Abstract
Cells express multiple G protein-coupled receptors that are simultaneously or sequentially activated by agonists. The consequences of activating one receptor on signaling and trafficking of another receptor are unknown. We examined the effects of selective activation of the neurokinin 1 receptor (NK1R) on signaling and trafficking of the NK3R and vice versa. Selective agonists of NK1R and NK3R induced membrane translocation of β-arrestins (β-ARRs). Dominant negative β-ARR319–418 inhibited endocytosis of NK1R and NK3R. Whereas an NK1R agonist caused sequestration of NK1R with β-ARR in the same endosomes, thereby depleting them from the cytosol, β-ARRs did not prominently sequester with the activated NK3R and rapidly returned to the cytosol. In cells coexpressing both receptors, prior activation of the NK1R inhibited endocytosis and homologous desensitization of the NK3R, which was dose-dependently reversed by overexpression of β-ARR1. Similar results were obtained in enteric neurons that naturally coexpress the NK1R and NK3R. In contrast, activation of the NK3R did not affect NK1R endocytosis or desensitization. Thus, the high-affinity and prolonged interaction of the NK1R with β-ARRs depletes β-ARRs from the cytosol and limits their role in desensitization and endocytosis of the NK3R. Because β-ARRs are critical for desensitization, endocytosis, and mitogenic signaling of many receptors, this sequestration is likely to have important and widespread implications.
A common theme of signaling by G protein-coupled receptors (GPCRs) for neurotransmitters and hormones is that a single agonist can interact with different receptors. For example, the tachykinins substance P (SP), neurokinin A (NKA), and NKB bind the three neurokinin receptors (NKR) with graded affinities (NK1R, SP > NKA > NKB; NK2R, NKA > NKB > SP; NK3R, NKB > NKA > SP). Thus, one agonist could regulate a cell although distinct receptors once the optimal concentration is achieved. Furthermore, different agonists may simultaneously or sequentially activate distinct receptors on the same cell. The functional relevance of this activation is unknown.
Activation of one receptor could trigger processes that regulate the same or a different receptor (homologous or heterologous regulation, respectively) (1). Most information about interactions between GPCRs derives from studies of desensitization. G protein receptor kinases and β-arrestins (β-ARRs) interact with agonist-occupied receptors to uncouple them from heterotrimeric G proteins and mediate homologous desensitization, whereas second-messenger kinases are responsible for heterologous desensitization (2, 3). Less is known about whether activation of one receptor affects trafficking of another. β-ARRs are clathrin adapters for receptor endocytosis (4–7), which is necessary for down-regulation (8), resensitization (9), and mitogenic signaling (10–13). Thus, control over the capacity of a GPCR to interact with β-ARRs could have major effects on signaling and trafficking of many receptors. This possibility has not been studied.
We examined interactions between NKRs, which play important roles in inflammation, pain, depression, smooth muscle contraction, and exocrine secretion (14). Some cells, such as enteric neurons (15, 16) and microvascular endothelial cells (17), coexpress NK1R and NK3R. However, the consequences of selectively activating one NKR on signaling and trafficking of another are unknown.
Our aims were to (i) determine the consequences of selectively activating the NK1R on trafficking and signaling of the NK3R and vice versa; (ii) define the role of β-ARRs in trafficking of these receptors; and (iii) evaluate the role of β-ARRs in heterologous regulation of trafficking and signaling.
Materials and Methods
Reagents.
NK1R agonist [Sar9MetO211]SP (SM-SP) and NK3R agonist [MePhe7]NKB (MP-NKB) were from Phoenix Pharmaceuticals (St. Joseph, MO). 3H-SM-SP (47 Ci/mmol) and 125I-MP-NKB (2,300 Ci/mmol) were from NEN. SM-SP and MP-NKB were labeled with Alexa-488 and -594 (Molecular Probes) (18). By measuring Ca2+ mobilization and binding of fluorescent peptides in cells expressing NK1R or NK3R, we verified that SM-SP and MP-NKB are selective agonists of the NK1R and NK3R, respectively, up to 10 nM (not shown). NK1R antagonist SR140333 and NK3R antagonist SR142801 were from Sanofi, Paris. Antibodies to β-ARR1/2 (rabbit) (3) and β-ARR1 (mouse) (6) were from R. J. Lefkowitz (Duke University) and Transduction Laboratories (Lexington, KY), respectively. Other reagents have been described (6, 7, 11, 12, 15, 19–23).
Generation of Transfected Cells.
Kirsten murine sarcoma virus-transformed rat kidney epithelial cells (KNRK) were stably transfected with either rat NK1R (pcDNANeo, designated KNRK-NK1R) or rat NK3R (pcDNAHygro, KNRK-NK3R) or both NK1R and NK3R (KNRK-NK1R/NK3R) (6, 7, 11, 12). Cells were transiently or stably transfected with β-ARR1 or dominant negative β-ARR319–418 or β-ARRV53D [C-terminal green fluorescent protein (GFP)] (6, 7, 11). Cells were screened by fluorescence microscopy and FACS by using Alexa-tagged peptides or GFP to identify cells with graded expression (6, 7).
Quantification of NK1R and NK3R.
Cells were incubated in 250 μl DMEM/0.1% BSA containing 0.5 nM 3H-SM-SP or 0.05 nM 125I-MP-NKB plus 0–100 nM of the unlabeled agonists for 60 min at 4°C. Nonspecific binding was determined in the presence of 1 μM unlabeled agonists. Receptor number was calculated by Scatchard analysis. Unless stated otherwise, KNRK-NK1R cells expressed 1.5 × 10−3 fmol NK1R/cell; KNRK-NK3R cells expressed 2.5 × 10−3 fmol NK3R/cell; and KNRK-NK1R/NK3R cells expressed 7 × 10−3 fmol NK1R/cell and 1.5 × 10−3 fmol NK3R/cell.
Quantification of β-ARR.
β-ARR1 was fused to GEX-4T-1 by PCR. Bacteria were transformed, and β-ARR1-GST fusion protein was extracted in 1% Triton X-100/10 mM 2-mercaptoethanol and purified by using glutathione-Sepharose beads. β-ARR1-GST was separated by SDS/PAGE and quantified by Coomassie staining and densitometry, using BSA standards. To quantify β-ARR1, cell lysates and β-ARR1-GST standards were fractionated by SDS/PAGE and analyzed by Western blotting with an antibody to β-ARR1 (11, 12). Band intensities were measured by densitometry. Unless stated otherwise, KNRK-NK1R/NK3R cells expressed 7.7 ± 1.5 × 10−3 fmol β-ARR1/cell.
Dispersion and Culture of Myenteric Neurons.
Myenteric neurons were dissociated from the guinea pig small intestine and studied after 7–14 days (22, 23).
Endocytosis of 3H-SM-SP and 125I-MP-NKB.
