Abstract
Amide-resolved hydrogen-deuterium exchange-rate constants were measured for backbone amides of alamethicin reconstituted in dioleoylphosphatidylcholine vesicles by an exchange-trapping method combined with high-resolution nuclear magnetic resonance spectroscopy. In vesicles containing alamethicin at molar ratios between 1:20 and 1:100 relative to lipid, the exchange-rate constants increased with increasing volume of the D20 buffer in which the vesicles were suspended, indicating that exchange under these conditions is dominated by partitioning of the peptide into the aqueous phase. This was supported by observation of a linear relationship between the exchange-rate constants for amides in membrane-reconstituted alamethicin and those for amides in alamethicin dissolved directly into D2O buffer. Significant protection of amides from exchange with D2O buffer in membrane-reconstituted alamethicin is interpreted in terms of stabilization by helical hydrogen bonding. Under conditions in which amide exchange occurred by partitioning of the peptide into solution, only lower limits for hydrogen-bond stabilities in the membrane were determined; all the potentially hydrogen-bonded amides of alamethicin are at least 1000-fold exchange protected in the membrane-bound state. When partitioning of alamethicin into the aqueous phase was suppressed by hydration of reconstituted vesicles in a limiting volume of water [D2O:dioleoylphosphatidylcholine:alamethicin; 220:1:0.05; (M:M:M)], the exchange-protection factors exhibited helical periodicity with highly exchange-protected, and less well-protected, amides on the nonpolar and polar helix faces, respectively. The exchange data indicate that, under the conditions studied, alamethicin adopts a