Abstract
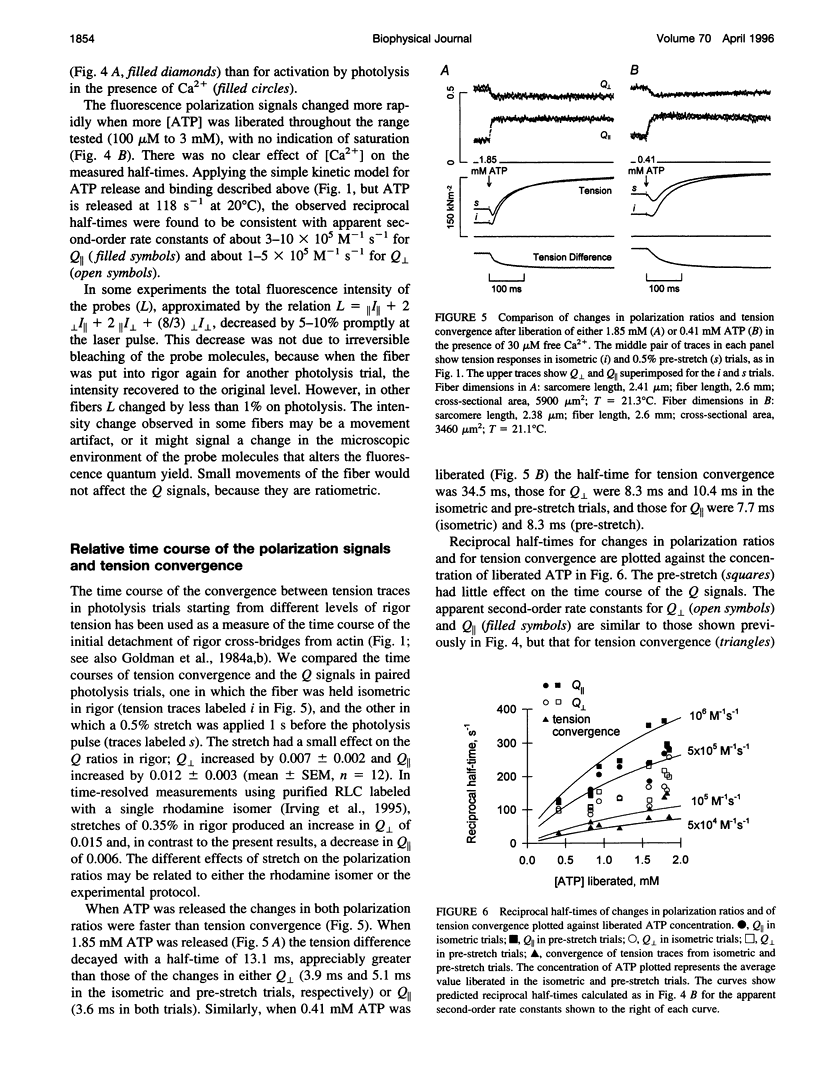
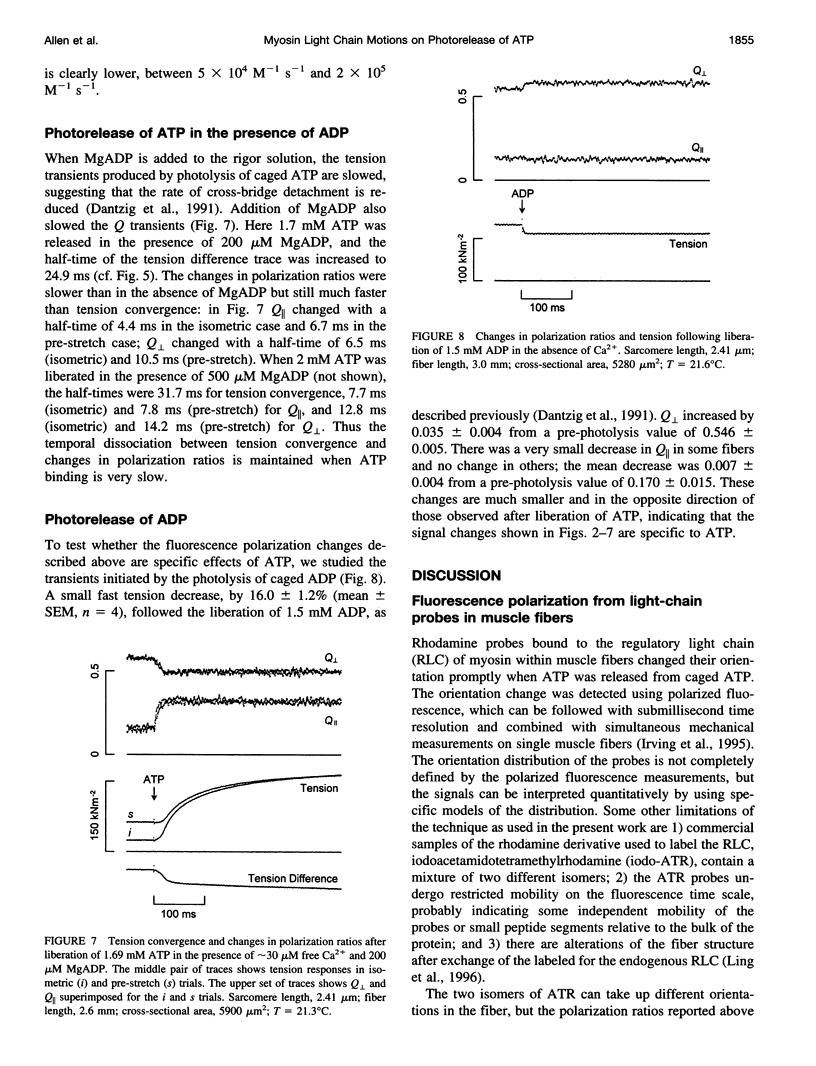
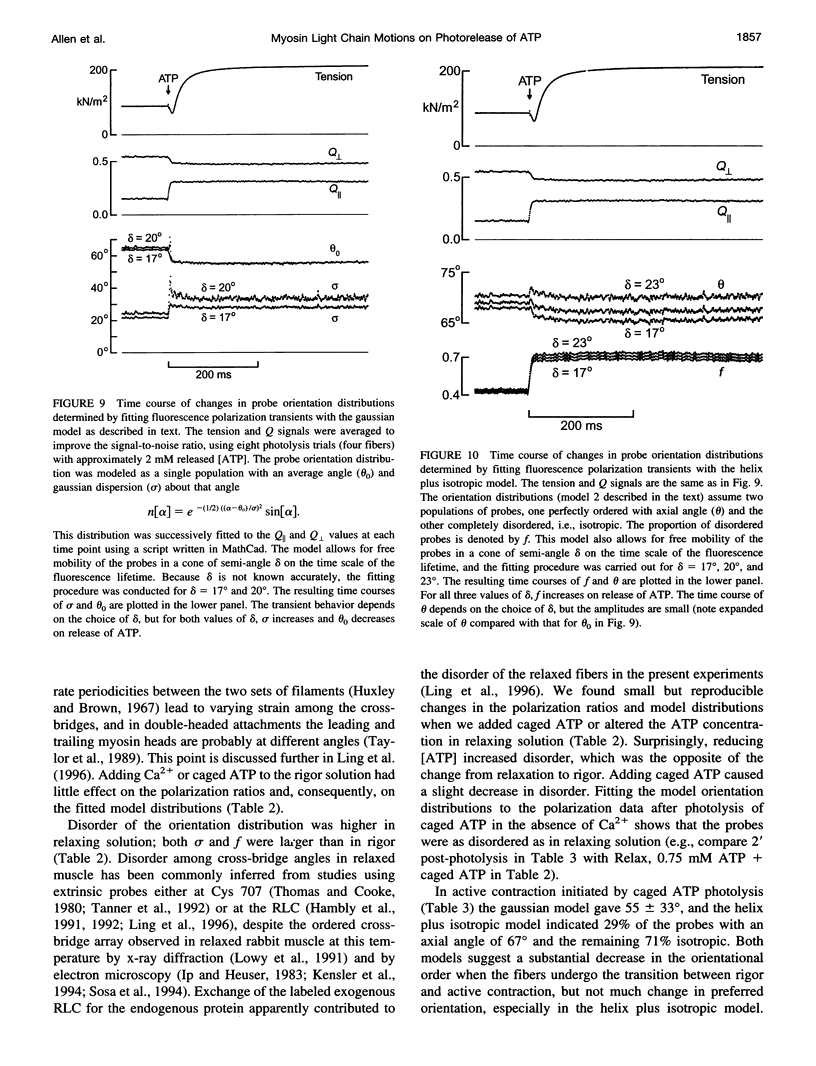
The orientation of the light-chain region of myosin heads in muscle fibers was followed by polarized fluorescence from an extrinsic probe during tension transients elicited by photolysis of caged ATP. Regulatory light chain from chicken gizzard myosin was covalently modified with iodoacetamidotetramethylrhodamine and exchanged into skinned fibers from rabbit psoas muscle without significant effect of the tension transients. Fluorescence polarization ratios Q parallel = (parallel I parallel-perpendicular I parallel)/ (parallel I parallel+perpendicular I parallel) and Q perpendicular = perpendicular I perpendicular - parallel I perpendicular)/ (perpendicular I perpendicular + parallel I perpendicular), where mIn denote fluorescence intensities for excitation (pre-subscript) and emission (post-subscript) parallel or perpendicular to the fiber axis, were simultaneously measured at 0.5 ms time resolution. Q perpendicular decreased and Q parallel increased promptly after ATP release in the presence or absence of CA2+, indicating changes in orientation of the light-chain region associated with ATP binding or cross-bridge detachment. Little further change in the Q signals accompanied either active tension development (+Ca2+) or the final relaxation (-Ca2+). The Q and tension transients slowed when liberated ATP concentration was reduced. Assuming that ATP is released at 118 s-1 (20 degrees C), the apparent second-order rate constants were 3-10 x 10(5) M-1 s-1 for Q parallel, 1-5 x 10(5) M-1 s-1 for Q perpendicular, and 0.5-2 x 10(5) M-1 s-1 for the convergence of tension traces starting from different rigor values. Fitting of model orientation distributions to the Q signals indicated that the angular disorder increases after ATP binding. This orientation change is specific to ATP because photo release of ADP caused much smaller changes in the Q signals.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barabás K., Keszthelyi L. Temperature dependence of ATP release from "caged" ATP. Acta Biochim Biophys Acad Sci Hung. 1984;19(3-4):305–309. [PubMed] [Google Scholar]
- Berger C. L., Craik J. S., Trentham D. R., Corrie J. E., Goldman Y. E. Fluorescence polarization from isomers of tetramethylrhodamine at SH-1 in rabbit psoas muscle fibers. Biophys J. 1995 Apr;68(4 Suppl):78S–80S. [PMC free article] [PubMed] [Google Scholar]