KNRK cells were incubated with 10 nM 3H-SM-SP or 125I-MP-NKB for 60 min at 4°C. They were washed and incubated for 0–30 min at 37°C. Cells were washed with acid to separate cell-surface (acid-labile) from internalized (acid-resistant) ligand (19, 20).
Fluorescence Microscopy.
Cells were incubated with 10 nM unlabeled or Alexa-SM-SP or MP-NKB for 60 or 120 min at 4°C, washed, and incubated for 0–30 min at 37°C (6, 7, 20, 23). β-ARR1/2 were localized by immunofluorescence (6, 11, 12, 23). Cells were observed by confocal microscopy.
Measurement of [Ca2+]i.
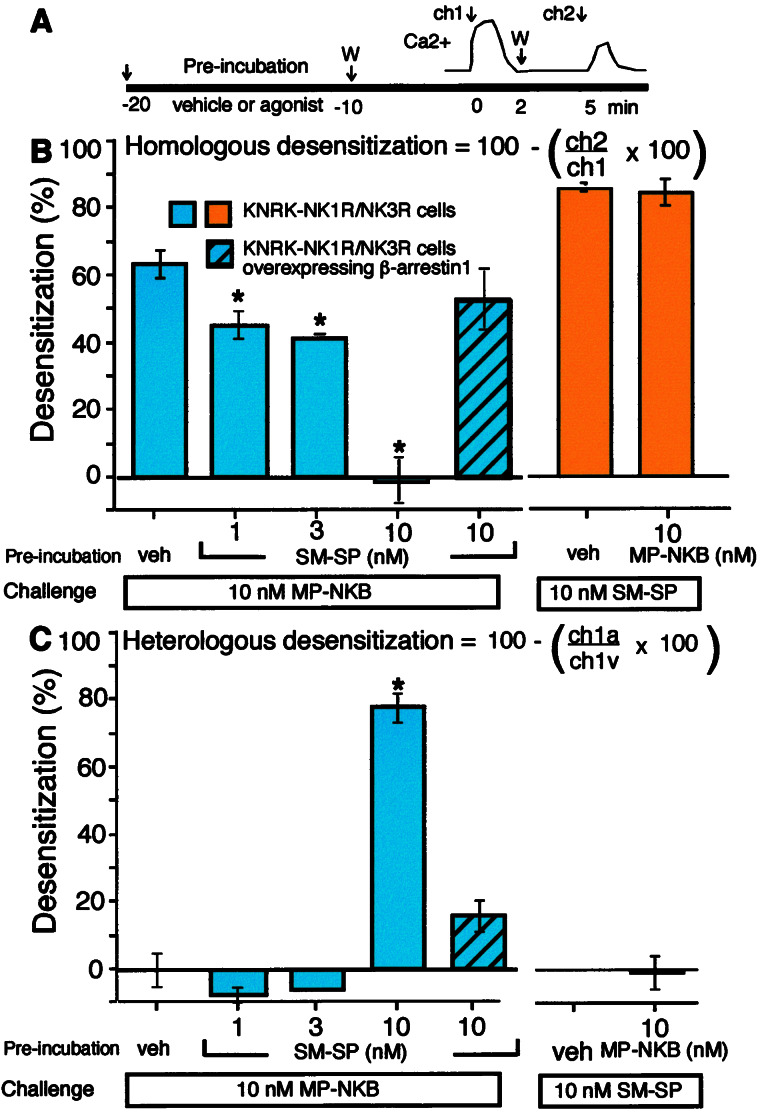
[Ca2+]i was measured by using Fura-2/AM (6, 7, 21). Cells were preincubated with agonist or vehicle, washed, and challenged repetitively. Homologous and heterologous desensitization were assessed (see Fig. 6 for details).
Figure 6.
Homologous and heterologous desensitization of the NK1R and NK3R in KNRK-NK1R/NK3R cells. (A) Experimental design. Cells were preincubated with vehicle or 1, 3, or 10 nM SM-SP or MP-NKB for 10 min at 37°C and washed (W). To assess desensitization, cells then were challenged 10 min later with 10 nM SM-SP or MP-NKB for 2 min (ch1), washed, and challenged again 5 min later with 10 nM of the same agonist (ch2). In B and C, blue bars refer to NK3R desensitization and orange refer to NK1R desensitization. (B) Homologous desensitization was assessed by comparing the magnitude of the second response (ch2) with that of the first (ch1) by using the same agonist on the same cells (see formula). Preincubation with SM-SP (1–10 nM) inhibited homologous desensitization of the NK3R to repeated challenge with MP-NKB. Overexpression of β-ARR1 rescued this desensitization (shaded blue box, for the 2+ cell line, see Fig. 5C). Preincubation with MP-NKB (10 nM) did not affect homologous desensitization of the NK1R to repeated challenge with SM-SP. (C) Heterologous desensitization was assessed by comparing the magnitude of the first response (ch1) in different cells preincubated with agonist (ch1a) or vehicle (ch1v) (see formula). Preincubation with high (10 nM) but not low (1–3 nM) SM-SP desensitized responses of the NK3R to MP-NKB. This heterologous desensitization was decreased by overexpression of β-ARR1. Preincubation with 10 nM MP-NKB did not desensitize NK1R responses to SM-SP. (n = 3; *, P < 0.05 compared with untreated cells.)
Statistics.
Observations were in n > 3 experiments in triplicate. Results are mean ± SE. Differences were analyzed by ANOVA and the Student–Newman–Keul's test.
Results
Activation of the NK1R Inhibits Endocytosis of the NK3R but Not Vice Versa.