stable helical structure in DOPC bilayers in which all the potentially hydrogen-bonded amides are stabilized by helical hydrogen bonds. The protection factors define the orientation of the peptide helix with respect to an aqueous phase, which is either the bulk solution or water within parallel or antiparallel transmembrane arrays of reconstituted alamethicin.
Full text
PDF

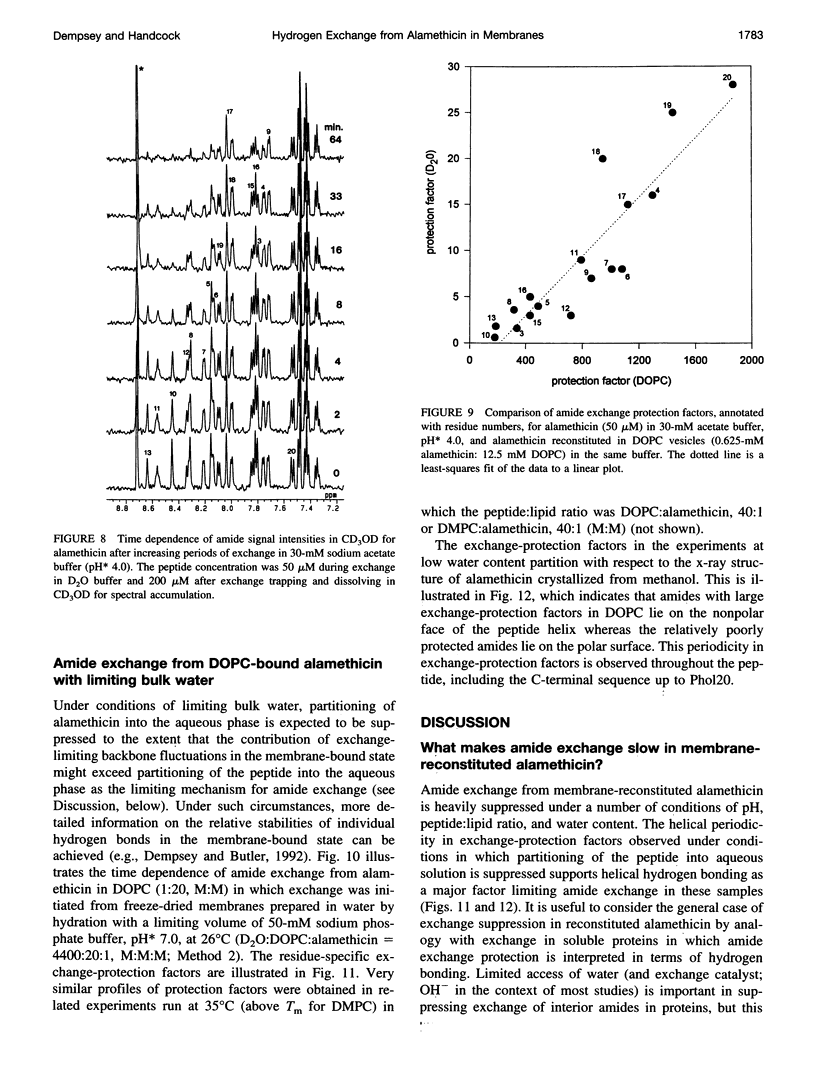
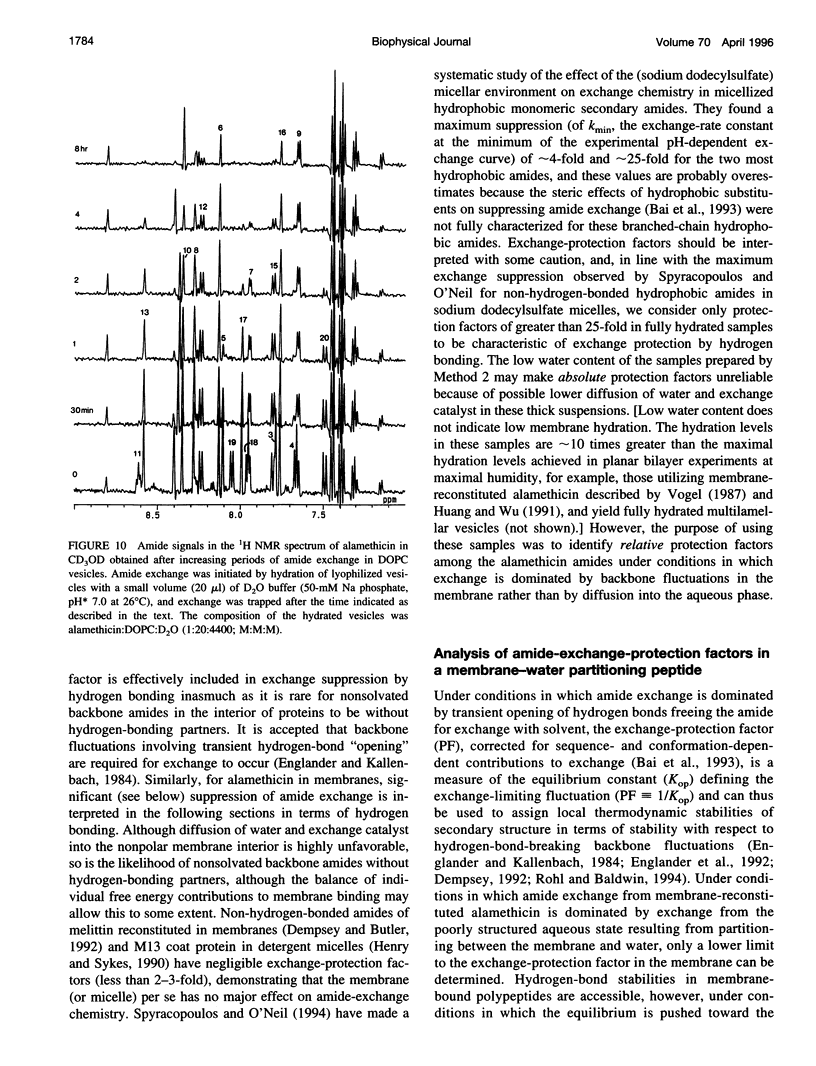
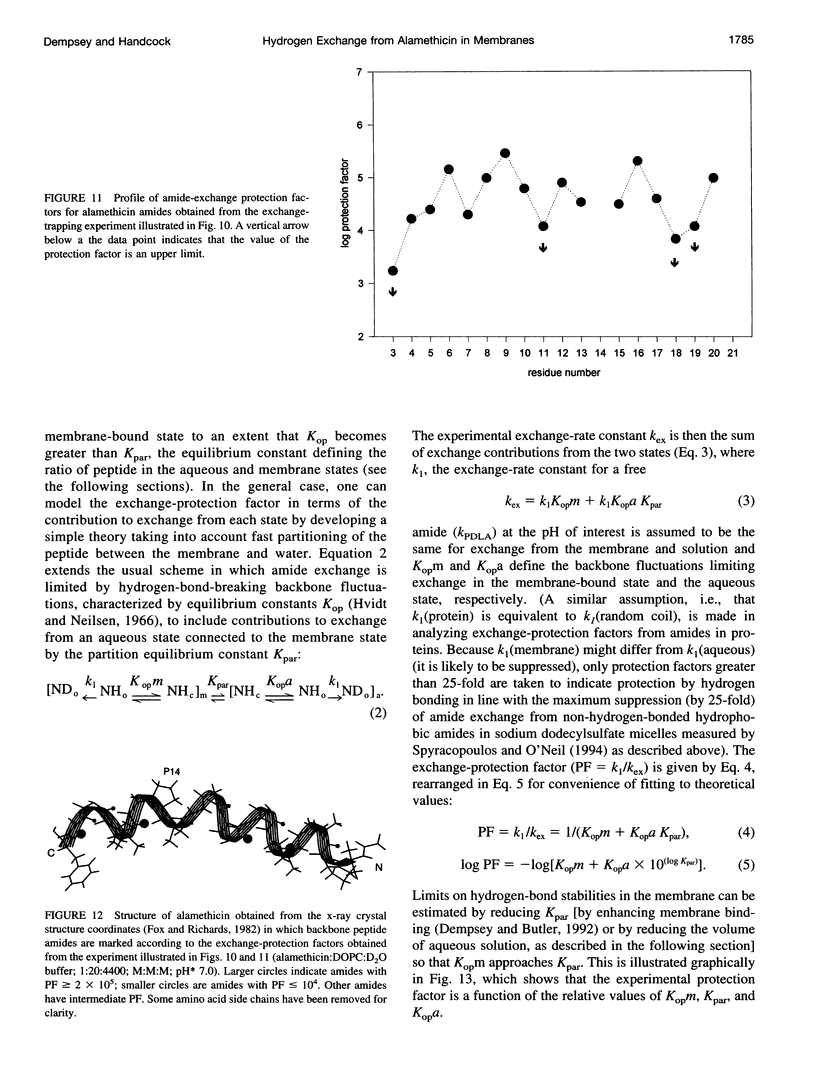
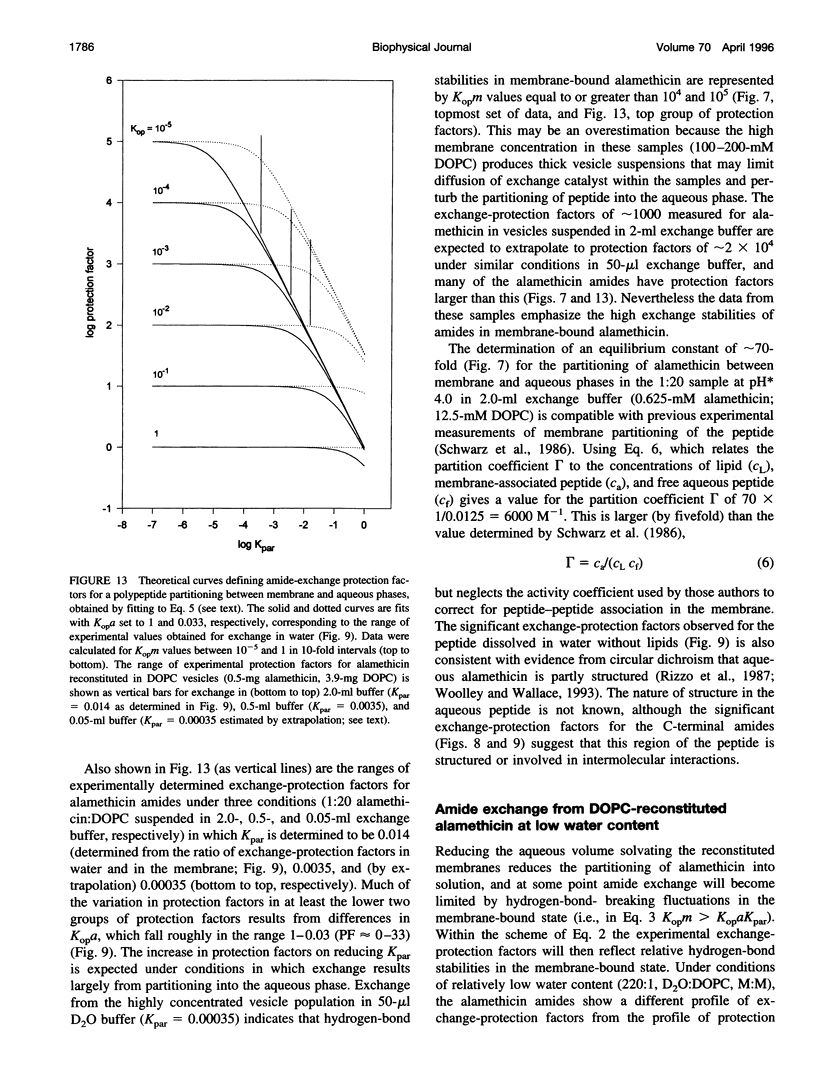


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bai Y., Milne J. S., Mayne L., Englander S. W. Primary structure effects on peptide group hydrogen exchange. Proteins. 1993 Sep;17(1):75–86. doi: 10.1002/prot.340170110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee U., Zidovetzki R., Birge R. R., Chan S. I. Interaction of alamethicin with lecithin bilayers: a 31P and 2H NMR study. Biochemistry. 1985 Dec 17;24(26):7621–7627. doi: 10.1021/bi00347a019. [DOI] [PubMed] [Google Scholar]
- Baumann G., Mueller P. A molecular model of membrane excitability. J Supramol Struct. 1974;2(5-6):538–557. doi: 10.1002/jss.400020504. [DOI] [PubMed] [Google Scholar]
- Boheim G. Statistical analysis of alamethicin channels in black lipid membranes. J Membr Biol. 1974;19(3):277–303. doi: 10.1007/BF01869983. [DOI] [PubMed] [Google Scholar]
- Dempsey C. E., Butler G. S. Helical structure and orientation of melittin in dispersed phospholipid membranes from amide exchange analysis in situ. Biochemistry. 1992 Dec 8;31(48):11973–11977. doi: 10.1021/bi00163a003. [DOI] [PubMed] [Google Scholar]
