Abstract
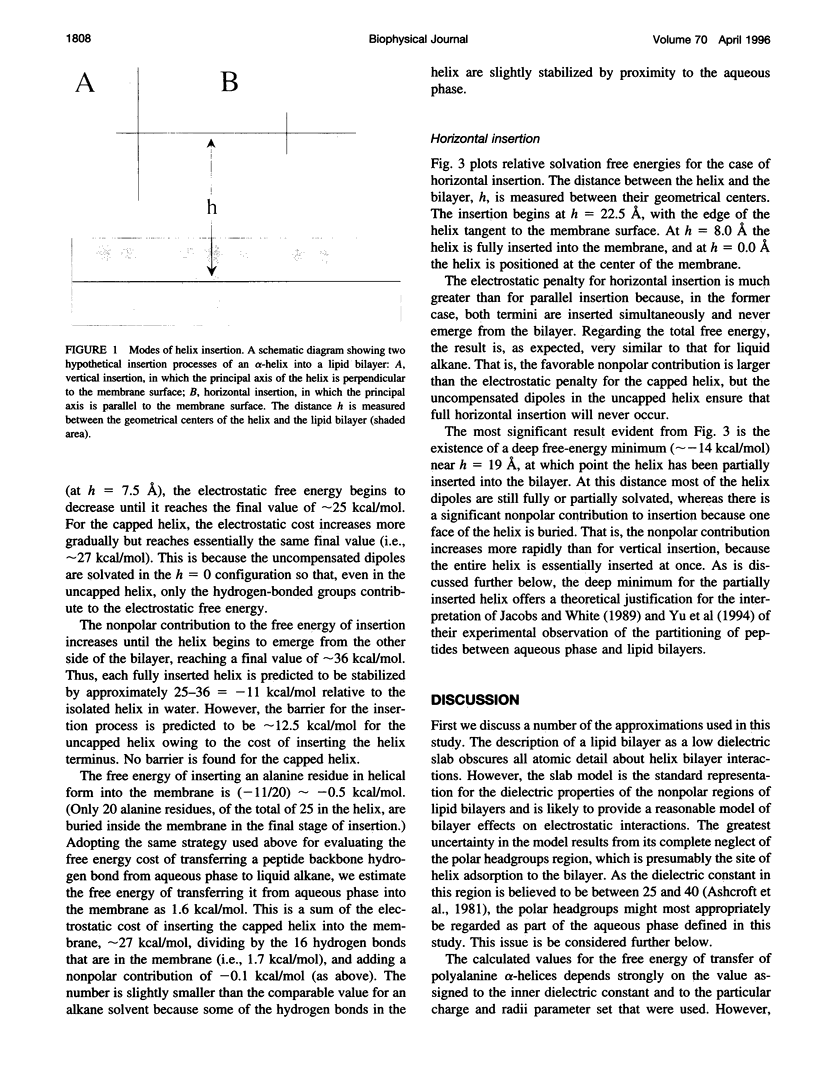
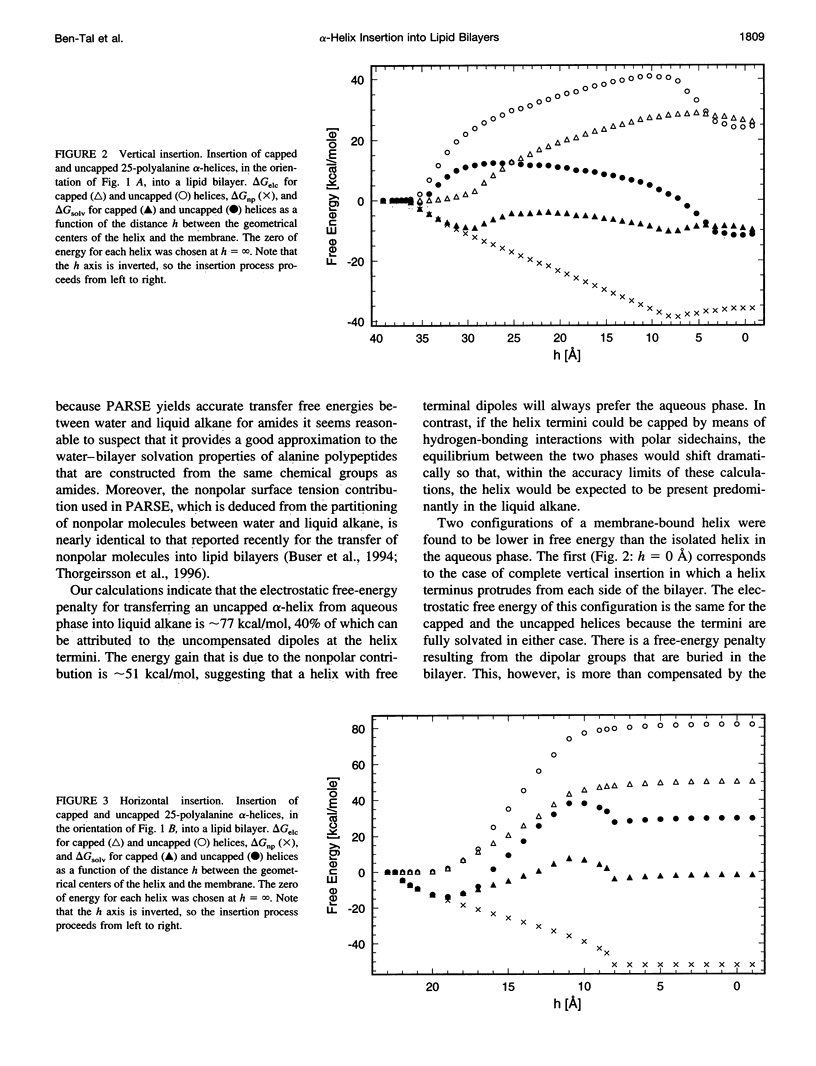
A detailed treatment is provided of the various free-energy terms that contribute to the transfer of a polyalanine alpha-helix from the aqueous phase into lipid bilayers. In agreement with previous work, the hydrophobic effect is found to provide the major driving force for helix insertion. However, an opposing effect of comparable magnitude is also identified and is attributed to the large free-energy penalty associated with the desolvation of peptide hydrogen bonds on transfer to the low dielectric environment of the bilayer. Lipid perturbation effects as well as the entropy loss associated with helix immobilization in the bilayer are also evaluated. Two configurations of a membrane-bound 25mer polyalanine helix were found to be lower in free energy than the isolated helix in the aqueous phase. The first corresponds to the case of vertical insertion, in which a helix terminus protrudes from each side of the bilayer. The second minimum is for the case of horizontal insertion, for which the helix is adsorbed upon the surface of the bilayer. The calculated free-energy minima are found to be in good agreement with recent measurements of related systems. Large free-energy barriers resulting from desolvation of unsatisfied hydrogen-bonding groups at the helix termini are obtained for both insertion processes. The barriers for insertion are significantly reduced if the helix termini are assumed to be "capped" through the formation of hydrogen bonds with polar sidechains. For uncapped helices, our results support recently proposed models in which helices are inserted by first adsorbing on the membrane surface and then having one terminus "swing around" so as to penetrate the bilayer.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashcroft R. G., Coster H. G., Smith J. R. The molecular organisation of bimolecular lipid membranes. The dielectric structure of the hydrophilic/hydrophobic interface. Biochim Biophys Acta. 1981 Apr 22;643(1):191–204. doi: 10.1016/0005-2736(81)90232-7. [DOI] [PubMed] [Google Scholar]
- Buser C. A., Sigal C. T., Resh M. D., McLaughlin S. Membrane binding of myristylated peptides corresponding to the NH2 terminus of Src. Biochemistry. 1994 Nov 8;33(44):13093–13101. doi: 10.1021/bi00248a019. [DOI] [PubMed] [Google Scholar]
- Dilger J. P., Benz R. Optical and electrical properties of thin monoolein lipid bilayers. J Membr Biol. 1985;85(2):181–189. doi: 10.1007/BF01871270. [DOI] [PubMed] [Google Scholar]
- Engelman D. M., Steitz T. A., Goldman A. Identifying nonpolar transbilayer helices in amino acid sequences of membrane proteins. Annu Rev Biophys Biophys Chem. 1986;15:321–353. doi: 10.1146/annurev.bb.15.060186.001541. [DOI] [PubMed] [Google Scholar]