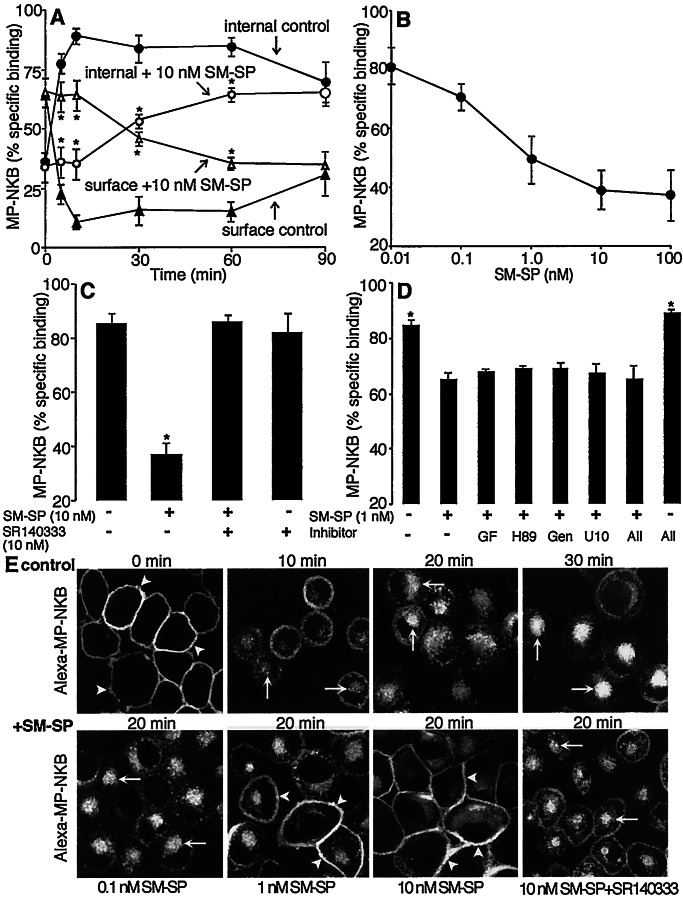
We examined the effects of activation of the NK1R with SM-SP (NK1R agonist) on endocytosis of MP-NKB (NK3R agonist) in KNRK coexpressing NK1R and NK3R (KNRK-NK1R/NK3R cells: 7.0 × 10−3 fmol NK1R/cell; 1.5 × 10−3 fmol NK3R/cell; 7.7 × 10−3 fmol β-ARR1/cell). Cells were preincubated with SM-SP or vehicle (30 min, 4°C) and then incubated with SM-SP plus 125I-MP-NKB (60 min, 4°C), washed, and warmed to 37°C for 0–90 min. Internalized 125I-MP-NKB was quantified by using an acid wash. In vehicle-treated cells, warming resulted in rapid endocytosis of 125I-MP-NKB that was maximal at 10 min (89.5 ± 2.8% internalized) (Fig. 1A). Prior activation of the NK1R inhibited endocytosis of 125I-MP-NKB (35.5 ± 6.0% internalized, 10 min, 10 nM SM-SP) for up to 60 min (Fig 1A). Inhibition by SM-SP was concentration-dependent (IC50 ≈ 0.5 nM; maximal, ≈10 nM) and, thus, dependent on the number of activated NK1Rs (Fig. 1B). A similar degree of inhibition of endocytosis of 125I-MP-NKB was observed in cell lines expressing approximately equivalent numbers of NK1R and NK3R (32.5 ± 1.7% internalized, 10 min, 10 nM SM-SP: 2.7 × 10−3 fmol NK1R/cell and 2.0 × 10−3 fmol NK3R/cell). Comparable results were obtained in 11 different cell lines expressing varying ratios of NK1R/NK3R from ≈1.4:1 to 6.7:1 (not shown).
Figure 1.
SM-SP (NK1R agonist) inhibits endocytosis of MP-NKB (NK3R agonist) in KNRK-NK1R/NK3R cells. (A) Endocytosis of 125I-MP-NKB. Cells were preincubated with SM-SP (10 nM, 30 min, 4°C) and coincubated with 125I-MP-NKB (60 min, 4°C). They were washed and incubated at 37°C for 0–90 min, and surface and internalized labels were determined by using an acid wash. Note that SM-SP inhibits endocytosis of 125I-MP-NKB. (B) Effects of graded concentrations of SM-SP on endocytosis of 125I-MP-NKB after 10 min at 37°C. (C) Specificity of inhibition by 10 nM SM-SP on endocytosis of 125I-MP-NKB after 10 min at 37°C. Cells were preincubated with SR140333 (NK1R antagonist, 10 nM, 30 min, 4°C) before incubation with SM-SP. Note that SR140333 abolished inhibition by SM-SP, confirming specificity. (D) Effects of kinase inhibitors on inhibition by 1 nM SM-SP on endocytosis of 125I-MP-NKB after 10 min at 37°C. Cells were preincubated with GF109203X (GFX, 100 nM), H-89 (500 nM), genistein (Gen, 10 μM), U1026 (U10, 10 μM), or a combination (30 min at 4°C) before incubation with SM-SP. Note that kinase inhibitors had no effect even on minor inhibition by 1 nM SM-SP. For A–D, n = 3. *, P < 0 05. (E) Endocytosis of fluorescent Alexa-MP-NKB, assessed by confocal microscopy. (Upper) Cells were incubated with 10 nM Alexa-MP-NKB (60 min, 4°C), washed, and incubated for 0–30 min at 37°C. Note trafficking of Alexa-MP-NKB from the plasma membrane (arrowheads) to endosomes (arrows). (Lower) Effects of SM-SP on endocytosis of MP-NKB after 20 min at 37°C. Cells were preincubated with graded concentrations of SM-SP (30 min, 4°C) before incubation with Alexa-MP-NKB or with 10 nM SR140333 before addition of SM-SP. Note that 1 and 10 nM SM-SP cause retention of Alexa-MP-NKB at the plasma membrane, which is reversed by SR140333, confirming specificity. (Scale = 5 μm.)
To confirm the specificity of inhibition, KNRK-NK1R/NK3R cells were pretreated with the NK1R antagonist SR140333 (10 nM) for 30 min before incubation with SM-SP. SR140333 abolished the inhibitory effect of 10 nM SM-SP (Fig. 1C). To investigate the possible role of kinases known to participate in heterologous desensitization of GPCRs (2, 3), cells were pretreated with inhibitors of protein kinase C (GF109203X), PKA (H-89), tyrosine kinases (genistein), and mitogen-activated protein (MAP) kinase MEK (U1026) for 30 min before incubation with SM-SP. The kinase inhibitors had no effect even on the small inhibition by 1 nM SM-SP (Fig. 1D).
We examined the effects of activating the NK1R on endocytosis of the NK3R agonist MP-NKB tagged with the fluorophore Alexa (Alexa-MP-NKB) by using confocal microscopy. In vehicle-treated cells, Alexa-MP-NKB was confined to the plasma membrane at 4°C (Fig. 1E Upper). After 5 or 10 min at 37°C, Alexa-MP-NKB was detected in superficially located endosomes that translocated to a perinuclear region at 20 or 30 min. Prior activation of the NK1R with SM-SP caused retention of label at the plasma membrane (Fig. 1E Lower). The effect was concentration-dependent and abolished by the NK1R antagonist SR140333.
