Abstract
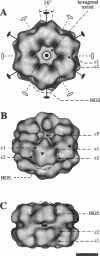
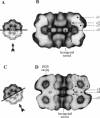
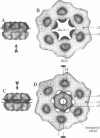
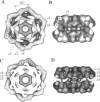
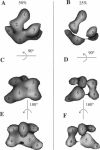
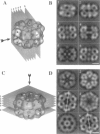
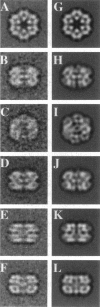
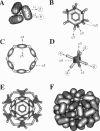
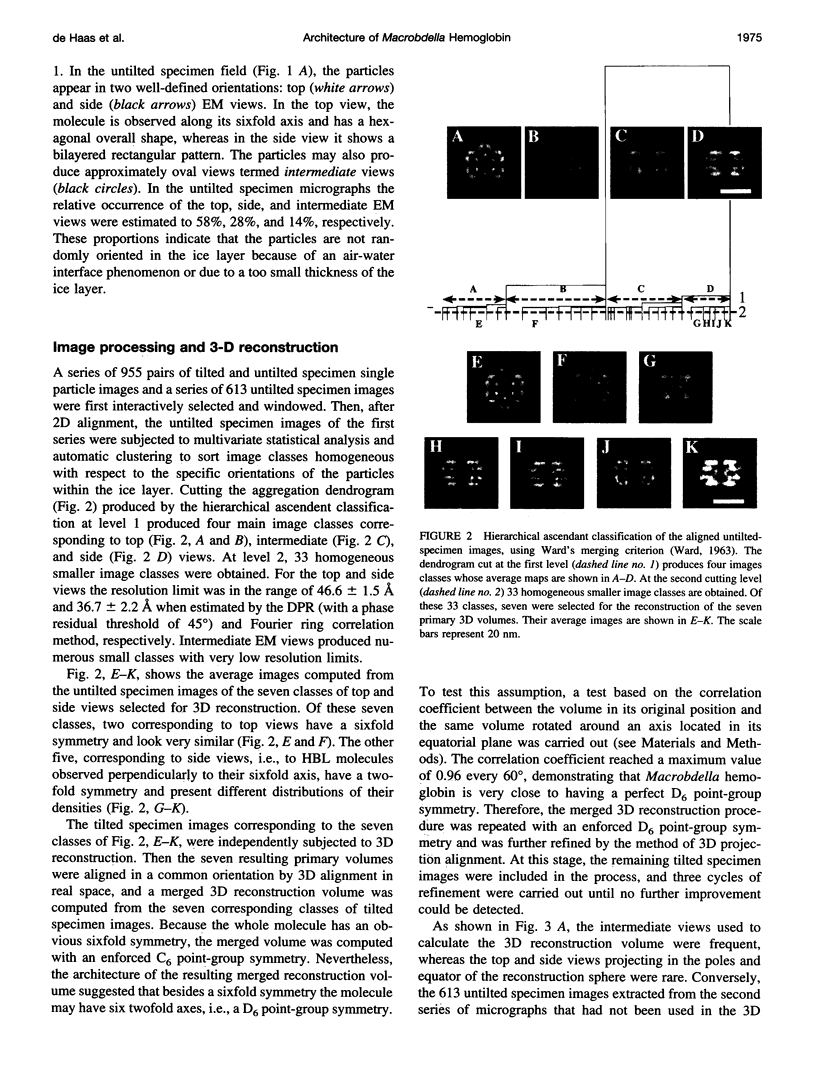
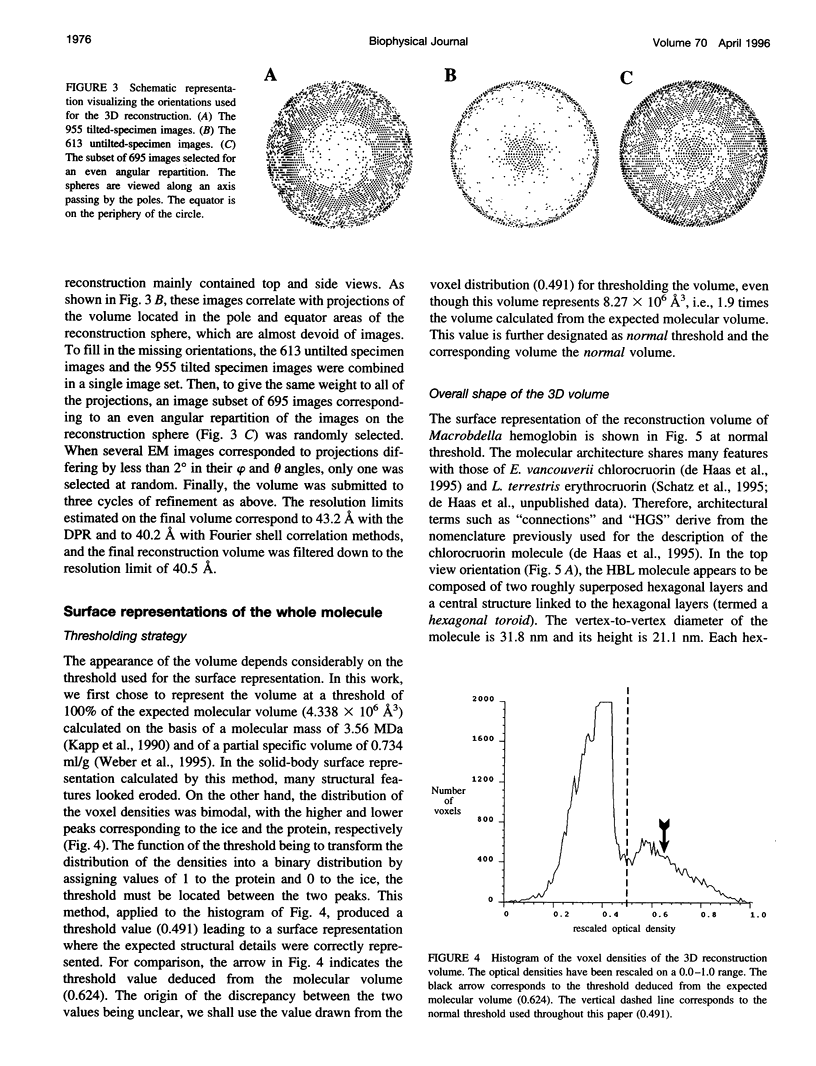
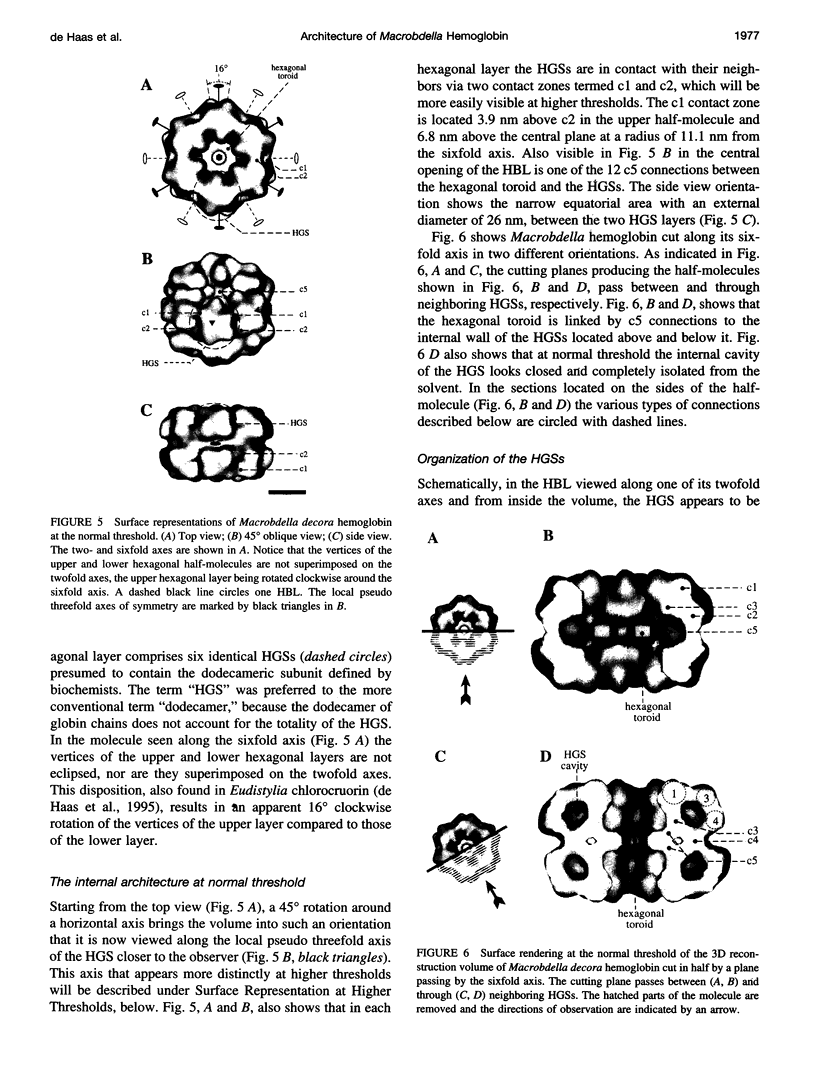
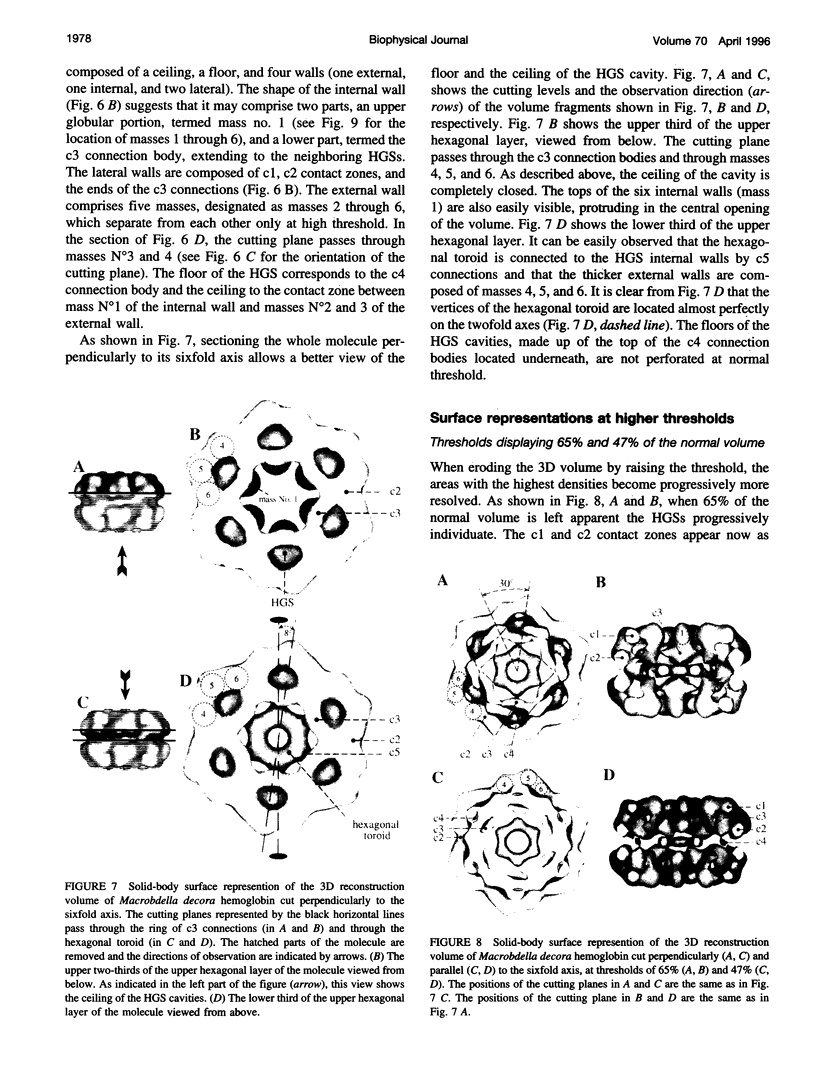
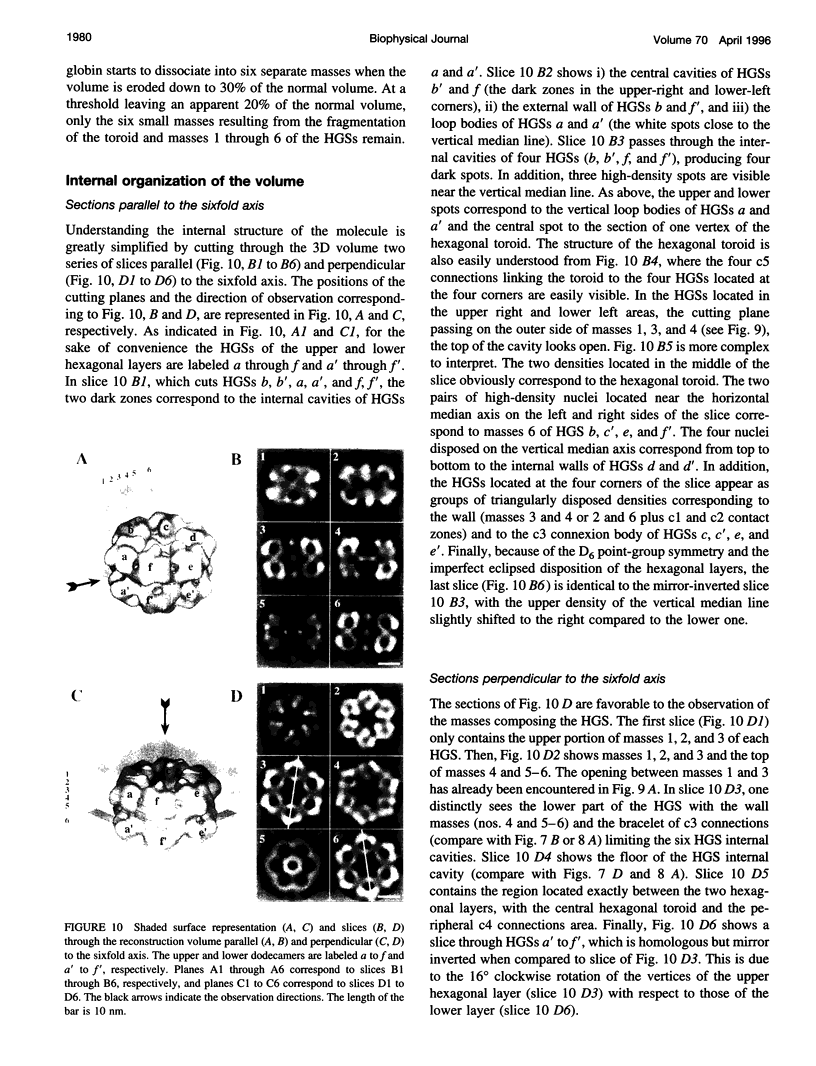
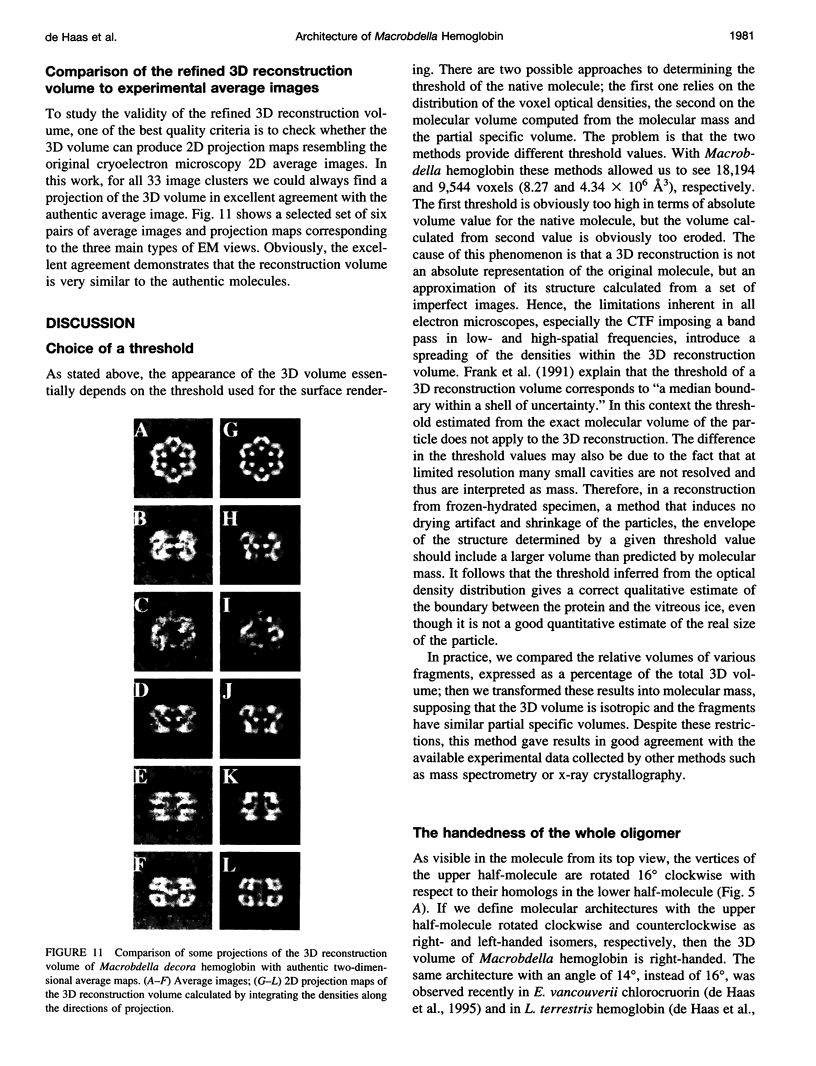
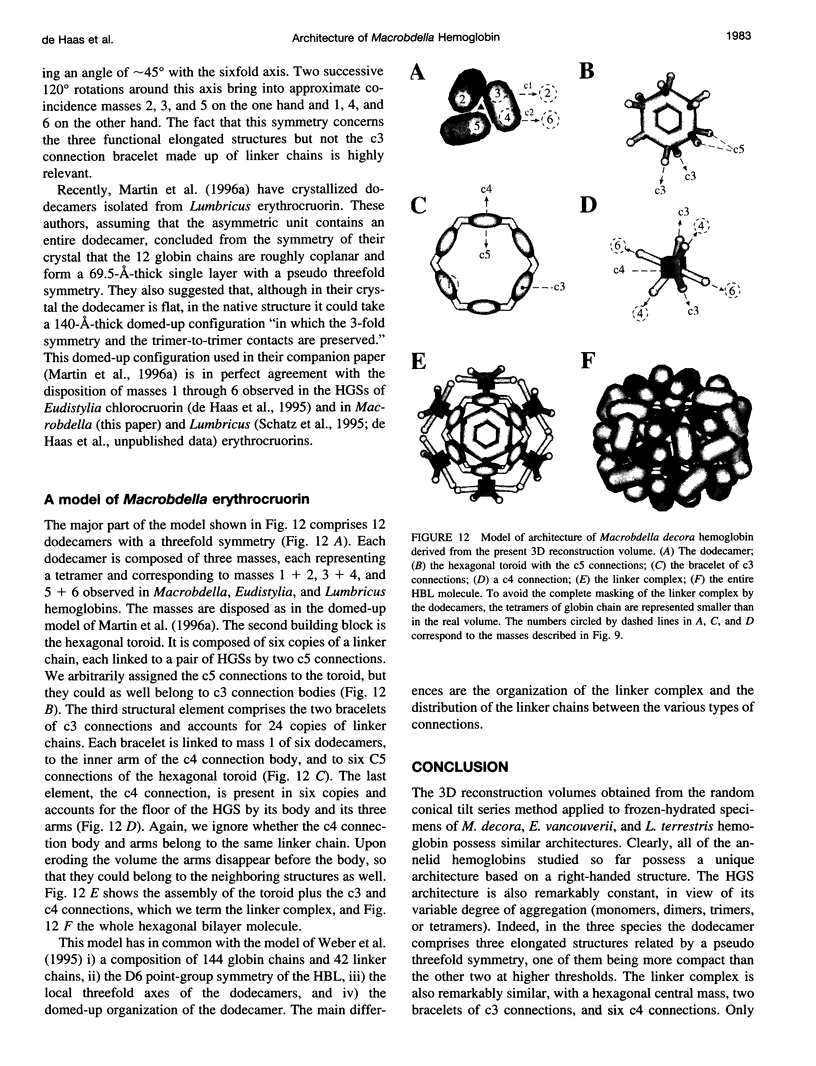
Macrobdella decora hemoglobin was observed in vitreous ice by cryoelectron microscopy and subjected to three-dimensional reconstruction by the method of random conical tilt series. The refined volume has a resolution of 40 A and a D6 point-group symmetry. Its architecture, with its hexagonal bilayer appearance, resembles those of Lumbricus terrestris (oligochaete) and Eudistylia vancouverii (polychaete). When the reconstruction volume is viewed along its sixfold axis, the vertices of the upper hexagonal layer are rotated 16 degrees clockwise compared to those of the lower layer. In agreement with the "bracelet" model of Vinogradov et al., a central linker complex is decorated by 12 hollow globular substructures. The linker complex is made up of a central hexagonal toroid linked by 12 c5 connections to two bracelets of c3 connections, which are themselves linked via six c4 connections. The portion of the hollow globular substructure corresponding to the dodecamer of globin chains has a local pseudo threefold symmetry and is composed of three elongated structures visible when the volume is displayed at high threshold. The main difference between Macrobdella, Lumbricus, and Eudistylia hemoglobins is the presence in Macrobdella of a central hexagonal toroid instead of a compact flat hexagonal structure.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adrian M., Dubochet J., Lepault J., McDowall A. W. Cryo-electron microscopy of viruses. Nature. 1984 Mar 1;308(5954):32–36. doi: 10.1038/308032a0. [DOI] [PubMed] [Google Scholar]
- Antonini E., Chiancone E. Assembly of multisubunit respiratory proteins. Annu Rev Biophys Bioeng. 1977;6:239–271. doi: 10.1146/annurev.bb.06.060177.001323. [DOI] [PubMed] [Google Scholar]
- Boekema E. J., van Heel M. Molecular shape of Lumbricus terrestris erythrocruorin studied by electron microscopy and image analysis. Biochim Biophys Acta. 1988 Dec 2;957(3):370–379. doi: 10.1016/0167-4838(88)90228-2. [DOI] [PubMed] [Google Scholar]
- Boisset N., Penczek P., Pochon F., Frank J., Lamy J. Three-dimensional architecture of human alpha 2-macroglobulin transformed with methylamine. J Mol Biol. 1993 Jul 20;232(2):522–529. doi: 10.1006/jmbi.1993.1408. [DOI] [PubMed] [Google Scholar]
- Dubochet J., Adrian M., Chang J. J., Homo J. C., Lepault J., McDowall A. W., Schultz P. Cryo-electron microscopy of vitrified specimens. Q Rev Biophys. 1988 May;21(2):129–228. doi: 10.1017/s0033583500004297. [DOI] [PubMed] [Google Scholar]
- Frank J., Penczek P., Grassucci R., Srivastava S. Three-dimensional reconstruction of the 70S Escherichia coli ribosome in ice: the distribution of ribosomal RNA. J Cell Biol. 1991 Nov;115(3):597–605. doi: 10.1083/jcb.115.3.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank J., Verschoor A., Boublik M. Computer averaging of electron micrographs of 40S ribosomal subunits. Science. 1981 Dec 18;214(4527):1353–1355. doi: 10.1126/science.7313694. [DOI] [PubMed] [Google Scholar]