- Engelman D. M., Steitz T. A. The spontaneous insertion of proteins into and across membranes: the helical hairpin hypothesis. Cell. 1981 Feb;23(2):411–422. doi: 10.1016/0092-8674(81)90136-7. [DOI] [PubMed] [Google Scholar]
- Fattal D. R., Ben-Shaul A. A molecular model for lipid-protein interaction in membranes: the role of hydrophobic mismatch. Biophys J. 1993 Nov;65(5):1795–1809. doi: 10.1016/S0006-3495(93)81249-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein A. V., Janin J. The price of lost freedom: entropy of bimolecular complex formation. Protein Eng. 1989 Oct;3(1):1–3. doi: 10.1093/protein/3.1.1. [DOI] [PubMed] [Google Scholar]
- Hagler A. T., Huler E., Lifson S. Energy functions for peptides and proteins. I. Derivation of a consistent force field including the hydrogen bond from amide crystals. J Am Chem Soc. 1974 Aug 21;96(17):5319–5327. doi: 10.1021/ja00824a004. [DOI] [PubMed] [Google Scholar]
- Honig B., Nicholls A. Classical electrostatics in biology and chemistry. Science. 1995 May 26;268(5214):1144–1149. doi: 10.1126/science.7761829. [DOI] [PubMed] [Google Scholar]
- Honig B., Yang A. S. Free energy balance in protein folding. Adv Protein Chem. 1995;46:27–58. doi: 10.1016/s0065-3233(08)60331-9. [DOI] [PubMed] [Google Scholar]
- Jacobs R. E., White S. H. The nature of the hydrophobic binding of small peptides at the bilayer interface: implications for the insertion of transbilayer helices. Biochemistry. 1989 Apr 18;28(8):3421–3437. doi: 10.1021/bi00434a042. [DOI] [PubMed] [Google Scholar]
- Jähnig F. Thermodynamics and kinetics of protein incorporation into membranes. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3691–3695. doi: 10.1073/pnas.80.12.3691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klotz I. M., Farnham S. B. Stability of an amide-hydrogen bond in an apolar environment. Biochemistry. 1968 Nov;7(11):3879–3882. doi: 10.1021/bi00851a013. [DOI] [PubMed] [Google Scholar]
- Leto T. L., Holloway P. W. Mechanism of cytochrome b5 binding to phosphatidylcholine vesicles. J Biol Chem. 1979 Jun 25;254(12):5015–5019. [PubMed] [Google Scholar]
- Marqusee S., Robbins V. H., Baldwin R. L. Unusually stable helix formation in short alanine-based peptides. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5286–5290. doi: 10.1073/pnas.86.14.5286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milik M., Skolnick J. Insertion of peptide chains into lipid membranes: an off-lattice Monte Carlo dynamics model. Proteins. 1993 Jan;15(1):10–25. doi: 10.1002/prot.340150104. [DOI] [PubMed] [Google Scholar]