- Dempsey C. E. Quantitation of the effects of an internal proline residue on individual hydrogen bond stabilities in an alpha-helix: pH-dependent amide exchange in melittin and [Ala-14]melittin. Biochemistry. 1992 May 19;31(19):4705–4712. doi: 10.1021/bi00134a025. [DOI] [PubMed] [Google Scholar]
- Englander S. W., Englander J. J., McKinnie R. E., Ackers G. K., Turner G. J., Westrick J. A., Gill S. J. Hydrogen exchange measurement of the free energy of structural and allosteric change in hemoglobin. Science. 1992 Jun 19;256(5064):1684–1687. doi: 10.1126/science.256.5064.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englander S. W., Kallenbach N. R. Hydrogen exchange and structural dynamics of proteins and nucleic acids. Q Rev Biophys. 1983 Nov;16(4):521–655. doi: 10.1017/s0033583500005217. [DOI] [PubMed] [Google Scholar]
- Esposito G., Carver J. A., Boyd J., Campbell I. D. High-resolution 1H NMR study of the solution structure of alamethicin. Biochemistry. 1987 Feb 24;26(4):1043–1050. doi: 10.1021/bi00378a010. [DOI] [PubMed] [Google Scholar]
- Fox R. O., Jr, Richards F. M. A voltage-gated ion channel model inferred from the crystal structure of alamethicin at 1.5-A resolution. Nature. 1982 Nov 25;300(5890):325–330. doi: 10.1038/300325a0. [DOI] [PubMed] [Google Scholar]
- Hall J. E., Vodyanoy I., Balasubramanian T. M., Marshall G. R. Alamethicin. A rich model for channel behavior. Biophys J. 1984 Jan;45(1):233–247. doi: 10.1016/S0006-3495(84)84151-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haris P. I., Chapman D. Fourier transform infrared spectra of the polypeptide alamethicin and a possible structural similarity with bacteriorhodopsin. Biochim Biophys Acta. 1988 Aug 18;943(2):375–380. doi: 10.1016/0005-2736(88)90571-8. [DOI] [PubMed] [Google Scholar]
