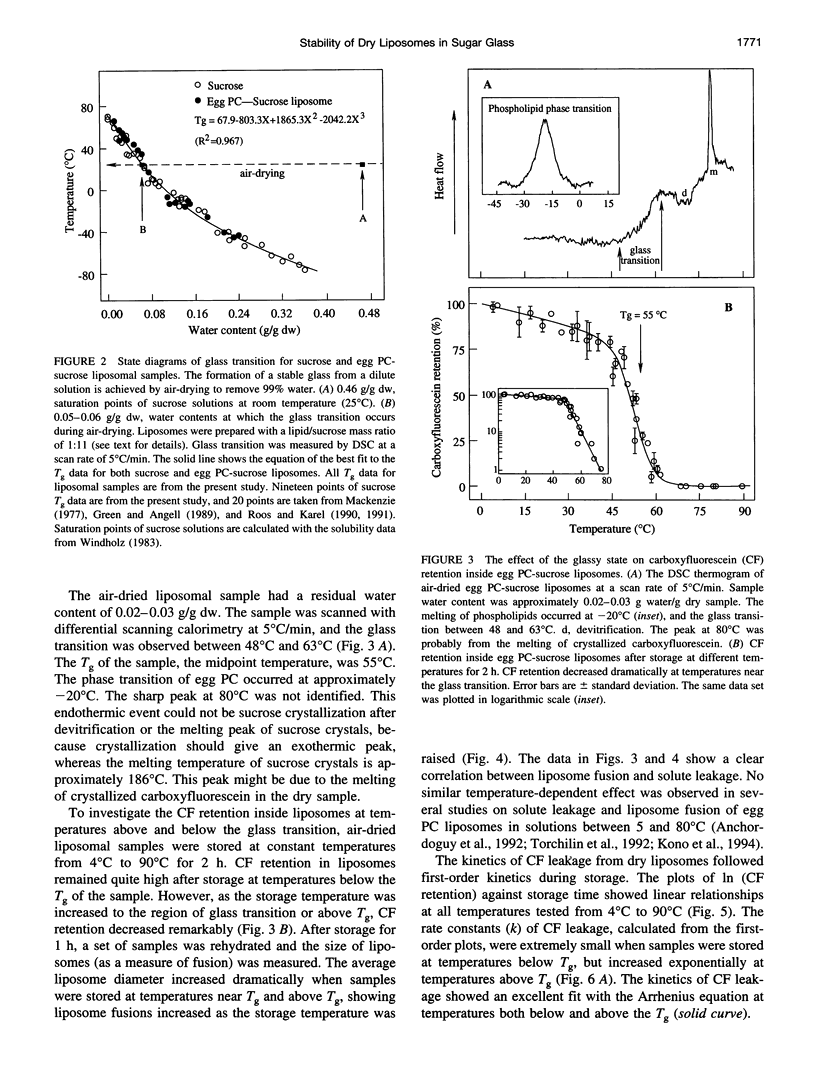
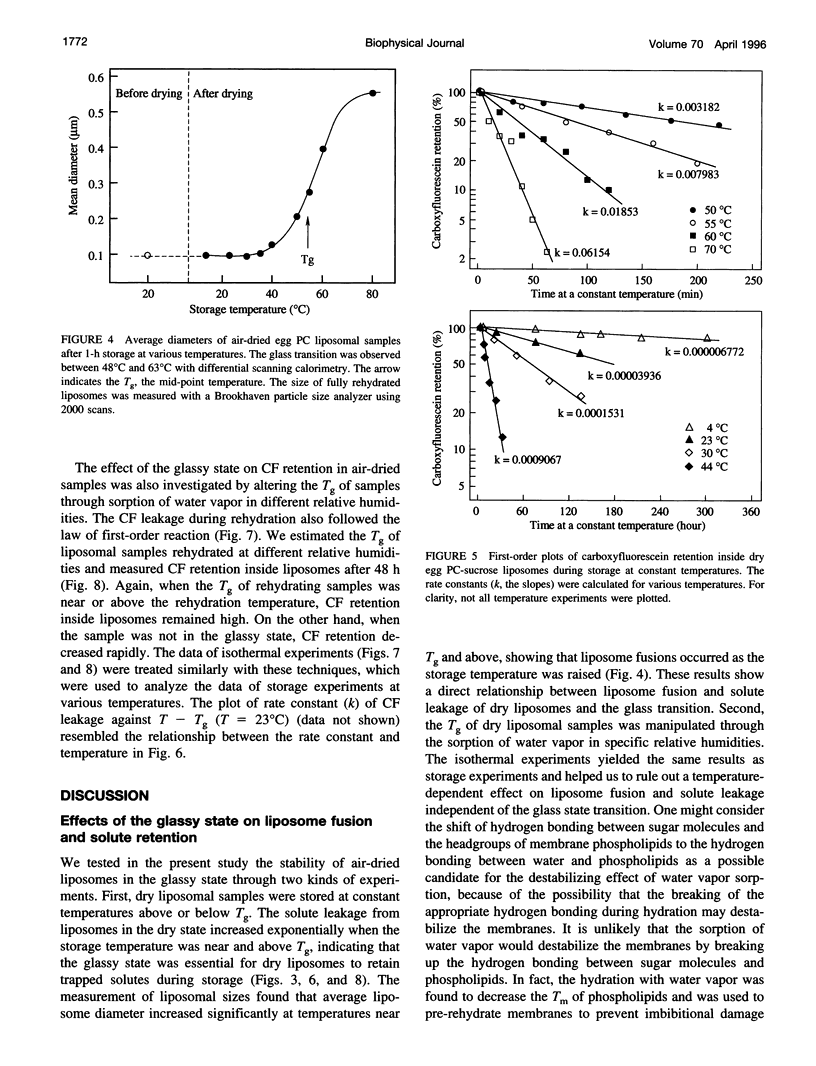
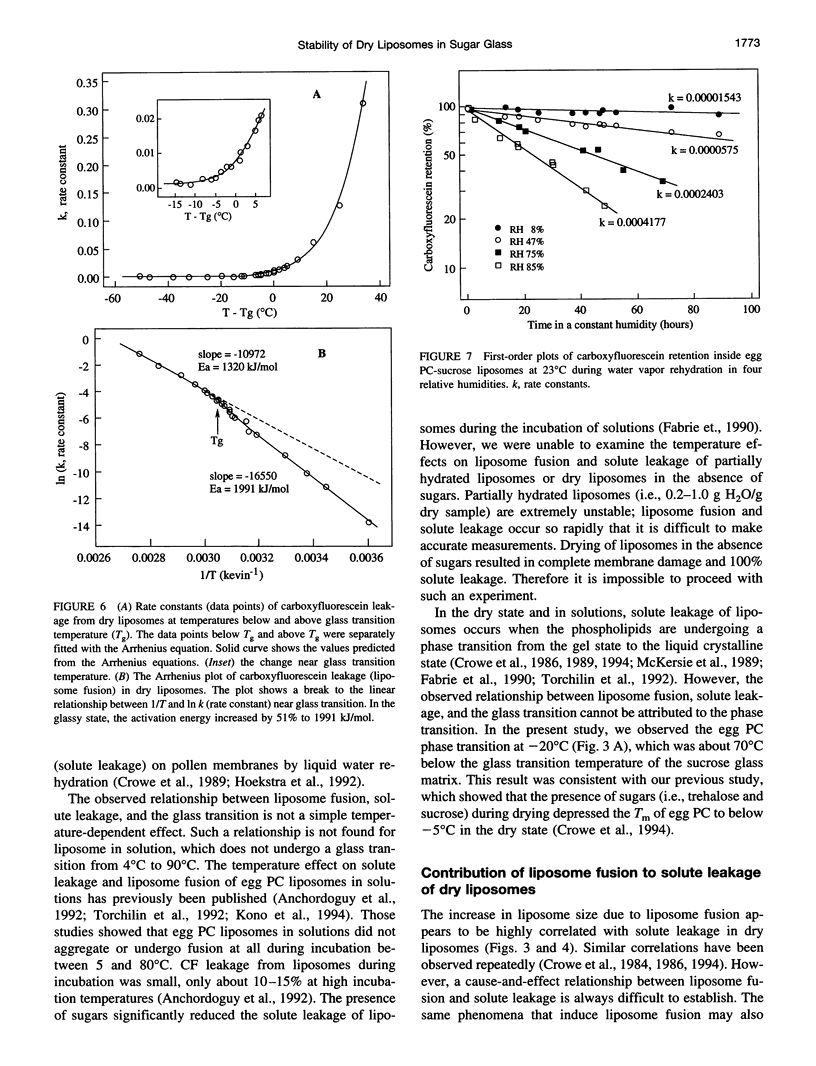
Sugars, particularly trehalose and sucrose, are used to stabilize liposomes during hydration (freeze-drying and air-drying). As a result, dry liposomes are trapped in a sugar glass, a supersaturated and thermodynamically unstable solid solution. We investigated the effects of the glassy state on liposome fusion and solute retention in the dry state. Solute leakage from dry liposomes was extremely slow at temperatures below the glass transition temperature (Tg); however, it increased exponentially as temperature increased to near or above the Tg, indicating that the glassy state had to be maintained for dry liposomes to retain trapped solutes. The leakage of solutes from dry liposomes followed the law of first-order kinetics and was correlated linearly with liposome fusion. The kinetics of solute leakage showed an excellent fit with the Arrhenius equation at temperatures both above and below the Tg, with a transitional break near the Tg. The activation energy of solute leakage was 1320 kJ/mol at temperatures above the Tg, but increased to 1991 kJ/mol at temperatures below the Tg. The stabilization effect of sugar glass on dry liposomes may be associated with the