- Burghardt T. P., Ando T., Borejdo J. Evidence for cross-bridge order in contraction of glycerinated skeletal muscle. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7515–7519. doi: 10.1073/pnas.80.24.7515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHEN R. F., BOWMAN R. L. FLUORESCENCE POLARIZATION: MEASUREMENT WITH ULTRAVIOLET-POLARIZING FILTERS IN A SPECTROPHOTOFLUOROMETER. Science. 1965 Feb 12;147(3659):729–732. doi: 10.1126/science.147.3659.729. [DOI] [PubMed] [Google Scholar]
- Chase P. B., Martyn D. A., Kushmerick M. J., Gordon A. M. Effects of inorganic phosphate analogues on stiffness and unloaded shortening of skinned muscle fibres from rabbit. J Physiol. 1993 Jan;460:231–246. doi: 10.1113/jphysiol.1993.sp019469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke R., Crowder M. S., Thomas D. D. Orientation of spin labels attached to cross-bridges in contracting muscle fibres. Nature. 1982 Dec 23;300(5894):776–778. doi: 10.1038/300776a0. [DOI] [PubMed] [Google Scholar]
- Cooke R. The mechanism of muscle contraction. CRC Crit Rev Biochem. 1986;21(1):53–118. doi: 10.3109/10409238609113609. [DOI] [PubMed] [Google Scholar]
- Dantzig J. A., Hibberd M. G., Trentham D. R., Goldman Y. E. Cross-bridge kinetics in the presence of MgADP investigated by photolysis of caged ATP in rabbit psoas muscle fibres. J Physiol. 1991 Jan;432:639–680. doi: 10.1113/jphysiol.1991.sp018405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferenczi M. A., Homsher E., Trentham D. R. The kinetics of magnesium adenosine triphosphate cleavage in skinned muscle fibres of the rabbit. J Physiol. 1984 Jul;352:575–599. doi: 10.1113/jphysiol.1984.sp015311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferenczi M. A. Phosphate burst in permeable muscle fibers of the rabbit. Biophys J. 1986 Sep;50(3):471–477. doi: 10.1016/S0006-3495(86)83484-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher A. J., Smith C. A., Thoden J., Smith R., Sutoh K., Holden H. M., Rayment I. Structural studies of myosin:nucleotide complexes: a revised model for the molecular basis of muscle contraction. Biophys J. 1995 Apr;68(4 Suppl):19S–28S. [PMC free article] [PubMed] [Google Scholar]
- Goldman Y. E., Hibberd M. G., McCray J. A., Trentham D. R. Relaxation of muscle fibres by photolysis of caged ATP. Nature. 1982 Dec 23;300(5894):701–705. doi: 10.1038/300701a0. [DOI] [PubMed] [Google Scholar]
- Goldman Y. E., Hibberd M. G., Trentham D. R. Initiation of active contraction by photogeneration of adenosine-5'-triphosphate in rabbit psoas muscle fibres. J Physiol. 1984 Sep;354:605–624. doi: 10.1113/jphysiol.1984.sp015395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman Y. E., Hibberd M. G., Trentham D. R. Relaxation of rabbit psoas muscle fibres from rigor by photochemical generation of adenosine-5'-triphosphate. J Physiol. 1984 Sep;354:577–604. doi: 10.1113/jphysiol.1984.sp015394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman Y. E., Simmons R. M. Active and rigor muscle stiffness [proceedings]. J Physiol. 1977 Jul;269(1):55P–57P. [PubMed] [Google Scholar]