- Henry G. D., Sykes B. D. Hydrogen exchange kinetics in a membrane protein determined by 15N NMR spectroscopy: use of the INEPT experiment to follow individual amides in detergent-solubilized M13 coat protein. Biochemistry. 1990 Jul 3;29(26):6303–6313. doi: 10.1021/bi00478a027. [DOI] [PubMed] [Google Scholar]
- Huang H. W., Wu Y. Lipid-alamethicin interactions influence alamethicin orientation. Biophys J. 1991 Nov;60(5):1079–1087. doi: 10.1016/S0006-3495(91)82144-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hvidt A., Nielsen S. O. Hydrogen exchange in proteins. Adv Protein Chem. 1966;21:287–386. doi: 10.1016/s0065-3233(08)60129-1. [DOI] [PubMed] [Google Scholar]
- Kelsh L. P., Ellena J. F., Cafiso D. S. Determination of the molecular dynamics of alamethicin using 13C NMR: implications for the mechanism of gating of a voltage-dependent channel. Biochemistry. 1992 Jun 9;31(22):5136–5144. doi: 10.1021/bi00137a007. [DOI] [PubMed] [Google Scholar]
- Lau S. Y., Taneja A. K., Hodges R. S. Synthesis of a model protein of defined secondary and quaternary structure. Effect of chain length on the stabilization and formation of two-stranded alpha-helical coiled-coils. J Biol Chem. 1984 Nov 10;259(21):13253–13261. [PubMed] [Google Scholar]
- McMullen A. I., Stirrup J. A. The aggregation of alamethicin. Biochim Biophys Acta. 1971 Sep 14;241(3):807–814. doi: 10.1016/0005-2736(71)90008-3. [DOI] [PubMed] [Google Scholar]
- Mueller P., Rudin D. O. Action potentials induced in biomolecular lipid membranes. Nature. 1968 Feb 24;217(5130):713–719. doi: 10.1038/217713a0. [DOI] [PubMed] [Google Scholar]
- Rizzo V., Stankowski S., Schwarz G. Alamethicin incorporation in lipid bilayers: a thermodynamic study. Biochemistry. 1987 May 19;26(10):2751–2759. doi: 10.1021/bi00384a015. [DOI] [PubMed] [Google Scholar]