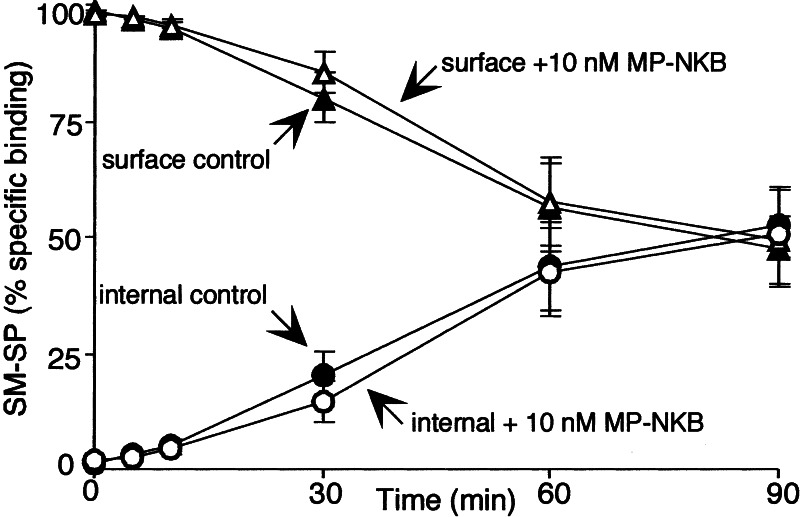
In contrast, prior activation of the NK3R with MP-NKB did not affect binding or endocytosis of 3H-SM-SP (Fig. 2) or fluorescent Alexa-SM-SP (not shown).
Figure 2.
MP-NKB (NK3R agonist) does not inhibit endocytosis of 3H-SM-SP (NK1R agonist) in KNRK-NK1R/NK3R cells. Cells were preincubated with vehicle or MP-NKB (10 nM, 30 min, 4°C) and coincubated with 3H-SM-SP (60 min, 4°C). They were washed and incubated at 37°C for indicated times, and surface and internalized labels were determined by using an acid wash. (n = 3.)
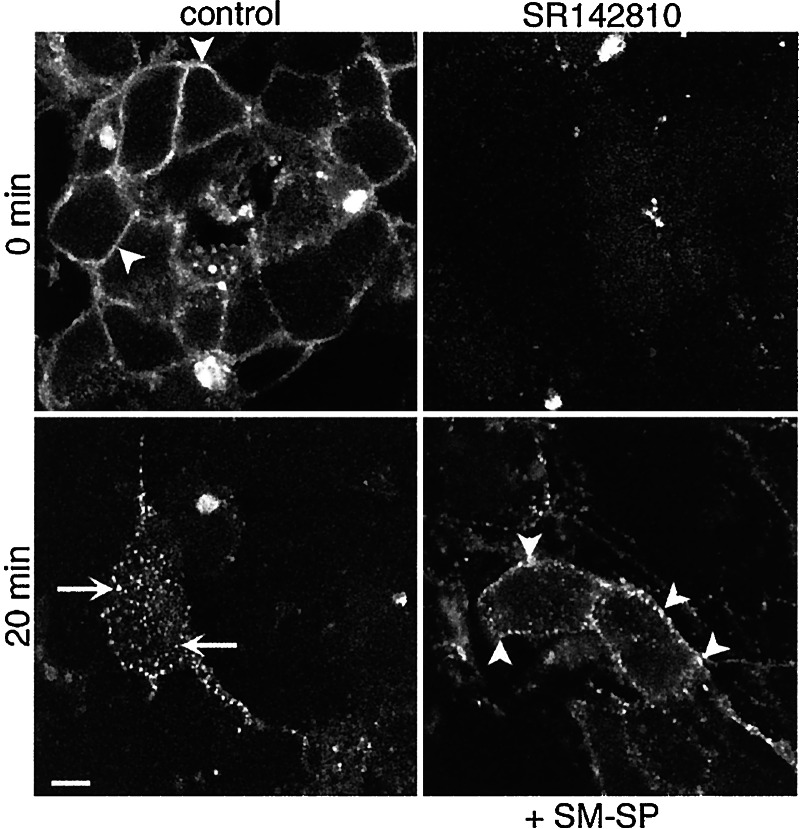
The effects of NK1R activation on endocytosis of NK3R were examined in enteric neurons that naturally express these receptors. In controls, Alexa-SM-SP (not shown) and Alexa-MP-NKB (Fig. 3) specifically bound to the plasma membrane of neurons at 4°C and were detected in endosomes after 20 min at 37°C. Preincubation with 10 nM SM-SP (30 min, 4°C) followed by coincubation with Alexa-MP-NKB caused retention of Alexa-MP-NKB at the cell surface.
Figure 3.
SM-SP (NK1R agonist) inhibits endocytosis of Alexa-MP-NKB (NK3R agonist) in neurons. Neurons were incubated with Alexa-MP-NKB (10 nM, 120 min, 4°C), washed, and incubated for 0 or 20 min at 37°C. In controls, MP-NKB translocated from the plasma membrane (arrowheads) to endosomes (arrows) at 20 min. Preincubation with NK3R antagonist SR142801 (10 nM, 30 min, 4°C) abolished binding, confirming specificity. Preincubation with SM-SP (10 nM, 30 min, 4°C) caused retention of Alexa-MP-NKB at or near the plasma membrane (arrowheads). (Scale = 5 μm.)
Thus, an NK1R agonist causes a concentration-dependent inhibition of endocytosis of the NK3R in transfected cells and enteric neurons. Inhibition is observed in cells expressing both receptors at various ratios. Therefore, the inhibition depends on the number of activated receptors but is independent of the relative levels of receptor expression. In contrast, activation of the NK3R does not affect NK1R trafficking.
The NK1R Sequesters β-ARRs in Endosomes and Thereby Inhibits Agonist-Dependent Endocytosis of the NK3R.