elevated energy barrier for liposome fusion and the physical separation of dry liposomes in the glassy state. The half-life of solute retention in dry liposomes may be prolonged by storing dry liposomes at temperatures below the Tg and by increasing the Tg of the dry liposome preparation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anchordoguy T. J., Rudolph A. S., Carpenter J. F., Crowe J. H. Modes of interaction of cryoprotectants with membrane phospholipids during freezing. Cryobiology. 1987 Aug;24(4):324–331. doi: 10.1016/0011-2240(87)90036-8. [DOI] [PubMed] [Google Scholar]
- Crowe J. H., Crowe L. M., Chapman D. Preservation of membranes in anhydrobiotic organisms: the role of trehalose. Science. 1984 Feb 17;223(4637):701–703. doi: 10.1126/science.223.4637.701. [DOI] [PubMed] [Google Scholar]
- Crowe J. H., Hoekstra F. A., Crowe L. M. Membrane phase transitions are responsible for imbibitional damage in dry pollen. Proc Natl Acad Sci U S A. 1989 Jan;86(2):520–523. doi: 10.1073/pnas.86.2.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowe J. H., Leslie S. B., Crowe L. M. Is vitrification sufficient to preserve liposomes during freeze-drying? Cryobiology. 1994 Aug;31(4):355–366. doi: 10.1006/cryo.1994.1043. [DOI] [PubMed] [Google Scholar]