- Goldman Y. E., Simmons R. M. Control of sarcomere length in skinned muscle fibres of Rana temporaria during mechanical transients. J Physiol. 1984 May;350:497–518. doi: 10.1113/jphysiol.1984.sp015215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hambly B., Franks K., Cooke R. Orientation of spin-labeled light chain-2 exchanged onto myosin cross-bridges in glycerinated muscle fibers. Biophys J. 1991 Jan;59(1):127–138. doi: 10.1016/S0006-3495(91)82205-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hambly B., Franks K., Cooke R. Paramagnetic probes attached to a light chain on the myosin head are highly disordered in active muscle fibers. Biophys J. 1992 Nov;63(5):1306–1313. doi: 10.1016/S0006-3495(92)81717-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi H., Goldman Y. E. Sliding distance between actin and myosin filaments per ATP molecule hydrolysed in skinned muscle fibres. Nature. 1991 Jul 25;352(6333):352–354. doi: 10.1038/352352a0. [DOI] [PubMed] [Google Scholar]
- Higuchi H., Goldman Y. E. Sliding distance per ATP molecule hydrolyzed by myosin heads during isotonic shortening of skinned muscle fibers. Biophys J. 1995 Oct;69(4):1491–1507. doi: 10.1016/S0006-3495(95)80020-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi H., Yanagida T., Goldman Y. E. Compliance of thin filaments in skinned fibers of rabbit skeletal muscle. Biophys J. 1995 Sep;69(3):1000–1010. doi: 10.1016/S0006-3495(95)79975-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirose K., Franzini-Armstrong C., Goldman Y. E., Murray J. M. Structural changes in muscle crossbridges accompanying force generation. J Cell Biol. 1994 Nov;127(3):763–778. doi: 10.1083/jcb.127.3.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirose K., Lenart T. D., Murray J. M., Franzini-Armstrong C., Goldman Y. E. Flash and smash: rapid freezing of muscle fibers activated by photolysis of caged ATP. Biophys J. 1993 Jul;65(1):397–408. doi: 10.1016/S0006-3495(93)81061-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homsher E., Millar N. C. Caged compounds and striated muscle contraction. Annu Rev Physiol. 1990;52:875–896. doi: 10.1146/annurev.ph.52.030190.004303. [DOI] [PubMed] [Google Scholar]
- Horiuti K., Sakoda T., Yamada K. Time course of rise of muscle stiffness at onset of contraction induced by photorelease of ATP. J Muscle Res Cell Motil. 1992 Dec;13(6):685–691. doi: 10.1007/BF01738257. [DOI] [PubMed] [Google Scholar]