- Green B. N., Suzuki T., Gotoh T., Kuchumov A. R., Vinogradov S. N. Electrospray ionization mass spectrometric determination of the complete polypeptide chain composition of Tylorrhynchus heterochaetus hemoglobin. J Biol Chem. 1995 Aug 4;270(31):18209–18211. doi: 10.1074/jbc.270.31.18209. [DOI] [PubMed] [Google Scholar]
- Ilan E., Haroun J. Oxygen-binding properties of extracellular hemoglobin from the leech, Hirudo medicinalis. Effects of pH, cations and temperature. Biochim Biophys Acta. 1993 Mar 5;1162(1-2):77–83. doi: 10.1016/0167-4838(93)90130-j. [DOI] [PubMed] [Google Scholar]
- Kapp O. H., Qabar A. N., Bonner M. C., Stern M. S., Walz D. A., Schmuck M., Pilz I., Wall J. S., Vinogradov S. N. Quaternary structure of the giant extracellular hemoglobin of the leech Macrobdella decora. J Mol Biol. 1990 May 5;213(1):141–158. doi: 10.1016/S0022-2836(05)80127-5. [DOI] [PubMed] [Google Scholar]
- Lambert O., Boisset N., Taveau J. C., Préaux G., Lamy J. N. Three-dimensional reconstruction of the alpha D and beta C-hemocyanins of Helix pomatia from frozen-hydrated specimens. J Mol Biol. 1995 Apr 28;248(2):431–448. doi: 10.1016/s0022-2836(95)80061-1. [DOI] [PubMed] [Google Scholar]
- Martin P. D., Eisele K. L., Doyle M. A., Kuchumov A. R., Walz D. A., Arutyunyan E. G., Vinogradov S. N., Edwards B. F. Molecular symmetry of the dodecamer subunit of Lumbricus terrestris hemoglobin. J Mol Biol. 1996 Jan 12;255(1):170–175. doi: 10.1006/jmbi.1996.0014. [DOI] [PubMed] [Google Scholar]