- Crowe L. M., Mouradian R., Crowe J. H., Jackson S. A., Womersley C. Effects of carbohydrates on membrane stability at low water activities. Biochim Biophys Acta. 1984 Jan 11;769(1):141–150. doi: 10.1016/0005-2736(84)90017-8. [DOI] [PubMed] [Google Scholar]
- Fabrie C. H., de Kruijff B., de Gier J. Protection by sugars against phase transition-induced leak in hydrated dimyristoylphosphatidylcholine liposomes. Biochim Biophys Acta. 1990 May 24;1024(2):380–384. doi: 10.1016/0005-2736(90)90368-x. [DOI] [PubMed] [Google Scholar]
- MacKenzie A. P. Non-equilibrium freezing behaviour of aqueous systems. Philos Trans R Soc Lond B Biol Sci. 1977 Mar 29;278(959):167–189. doi: 10.1098/rstb.1977.0036. [DOI] [PubMed] [Google Scholar]
- Torchilin V. P., Omelyanenko V. G., Lukyanov A. N. Temperature-dependent aggregation of pH-sensitive phosphatidyl ethanolamine-oleic acid-cholesterol liposomes as measured by fluorescent spectroscopy. Anal Biochem. 1992 Nov 15;207(1):109–113. doi: 10.1016/0003-2697(92)90510-e. [DOI] [PubMed] [Google Scholar]
- Womersley C., Uster P. S., Rudolph A. S., Crowe J. H. Inhibition of dehydration-induced fusion between liposomal membranes by carbohydrates as measured by fluorescence energy transfer. Cryobiology. 1986 Jun;23(3):245–255. doi: 10.1016/0011-2240(86)90050-7. [DOI] [PubMed] [Google Scholar]