- Huxley A. F., Simmons R. M. Proposed mechanism of force generation in striated muscle. Nature. 1971 Oct 22;233(5321):533–538. doi: 10.1038/233533a0. [DOI] [PubMed] [Google Scholar]
- Huxley H. E., Brown W. The low-angle x-ray diagram of vertebrate striated muscle and its behaviour during contraction and rigor. J Mol Biol. 1967 Dec 14;30(2):383–434. doi: 10.1016/s0022-2836(67)80046-9. [DOI] [PubMed] [Google Scholar]
- Huxley H. E., Stewart A., Sosa H., Irving T. X-ray diffraction measurements of the extensibility of actin and myosin filaments in contracting muscle. Biophys J. 1994 Dec;67(6):2411–2421. doi: 10.1016/S0006-3495(94)80728-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxley H. E. The mechanism of muscular contraction. Science. 1969 Jun 20;164(3886):1356–1365. doi: 10.1126/science.164.3886.1356. [DOI] [PubMed] [Google Scholar]
- Ip W., Heuser J. Direct visualization of the myosin crossbridge helices on relaxed rabbit psoas thick filaments. J Mol Biol. 1983 Nov 25;171(1):105–109. doi: 10.1016/s0022-2836(83)80317-9. [DOI] [PubMed] [Google Scholar]
- Irving M., St Claire Allen T., Sabido-David C., Craik J. S., Brandmeier B., Kendrick-Jones J., Corrie J. E., Trentham D. R., Goldman Y. E. Tilting of the light-chain region of myosin during step length changes and active force generation in skeletal muscle. Nature. 1995 Jun 22;375(6533):688–691. doi: 10.1038/375688a0. [DOI] [PubMed] [Google Scholar]
- Irving M. Steady-state polarization from cylindrically symmetric fluorophores undergoing rapid restricted motion. Biophys J. 1996 Apr;70(4):1830–1835. doi: 10.1016/S0006-3495(96)79748-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kensler R. W., Peterson S., Norberg M. The effects of changes in temperature or ionic strength on isolated rabbit and fish skeletal muscle thick filaments. J Muscle Res Cell Motil. 1994 Feb;15(1):69–79. doi: 10.1007/BF00123834. [DOI] [PubMed] [Google Scholar]
- Ling N., Shrimpton C., Sleep J., Kendrick-Jones J., Irving M. Fluorescent probes of the orientation of myosin regulatory light chains in relaxed, rigor, and contracting muscle. Biophys J. 1996 Apr;70(4):1836–1846. doi: 10.1016/S0006-3495(96)79749-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardi V., Piazzesi G., Linari M. Rapid regeneration of the actin-myosin power stroke in contracting muscle. Nature. 1992 Feb 13;355(6361):638–641. doi: 10.1038/355638a0. [DOI] [PubMed] [Google Scholar]
- Lowy J., Popp D., Stewart A. A. X-ray studies of order-disorder transitions in the myosin heads of skinned rabbit psoas muscles. Biophys J. 1991 Oct;60(4):812–824. doi: 10.1016/S0006-3495(91)82116-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lymn R. W., Taylor E. W. Mechanism of adenosine triphosphate hydrolysis by actomyosin. Biochemistry. 1971 Dec 7;10(25):4617–4624. doi: 10.1021/bi00801a004. [DOI] [PubMed] [Google Scholar]