- Martin P. D., Kuchumov A. R., Green B. N., Oliver R. W., Braswell E. H., Wall J. S., Vinogradov S. N. Mass spectrometric composition and molecular mass of Lumbricus terrestris hemoglobin: a refined model of its quaternary structure. J Mol Biol. 1996 Jan 12;255(1):154–169. doi: 10.1006/jmbi.1996.0013. [DOI] [PubMed] [Google Scholar]
- Milligan R. A., Brisson A., Unwin P. N. Molecular structure determination of crystalline specimens in frozen aqueous solutions. Ultramicroscopy. 1984;13(1-2):1–9. doi: 10.1016/0304-3991(84)90051-2. [DOI] [PubMed] [Google Scholar]
- Ownby D. W., Zhu H., Schneider K., Beavis R. C., Chait B. T., Riggs A. F. The extracellular hemoglobin of the earthworm, Lumbricus terrestris. Determination of subunit stoichiometry. J Biol Chem. 1993 Jun 25;268(18):13539–13547. [PubMed] [Google Scholar]
- Penczek P. A., Grassucci R. A., Frank J. The ribosome at improved resolution: new techniques for merging and orientation refinement in 3D cryo-electron microscopy of biological particles. Ultramicroscopy. 1994 Mar;53(3):251–270. doi: 10.1016/0304-3991(94)90038-8. [DOI] [PubMed] [Google Scholar]