- Moll T. S., Thompson T. E. Semisynthetic proteins: model systems for the study of the insertion of hydrophobic peptides into preformed lipid bilayers. Biochemistry. 1994 Dec 27;33(51):15469–15482. doi: 10.1021/bi00255a029. [DOI] [PubMed] [Google Scholar]
- Nicholls A., Sharp K. A., Honig B. Protein folding and association: insights from the interfacial and thermodynamic properties of hydrocarbons. Proteins. 1991;11(4):281–296. doi: 10.1002/prot.340110407. [DOI] [PubMed] [Google Scholar]
- Roseman M. A. Hydrophobicity of the peptide C=O...H-N hydrogen-bonded group. J Mol Biol. 1988 Jun 5;201(3):621–623. doi: 10.1016/0022-2836(88)90642-0. [DOI] [PubMed] [Google Scholar]
- Sharp K. A., Nicholls A., Fine R. F., Honig B. Reconciling the magnitude of the microscopic and macroscopic hydrophobic effects. Science. 1991 Apr 5;252(5002):106–109. doi: 10.1126/science.2011744. [DOI] [PubMed] [Google Scholar]
- Shrake A., Rupley J. A. Environment and exposure to solvent of protein atoms. Lysozyme and insulin. J Mol Biol. 1973 Sep 15;79(2):351–371. doi: 10.1016/0022-2836(73)90011-9. [DOI] [PubMed] [Google Scholar]
- Sneddon S. F., Tobias D. J., Brooks C. L., 3rd Thermodynamics of amide hydrogen bond formation in polar and apolar solvents. J Mol Biol. 1989 Oct 20;209(4):817–820. doi: 10.1016/0022-2836(89)90609-8. [DOI] [PubMed] [Google Scholar]
- Thorgeirsson T. E., Russell C. J., King D. S., Shin Y. K. Direct determination of the membrane affinities of individual amino acids. Biochemistry. 1996 Feb 13;35(6):1803–1809. doi: 10.1021/bi952300c. [DOI] [PubMed] [Google Scholar]
- Vila J., Williams R. L., Grant J. A., Wójcik J., Scheraga H. A. The intrinsic helix-forming tendency of L-alanine. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7821–7825. doi: 10.1073/pnas.89.16.7821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel H. Incorporation of melittin into phosphatidylcholine bilayers. Study of binding and conformational changes. FEBS Lett. 1981 Nov 2;134(1):37–42. doi: 10.1016/0014-5793(81)80545-5. [DOI] [PubMed] [Google Scholar]
- Wolfenden R., Andersson L., Cullis P. M., Southgate C. C. Affinities of amino acid side chains for solvent water. Biochemistry. 1981 Feb 17;20(4):849–855. doi: 10.1021/bi00507a030. [DOI] [PubMed] [Google Scholar]
- Yang A. S., Honig B. Free energy determinants of secondary structure formation: I. alpha-Helices. J Mol Biol. 1995 Sep 22;252(3):351–365. doi: 10.1006/jmbi.1995.0502. [DOI] [PubMed] [Google Scholar]
- Yu Y. G., Thorgeirsson T. E., Shin Y. K. Topology of an amphiphilic mitochondrial signal sequence in the membrane-inserted state: a spin labeling study. Biochemistry. 1994 Nov 29;33(47):14221–14226. doi: 10.1021/bi00251a034. [DOI] [PubMed] [Google Scholar]