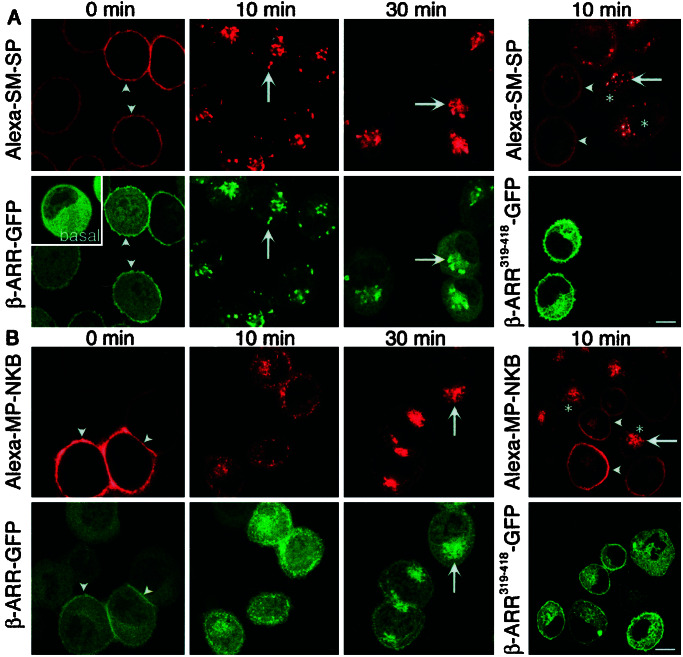
We hypothesized that NK1R-mediated inhibition of NK3R endocytosis was a result of competition between receptors for interaction with β-ARRs. To determine the role of β-ARRs in NK1R and NK3R endocytosis, we transiently expressed β-ARR1 (control) or dominant negative β-ARRV53D or β-ARR319–418 in cells expressing either NK1R or NK3R (KNRK-NK1R or KNRK-NK3R cells) and examined endocytosis of fluorescent agonists. In KNRK-NK1R cells (1.5 × 10−3 fmol NK1R/cell) expressing β-ARR1-GFP, Alexa-SM-SP was confined to the cell surface after 60 min at 4°C, and β-ARR1-GFP had translocated from the cytosol to the plasma membrane (Fig. 4A). After 10 or 30 min at 37°C, Alexa-SM-SP and β-ARR1 were found in the same endosomes. Notably, translocation of β-ARR1 to NK1R endosomes markedly depleted cytoplasmic pools of β-ARR1 (e.g., 10 min). Transient expression of β-ARRV53D-GFP (not shown) or β-ARR319–418-GFP prevented endocytosis of Alexa-SM-SP (Fig. 4A) compared with untransfected cells (Fig. 4A). In KNRK-NK3R cells (2.5 × 10−3 fmol NK3R/cell) expressing β-ARR1-GFP, Alexa-MP-NKB was confined to the cell surface at 4°C, and β-ARR1-GFP had translocated from the cytosol to the plasma membrane (Fig. 4B). After 10 or 30 min at 37°C, Alexa-MP-NKB redistributed to endosomes. In contrast, β-ARR1 was not sequestered prominently into endosomes after warming, but was distributed diffusely throughout the cytosol. Transient expression of β-ARRV53D-GFP (not shown) or β-ARR319–418-GFP prevented endocytosis of Alexa-MP-NKB (Fig. 4B) compared with untransfected cells (Fig. 4B). Thus, β-ARRs mediate agonist-stimulated endocytosis of the NK1R and NK3R. Whereas β-ARR1 remains sequestered with the NK1R in endosomes, which depletes cytosolic pools, β-ARR1 does not prominently sequester in endosomes with the NK3R and, instead, returns to the cytoplasm. The reduced sequestration of β-ARRs in endosomes with the NK3R is not due to a lower expression of NK3R because it was more highly expressed than NK1R.
Figure 4.
Agonist-induced trafficking of Alexa-SM-SP (NK1R agonist) in KNRK-NK1R cells (A) or Alexa-MP-NKB (NK3R agonist) in KNRK-NK3R cells (B). Cells were transiently transfected with wild-type β-ARR1-GFP (Left) or β-ARR (319–418)-GFP (Right). Cells were incubated with Alexa-SM-SP or Alexa-MP-NKB (10 nM, 60 min, 4°C), washed, and incubated at 37°C for 0–30 min. Under basal conditions (no agonist), β-ARR-GFP was cytosolic (see A Inset). (A) Alexa-SM-SP induced translocation of β-ARR-GFP to the plasma membrane (arrowheads, 0 min) followed by sequestration of β-ARR-GFP and Alexa-SM-SP into the same endosomes at 10 and 30 min (arrows), which depleted β-ARR-GFP from the cytosol (e.g., see 10 min). Expression of β-ARR319–418-GFP caused retention of Alexa-SM-SP at the cell surface (arrowheads) compared with marked endocytosis in untransfected cells (*). (B) Alexa-MP-NKB induced translocation of β-ARR-GFP to the plasma membrane (arrowheads, 0 min) followed by endocytosis of β-ARR-GFP and Alexa-MP-NKB at 10 and 30 min (arrows). Note that although β-ARR-GFP colocalized to a minor extent with Alexa-MP-NKB in endosomes, β-ARR-GFP was not depleted from the cytosol (e.g., see 10 min). Expression of β-ARR319–418-GFP caused retention of Alexa-MP-NKB at the cell surface (arrowheads) compared with marked endocytosis in untransfected cells (*). (Scale = 5 μm.)
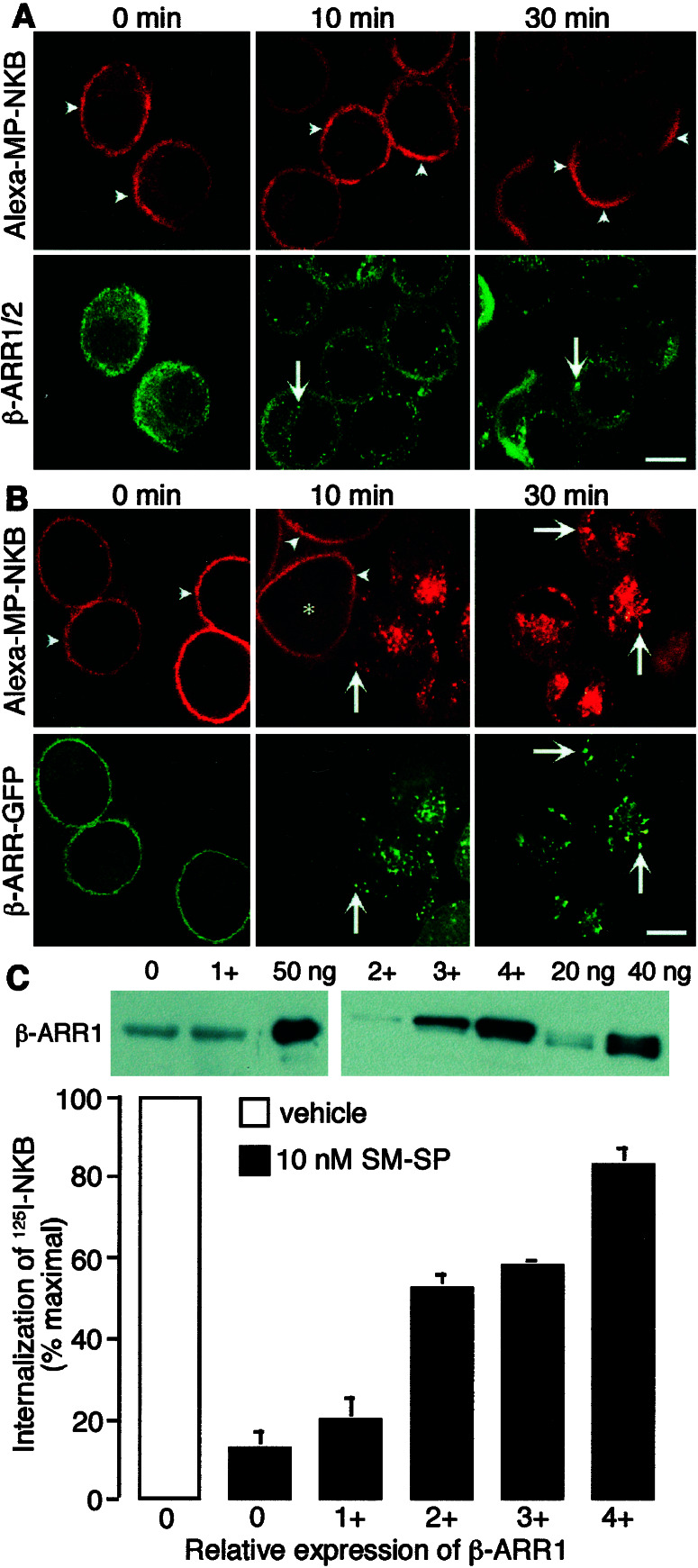
Prior activation of the NK1R may induce sequestration of endogenous β-ARRs into endosomes. This sequestration could deplete β-ARRs from the cytosol and thereby limit interactions between β-ARRs and the NK3R. Therefore, we localized endogenous β-ARR1/2 in cells coexpressing the NK1R and NK3R. KNRK-NK1R/NK3R cells were incubated with SM-SP for 30 min at 4°C plus 10 nM Alexa-MP-NKB for an additional 60 min at 4°C. Cells were washed and incubated at 37°C for 0–30 min, and β-ARR1/2 was detected by immunofluorescence. SM-SP induced membrane translocation of β-ARR1/2 followed by sequestration into endosomes and depletion from the cytosol (Fig. 5A). Alexa-MP-NKB was retained at the cell surface at all times, where it clustered in patches at 30 min. Thus, an NK1R agonist triggers sequestration of endogenous β-ARR1/2 into endosomes and depletes cytosolic pools.