- Penczek P., Radermacher M., Frank J. Three-dimensional reconstruction of single particles embedded in ice. Ultramicroscopy. 1992 Jan;40(1):33–53. [PubMed] [Google Scholar]
- Qabar A. N., Stern M. S., Walz D. A., Chiu J. T., Timkovich R., Wall J. S., Kapp O. H., Vinogradov S. N. Hierarchy of globin complexes. The quaternary structure of the extracellular chlorocruorin of Eudistylia vancouverii. J Mol Biol. 1991 Dec 20;222(4):1109–1129. doi: 10.1016/0022-2836(91)90596-x. [DOI] [PubMed] [Google Scholar]
- Radermacher M. Three-dimensional reconstruction of single particles from random and nonrandom tilt series. J Electron Microsc Tech. 1988 Aug;9(4):359–394. doi: 10.1002/jemt.1060090405. [DOI] [PubMed] [Google Scholar]
- Radermacher M., Wagenknecht T., Verschoor A., Frank J. Three-dimensional reconstruction from a single-exposure, random conical tilt series applied to the 50S ribosomal subunit of Escherichia coli. J Microsc. 1987 May;146(Pt 2):113–136. doi: 10.1111/j.1365-2818.1987.tb01333.x. [DOI] [PubMed] [Google Scholar]