- Rohl C. A., Baldwin R. L. Exchange kinetics of individual amide protons in 15N-labeled helical peptides measured by isotope-edited NMR. Biochemistry. 1994 Jun 28;33(25):7760–7767. doi: 10.1021/bi00191a003. [DOI] [PubMed] [Google Scholar]
- Sansom M. S. Structure and function of channel-forming peptaibols. Q Rev Biophys. 1993 Nov;26(4):365–421. doi: 10.1017/s0033583500002833. [DOI] [PubMed] [Google Scholar]
- Schwarz G., Stankowski S., Rizzo V. Thermodynamic analysis of incorporation and aggregation in a membrane: application to the pore-forming peptide alamethicin. Biochim Biophys Acta. 1986 Sep 25;861(1):141–151. doi: 10.1016/0005-2736(86)90573-0. [DOI] [PubMed] [Google Scholar]
- Vogel H. Comparison of the conformation and orientation of alamethicin and melittin in lipid membranes. Biochemistry. 1987 Jul 14;26(14):4562–4572. doi: 10.1021/bi00388a060. [DOI] [PubMed] [Google Scholar]
- Woolley G. A., Wallace B. A. Model ion channels: gramicidin and alamethicin. J Membr Biol. 1992 Aug;129(2):109–136. doi: 10.1007/BF00219508. [DOI] [PubMed] [Google Scholar]
- Woolley G. A., Wallace B. A. Temperature dependence of the interaction of alamethicin helices in membranes. Biochemistry. 1993 Sep 21;32(37):9819–9825. doi: 10.1021/bi00088a037. [DOI] [PubMed] [Google Scholar]
- Yee A. A., O'Neil J. D. Uniform 15N labeling of a fungal peptide: the structure and dynamics of an alamethicin by 15N and 1H NMR spectroscopy. Biochemistry. 1992 Mar 31;31(12):3135–3143. doi: 10.1021/bi00127a014. [DOI] [PubMed] [Google Scholar]
- Zhou N. E., Zhu B. Y., Kay C. M., Hodges R. S. The two-stranded alpha-helical coiled-coil is an ideal model for studying protein stability and subunit interactions. Biopolymers. 1992 Apr;32(4):419–426. doi: 10.1002/bip.360320419. [DOI] [PubMed] [Google Scholar]