Figure 5.
Expression of β-ARR in KNRK-NK1R/NK3R cells rescues inhibition of endocytosis. Cells were preincubated with SM-SP (10 nM, 30 min, 4°C) and coincubated with Alexa-MP-NKB (10 nM, 60 min, 4°C) (A and B) or 125I-MP-NKB (C). Cells were washed and incubated for 0–30 min (A and B) or 10 min (C) at 37°C. (A) Trafficking of Alexa-MP-NKB and β-ARR1/2 in KNRK-NK1R/NK3R cells. Endogenous β-ARR1/2 was detected by immunofluorescence with a FITC-conjugated secondary antibody. Note that whereas β-ARR1/2 was sequestered into prominent endosomes after 10 and 30 min (arrows), Alexa-MP-NKB remained at the cell surface (arrowheads). (B) Transient expression of β-ARR1 rescues inhibition of endocytosis of MP-NKB by SM-SP in KNRK-NK1R/NK3R cells. Alexa-MP-NKB internalized in cells coexpressing β-ARR (arrows), but was retained at the cell surface in untransfected cells (arrowheads, *). (Scale = 5 μm.) (C) Rescue of endocytosis of 125I-MP-NKB by graded overexpression of β-ARR1. Internalization was quantified by an acid wash and is expressed as a percentage of maximal observed in vehicle-treated cells (100%). Insets show quantification of β-ARR1 by Western blotting with standards of β-ARR1-GST (ng/lane). Different exposures are shown for various cell lines: 0 and 1+ required longer exposure for detection than lines 2+, 3+, and 4+. β-ARR1 levels were (×10−3 fmol β-ARR1/cell): 0, 7.7 ± 1.5; 1+, 6.3 ± 1.2; 2+, 49 ± 7; 3+, 106 ± 13; 4+, 156 ± 26, representing 7- to 22-fold over basal levels).
If sequestration of β-ARRs with the NK1R is the mechanism by which the NK1R inhibits endocytosis of the NK3R, overexpression of β-ARRs should rescue NK3R endocytosis. To test this hypothesis, we transiently transfected KNRK-NK1R/NK3R cells with β-ARR1. In untransfected cells, NK1R activation prevented endocytosis of the Alexa-MP-NKB (see 10 min, Fig. 5B). However, in cells overexpressing β-ARR1, activation of the NK1R did not prevent endocytosis of Alexa-MP-NKB, which was detected in endosomes after 10 and 30 min at 37°C. We quantified the rescue of NK3R endocytosis by β-ARR1 by examining internalization of 125I-MP-NKB by an acid wash. In KNRK-NK1R/NK3R cells pretreated with 10 nM SM-SP, internalization after 10 min at 37°C was 12.8 ± 4.2% of maximal (100% in vehicle-treated cells). Stable overexpression of graded amounts of β-ARR1 (7- to 22-fold over basal) caused a dose-related rescue (83.3 ± 3.73% of maximal in cells expressing 156 ± 26 × 10−3 fmol β-ARR1/cell, ≈22-fold over basal, Fig. 5C). These results suggest that NK1R agonists inhibit NK3R endocytosis by sequestering β-ARRs in endosomes and thereby depleting cytoplasmic pools.
Sequestration of β-ARRs with the NK1R Inhibits Homologous Desensitization of the NK3R.
We determined whether sequestration of β-ARRs with the NK1R leads to diminished desensitization of the NK3R. To examine desensitization, KNRK-NK1R/NK3R cells were preincubated with vehicle or 10 nM SM-SP or MP-NKB for 10 min at 37°C, washed, and incubated in peptide-free solution for 10 min. Cells were challenged with 10 nM of agonist (different from the preincubation) for 2 min, washed, and rechallenged with 10 nM of the same agonist 5 min after the first challenge (Fig. 6A). Homologous desensitization was assessed by comparing the magnitude of responses to the first and second challenges with the same agonists. Heterologous desensitization was assessed by comparing responses to the first agonist in cells preincubated with vehicle or a different agonist. In vehicle-treated cells, 10 nM MP-NKB caused a transient increase in [Ca2+]i that desensitized to repeated challenge (64 ± 4% desensitization) (Fig. 6B). In cells pretreated with 1, 3, or 10 nM SM-SP for 10 min, when β-ARRs were depleted from the cytosol (not shown), homologous desensitization was reduced (1 nM, 46 ± 4%; 3 nM, 42 ± 1%; 10 nM, 0 ± 6% desensitization). Inhibition by 10 nM SM-SP was abolished by overexpression of β-ARR1 at >7-fold basal, suggesting that the effect is mediated by NK1R-induced sequestration of β-ARRs. Pretreatment with 1 or 3 nM SM-SP did not affect the first responses to 10 nM MP-NKB, whereas 10 nM SM-SP caused marked heterologous desensitization (78.1 ± 4.3% desensitization) (Fig. 6C). Overexpression of β-ARR1 markedly diminished this effect. Thus, prior activation of the NK1R markedly reduces homologous desensitization of the NK3R, even at concentrations that do not cause heterologous desensitization. This diminished homologous desensitization likely is mediated by SM-SP-induced depletion of the cytosol of β-ARRs.
In contrast, activation of the NK3R did not affect homologous desensitization of the NK1R (85 ± 1% desensitization in vehicle-treated cells, 85 ± 4% desensitization in cells treated with 10 nM MP-NKB) (Fig. 6B). Preincubation with 10 nM-MP-NKB did not cause heterologous desensitization of the NK1R (Fig. 6C). Thus, activation of the NK3R does not impair the capacity of the NK1R to signal or desensitize.