- Radermacher M., Wagenknecht T., Verschoor A., Frank J. Three-dimensional structure of the large ribosomal subunit from Escherichia coli. EMBO J. 1987 Apr;6(4):1107–1114. doi: 10.1002/j.1460-2075.1987.tb04865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxton W. O., Baumeister W. The correlation averaging of a regularly arranged bacterial cell envelope protein. J Microsc. 1982 Aug;127(Pt 2):127–138. doi: 10.1111/j.1365-2818.1982.tb00405.x. [DOI] [PubMed] [Google Scholar]
- Schatz M., Orlova E. V., Dube P., Jäger J., van Heel M. Structure of Lumbricus terrestris hemoglobin at 30 A resolution determined using angular reconstitution. J Struct Biol. 1995 Jan-Feb;114(1):28–40. doi: 10.1006/jsbi.1995.1003. [DOI] [PubMed] [Google Scholar]
- Van Heel M. Angular reconstitution: a posteriori assignment of projection directions for 3D reconstruction. Ultramicroscopy. 1987;21(2):111–123. doi: 10.1016/0304-3991(87)90078-7. [DOI] [PubMed] [Google Scholar]
- Vinogradov S. N., Lugo S. D., Mainwaring M. G., Kapp O. H., Crewe A. V. Bracelet protein: a quaternary structure proposed for the giant extracellular hemoglobin of Lumbricus terrestris. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8034–8038. doi: 10.1073/pnas.83.21.8034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinogradov S. N., Sharma P. K., Qabar A. N., Wall J. S., Westrick J. A., Simmons J. H., Gill S. J. A dodecamer of globin chains is the principal functional subunit of the extracellular hemoglobin of Lumbricus terrestris. J Biol Chem. 1991 Jul 15;266(20):13091–13096. [PubMed] [Google Scholar]