- Milligan R. A., Flicker P. F. Structural relationships of actin, myosin, and tropomyosin revealed by cryo-electron microscopy. J Cell Biol. 1987 Jul;105(1):29–39. doi: 10.1083/jcb.105.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss R. L., Giulian G. G., Greaser M. L. Physiological effects accompanying the removal of myosin LC2 from skinned skeletal muscle fibers. J Biol Chem. 1982 Aug 10;257(15):8588–8591. [PubMed] [Google Scholar]
- Ostap E. M., Barnett V. A., Thomas D. D. Resolution of three structural states of spin-labeled myosin in contracting muscle. Biophys J. 1995 Jul;69(1):177–188. doi: 10.1016/S0006-3495(95)79888-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peckham M., Ferenczi M. A., Irving M. A birefringence study of changes in myosin orientation during relaxation of skinned muscle fibers induced by photolytic ATP release. Biophys J. 1994 Sep;67(3):1141–1148. doi: 10.1016/S0006-3495(94)80581-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole K. J., Maeda Y., Rapp G., Goody R. S. Dynamic X-ray diffraction measurements following photolytic relaxation and activation of skinned rabbit psoas fibres. Adv Biophys. 1991;27:63–75. doi: 10.1016/0065-227x(91)90008-2. [DOI] [PubMed] [Google Scholar]
- Rayment I., Holden H. M., Whittaker M., Yohn C. B., Lorenz M., Holmes K. C., Milligan R. A. Structure of the actin-myosin complex and its implications for muscle contraction. Science. 1993 Jul 2;261(5117):58–65. doi: 10.1126/science.8316858. [DOI] [PubMed] [Google Scholar]
- Reedy M. K., Goody R. S., Hofmann W., Rosenbaum G. Co-ordinated electron microscopy and X-ray studies of glycerinated insect flight muscle. I. X-ray diffraction monitoring during preparation for electron microscopy of muscle fibres fixed in rigor, in ATP and in AMPPNP. J Muscle Res Cell Motil. 1983 Feb;4(1):25–53. doi: 10.1007/BF00711957. [DOI] [PubMed] [Google Scholar]
- Reedy M. K., Holmes K. C., Tregear R. T. Induced changes in orientation of the cross-bridges of glycerinated insect flight muscle. Nature. 1965 Sep 18;207(5003):1276–1280. doi: 10.1038/2071276a0. [DOI] [PubMed] [Google Scholar]
- Sosa H., Popp D., Ouyang G., Huxley H. E. Ultrastructure of skeletal muscle fibers studied by a plunge quick freezing method: myofilament lengths. Biophys J. 1994 Jul;67(1):283–292. doi: 10.1016/S0006-3495(94)80479-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner J. W., Thomas D. D., Goldman Y. E. Transients in orientation of a fluorescent cross-bridge probe following photolysis of caged nucleotides in skeletal muscle fibres. J Mol Biol. 1992 Jan 5;223(1):185–203. doi: 10.1016/0022-2836(92)90725-y. [DOI] [PubMed] [Google Scholar]