Discussion
Activation of the NK1R caused sequestration of β-ARRs into endosomes and depletion of cytosolic β-ARRs, which impeded endocytosis and homologous desensitization of the NK3R. In view of the requirement of β-ARRs for desensitization, endocytosis, and signaling of many GPCRs, the sequestration of β-ARRs is likely to have marked effects on signaling.
β-ARR-Dependent Interactions Between Neurokinin Receptors.
Several observations suggest that sequestration of β-ARRs with the NK1R mediates inhibition of NK3R trafficking and desensitization. First, dominant negative β-ARRs caused surface retention of the NK3R, indicating a requirement of β-ARRs in endocytosis. β-ARRs are adapters for clathrin-dependent endocytosis of many GPCRs, including the NK1R, which supports our results (4–7). Although not examined, β-ARRs and G protein receptor kinases probably mediate homologous desensitization of the NK3R, as they do for the NK1R (6, 24). Second, NK1R agonists caused sequestration of β-ARR1 into endosomes and depletion of cytosolic β-ARR1. This effect was observed in cells expressing NK1R, NK3R, and β-ARR at similar levels (7 × 10−3 fmol NK1R/cell; 1.5 × 10−3 fmol NK3R/cell; 7.7 × 10−3 fmol β-ARR1/cell). Indeed, β-ARR1 remains with the NK1R in endosomes for several hours after SP-treatment (6). SP also causes rapid membrane translocation of β-ARR2, followed by prolonged association with the receptor in endosomes (25, 26). Thus, the NK1R belongs to “class B” GPCRs, including angiotensin II 1A, neurotensin 1, vasopressin V2, and thyroid releasing hormone receptors, that interact with β-ARR1/2 with high affinity and that internalize with β-ARRs in endosomes (26). Third, depletion of cytosolic β-ARRs by prior activation of the NK1R coincided with the inhibition of agonist-induced endocytosis and homologous desensitization of the NK3R. The inhibition of homologous desensitization occurred at concentrations of SM-SP (<3 nM) that failed to cause heterologous desensitization of the NK3R and, thus, cannot be attributed to diminished signaling of the NK3R. Finally, graded overexpression of β-ARR1 (7- to 22-fold over basal) rescued the NK1R-dependent inhibition of endocytosis of the NK3R.
Prior activation of the NK3R did not affect endocytosis or homologous desensitization of the NK1R. Although an NK3R agonist caused membrane translocation of β-ARR1, β-ARR1 rapidly returned to the cytosol and did not prominently sequester in endosomes, presumably because of the low-affinity interaction with the NK3R. Cytosolic β-ARRs then would be available to interact with the NK1R. Thus, the NK3R belongs to the “class A” GPCRs, including β2- and α1b-adrenergic, μ-opioid, endothelin A, and dopamine D1A receptors, that form low-affinity, unstable interactions with β-ARRs, dissociate from receptors near the plasma membrane, and largely are excluded from endosomes (26). The diminished sequestration of β-ARRs with NK3R compared with NK1R was observed in cell lines expressing comparable receptor levels and treated with the same agonist concentrations and, thus, is unlikely to be attributable to differences in expression or extent of activation of receptors.
The apparent difference in the affinity of the NK1R and NK3R for β-ARRs also may account for differences in trafficking and resensitization of these receptors. The internalized NK1R slowly (hours) recycles to the plasma membrane, and acidotropic agents and phosphatase inhibitors block recycling and resensitization (19–22). Recycling and resensitization entail ligand dissociation in acidified endosomes and receptor dephosphorylation and dissociation of β-ARRs. In contrast, internalized NK3R rapidly (30 min) recycles in KNRK cells (unpublished observations), possibly because of more rapid dissociation from β-ARRs, although there is no evidence for recycling in neurons (27). Interaction with β-ARRs also affects trafficking of other GPCRs. The β2-adrenergic receptor, which has a low-affinity interaction with β-ARRs, recycles and resensitizes rapidly, whereas the vasopressin V2 receptor, which interacts with β-ARRs with high affinity, slowly recycles and resensitizes (28).
Activation of the NK1R caused heterologous desensitization of the NK3R but not vice versa. A similar pattern of heterologous desensitization occurs in enteric neurons (29). An unexpected finding was that overexpression of β-ARR1 diminished heterologous desensitization of the NK3R. Prior activation of the NK1R caused redistribution of NK3R into patches at the plasma membrane (e.g., Fig. 5A, 30 min), which could affect NK3R coupling and, thus, induce heterologous desensitization. By restoring the normal distribution of the NK3R, β-ARRs thus could diminish heterologous desensitization.
Physiological Implications.
NK1R and NK3R are coexpressed by enteric neurons (15, 16) and endothelial cells (17). SP and NKA, the principal tachykinins in peripheral tissues, can interact with both NK1R and NK3R (14). Activation of the NK1R would sequester β-ARRs in endosomes and thereby cause retention of the NK3R at the cell surface, where it would be resistant to desensitization and internalization. Such regulation could permit cells to respond to tachykinins at a time when the NK1R was desensitized and internalized. This state may persist until β-ARRs return to the cytosol, when the NK3R could interact with β-ARRs, uncouple, and internalize and the NK1R would have recycled.
The sequestration of β-ARRs in endosomes with the NK1R similarly could impede β-ARR-dependent regulation of signaling by many GPCRs. Agonists of other “class B” GPCRs, which interact with high affinity with β-ARRs, also would affect β-ARR-dependent signaling of “class A,” low-affinity GPCRs. In view of the roles of β-ARRs in uncoupling, endocytosis, and mitogenic signaling, regulation of the interactions between GPCRs and β-ARR would be expected to have far-reaching, functional implications.
During review, a report was published indicating that activation of the vasopressin V2 receptor inhibits endocytosis of the β2AR by a mechanism similar to the one reported herein (30).
Acknowledgments
We thank R. J. Lefkowitz for β-ARR antibodies, constructs, and protein. This work was supported by National Institutes of Health Grants DK39957, DK43207, and DK52388 and Institut National de la Santé et de la Recherche Médicale.