- Weber R. E., Malte H., Braswell E. H., Oliver R. W., Green B. N., Sharma P. K., Kuchumov A., Vinogradov S. N. Mass spectrometric composition, molecular mass and oxygen binding of Macrobdella decora hemoglobin and its tetramer and monomer subunits. J Mol Biol. 1995 Sep 1;251(5):703–720. doi: 10.1006/jmbi.1995.0466. [DOI] [PubMed] [Google Scholar]
- de Haas F., Taveau J. C., Boisset N., Lambert O., Vinogradov S. N., Lamy J. N. Three-dimensional reconstruction of the chlorocruorin of the polychaete annelid Eudistylia vancouverii. J Mol Biol. 1996 Jan 12;255(1):140–153. doi: 10.1006/jmbi.1996.0012. [DOI] [PubMed] [Google Scholar]
- van Heel M., Frank J. Use of multivariate statistics in analysing the images of biological macromolecules. Ultramicroscopy. 1981;6(2):187–194. doi: 10.1016/0304-3991(81)90059-0. [DOI] [PubMed] [Google Scholar]
- van Heel M. Multivariate statistical classification of noisy images (randomly oriented biological macromolecules). Ultramicroscopy. 1984;13(1-2):165–183. doi: 10.1016/0304-3991(84)90066-4. [DOI] [PubMed] [Google Scholar]