- Taylor K. A., Reedy M. C., Córdova L., Reedy M. K. Three-dimensional image reconstruction of insect flight muscle. II. The rigor actin layer. J Cell Biol. 1989 Sep;109(3):1103–1123. doi: 10.1083/jcb.109.3.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thirlwell H., Corrie J. E., Reid G. P., Trentham D. R., Ferenczi M. A. Kinetics of relaxation from rigor of permeabilized fast-twitch skeletal fibers from the rabbit using a novel caged ATP and apyrase. Biophys J. 1994 Dec;67(6):2436–2447. doi: 10.1016/S0006-3495(94)80730-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thirlwell H., Sleep J. A., Ferenczi M. A. Inhibition of unloaded shortening velocity in permeabilized muscle fibres by caged ATP compounds. J Muscle Res Cell Motil. 1995 Apr;16(2):131–137. doi: 10.1007/BF00122531. [DOI] [PubMed] [Google Scholar]
- Thomas D. D., Cooke R. Orientation of spin-labeled myosin heads in glycerinated muscle fibers. Biophys J. 1980 Dec;32(3):891–906. doi: 10.1016/S0006-3495(80)85024-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D. D. Spectroscopic probes of muscle cross-bridge rotation. Annu Rev Physiol. 1987;49:691–709. doi: 10.1146/annurev.ph.49.030187.003355. [DOI] [PubMed] [Google Scholar]
- Tsukita S., Yano M. Actomyosin structure in contracting muscle detected by rapid freezing. Nature. 1985 Sep 12;317(6033):182–184. doi: 10.1038/317182a0. [DOI] [PubMed] [Google Scholar]
- Vibert P., Cohen C. Domains, motions and regulation in the myosin head. J Muscle Res Cell Motil. 1988 Aug;9(4):296–305. doi: 10.1007/BF01773873. [DOI] [PubMed] [Google Scholar]
- WEBER G. Polarization of the fluorescence of macromolecules. I. Theory and experimental method. Biochem J. 1952 May;51(2):145–155. doi: 10.1042/bj0510145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi K., Sugimoto Y., Tanaka H., Ueno Y., Takezawa Y., Amemiya Y. X-ray diffraction evidence for the extensibility of actin and myosin filaments during muscle contraction. Biophys J. 1994 Dec;67(6):2422–2435. doi: 10.1016/S0006-3495(94)80729-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White H. D., Taylor E. W. Energetics and mechanism of actomyosin adenosine triphosphatase. Biochemistry. 1976 Dec 28;15(26):5818–5826. doi: 10.1021/bi00671a020. [DOI] [PubMed] [Google Scholar]
- Yanagida T., Arata T., Oosawa F. Sliding distance of actin filament induced by a myosin crossbridge during one ATP hydrolysis cycle. Nature. 1985 Jul 25;316(6026):366–369. doi: 10.1038/316366a0. [DOI] [PubMed] [Google Scholar]
- Yount R. G. ATP analogs. Adv Enzymol Relat Areas Mol Biol. 1975;43:1–56. doi: 10.1002/9780470122884.ch1. [DOI] [PubMed] [Google Scholar]