Abbreviations
- GPCR
G protein-coupled receptor
- NKR
neurokinin receptor
- SP
substance P
- NKA and NKB
neurokinins A and B
- SM-SP
[Sar9MetO211]SP
- MP-NKB
[MePhe7]NKB
- β-ARR
β-arrestin
- KNRK
Kirsten sarcoma virus-transformed rat kidney epithelial cells
- KNRK-NK1R/NK3R
KNRK cells coexpressing NK1R and NK3R
- GFP
green fluorescent protein
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Böhm S, Grady E F, Bunnett N W. Biochem J. 1997;322:1–18. doi: 10.1042/bj3220001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lohse M J, Benovic J L, Codina J, Caron M G, Lefkowitz R J. Science. 1990;248:1547–1550. doi: 10.1126/science.2163110. [DOI] [PubMed] [Google Scholar]
- 3.Attramadal H, Arriza J L, Aoki C, Dawson T M, Codina J, Kwatra M M, Snyder S H, Caron M G, Lefkowitz R J. J Biol Chem. 1992;267:17882–17890. [PubMed] [Google Scholar]
- 4.Goodman O B, Jr, Krupnick J G, Santini F, Gurevich V V, Penn R B, Gagnon A W, Keen J H, Benovic J L. Nature (London) 1996;383:447–450. doi: 10.1038/383447a0. [DOI] [PubMed] [Google Scholar]
- 5.Ferguson S S, Downey W E, Colapietro A M, Barak L S, Menard L, Caron M G. Science. 1996;271:363–366. doi: 10.1126/science.271.5247.363. [DOI] [PubMed] [Google Scholar]
- 6.McConalogue K, Dery O, Lovett M, Wong H, Walsh J H, Grady E F, Bunnett N W. J Biol Chem. 1999;274:16257–16268. doi: 10.1074/jbc.274.23.16257. [DOI] [PubMed] [Google Scholar]
- 7.Dery O, Thoma M S, Wong H, Grady E F, Bunnett N W. J Biol Chem. 1999;274:18524–18535. doi: 10.1074/jbc.274.26.18524. [DOI] [PubMed] [Google Scholar]
- 8.Li J G, Benovic J L, Liu-Chen L Y. Mol Pharmacol. 2000;58:795–801. [PubMed] [Google Scholar]
- 9.Zhang J, Barak L S, Winkler K E, Caron M G, Ferguson S S. J Biol Chem. 1997;272:27005–27014. doi: 10.1074/jbc.272.43.27005. [DOI] [PubMed] [Google Scholar]
- 10.Luttrell L M, Ferguson S S, Daaka Y, Miller W E, Maudsley S, Della Rocca G J, Lin F, Kawakatsu H, Owada K, Luttrell D K, et al. Science. 1999;283:655–661. doi: 10.1126/science.283.5402.655. [DOI] [PubMed] [Google Scholar]
- 11.DeFea K A, Zalevsky J, Thoma M S, Dery O, Mullins R D, Bunnett N W. J Cell Biol. 2000;148:1267–1281. doi: 10.1083/jcb.148.6.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeFea K A, Vaughn Z D, O'Bryan E M, Nishijima D, Dery O, Bunnett N W. Proc Natl Acad Sci USA. 2000;97:11086–11091. doi: 10.1073/pnas.190276697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luttrell L M, Roudabush F L, Choy E W, Miller W E, Field M E, Pierce K L, Lefkowitz R J. Proc Natl Acad Sci USA. 2001;98:2449–2454. doi: 10.1073/pnas.041604898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Otsuka M, Yoshioka K. Physiol Rev. 1993;73:229–308. doi: 10.1152/physrev.1993.73.2.229. [DOI] [PubMed] [Google Scholar]
- 15.Grady E F, Baluk P, Böhm S, Gamp P, Wong H, Payan D G, Ansel J, Portbury A L, Furness J B, McDonald D M, et al. J Neurosci. 1996;16:6975–6986. doi: 10.1523/JNEUROSCI.16-21-06975.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mann P T, Southwell B R, Ding Y Q, Shigemoto R, Mizuno N, Furness J B. Cell Tissue Res. 1997;289:1–9. doi: 10.1007/s004410050846. [DOI] [PubMed] [Google Scholar]
- 17.Quinlan K L, Song I S, Bunnett N W, Letran E, Steinhoff M, Harten B, Olerud J E, Armstrong C A, Caughman S W, Ansel J C. Am J Physiol. 1998;275:C1580–C1590. doi: 10.1152/ajpcell.1998.275.6.C1580. [DOI] [PubMed] [Google Scholar]
- 18.Bunnett N W, Dazin P F, Payan D G, Grady E F. Peptides. 1995;16:733–740. doi: 10.1016/0196-9781(95)00042-i. [DOI] [PubMed] [Google Scholar]
- 19.Garland A M, Grady E F, Payan D G, Vigna S R, Bunnett N W. Biochem J. 1994;303:177–186. doi: 10.1042/bj3030177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grady E F, Garland A G, Gamp P D, Lovett M, Payan D G, Bunnett N W. Mol Biol Cell. 1995;6:509–524. doi: 10.1091/mbc.6.5.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garland A M, Grady E F, Lovett M, Vigna S R, Frucht M M, Krause J E, Bunnett N W. Mol Pharm. 1996;49:438–446. [PubMed] [Google Scholar]
- 22.Grady E F, Gamp P D, Baluk P, McDonald D M, Payan D G, Bunnett N W. Neuroscience. 1996;16:1239–1254. doi: 10.1016/0306-4522(96)00357-0. [DOI] [PubMed] [Google Scholar]
- 23.McConalogue K, Corvera C U, Gamp P D, Grady E F, Bunnett N W. Mol Biol Cell. 1998;9:2305–2324. doi: 10.1091/mbc.9.8.2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kwatra M M, Schwinn D A, Schreurs J, Blank J L, Kim C M, Benovic J L, Krause J E, Caron M G, Lefkowitz R J. J Biol Chem. 1993;268:9161–9164. [PubMed] [Google Scholar]
- 25.Barak L S, Warabi K, Feng X, Caron M G, Kwatra M M. J Biol Chem. 1999;274:7565–7569. doi: 10.1074/jbc.274.11.7565. [DOI] [PubMed] [Google Scholar]
- 26.Oakley R H, Laporte S A, Holt J A, Caron M G, Barak L S. J Biol Chem. 2000;275:17201–17210. doi: 10.1074/jbc.M910348199. [DOI] [PubMed] [Google Scholar]
- 27.Jenkinson K M, Mann P T, Southwell B R, Furness J B. Neuroscience. 2000;100:191–199. doi: 10.1016/s0306-4522(00)00259-1. [DOI] [PubMed] [Google Scholar]
- 28.Oakley R H, Laporte S A, Holt J A, Barak L S, Caron M G. J Biol Chem. 1999;274:32248–32257. doi: 10.1074/jbc.274.45.32248. [DOI] [PubMed] [Google Scholar]
- 29.Frieling T, Dobreva G, Weber E, Becker K, Rupprecht C, Neunlist M, Schemann M. Naunyn-Schmiedeberg's Arch Pharmacol. 1999;359:71–79. doi: 10.1007/pl00005327. [DOI] [PubMed] [Google Scholar]
- 30.Klein U, Muller C, Chu P, Birnbaumer M, von Zastrow M. J Biol Chem. 2001;276:17442–17447. doi: 10.1074/jbc.M009214200. [DOI] [PubMed] [Google Scholar]