Abstract
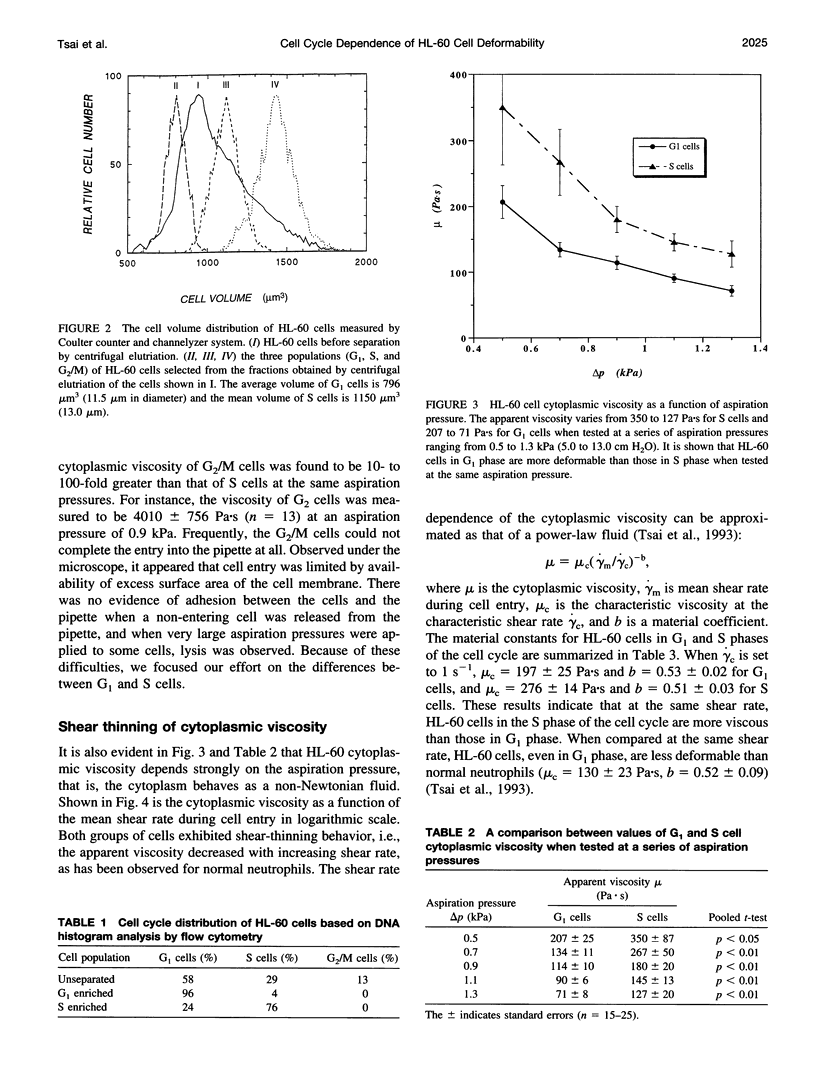
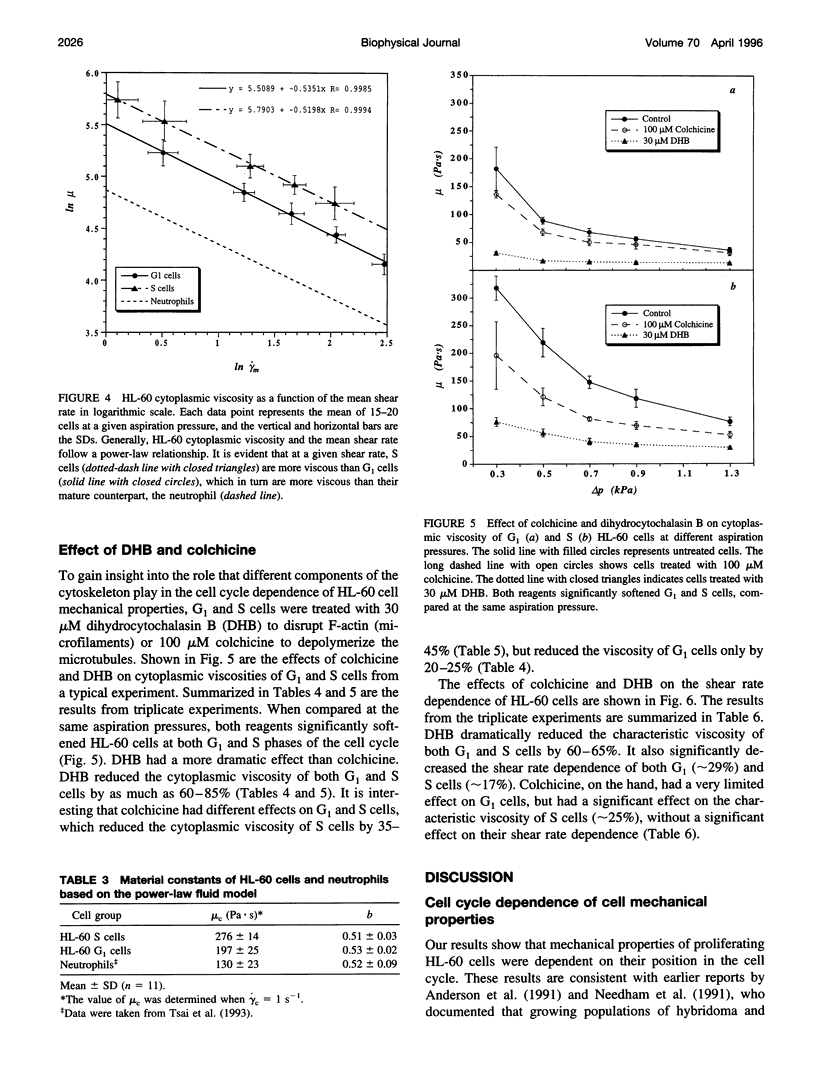
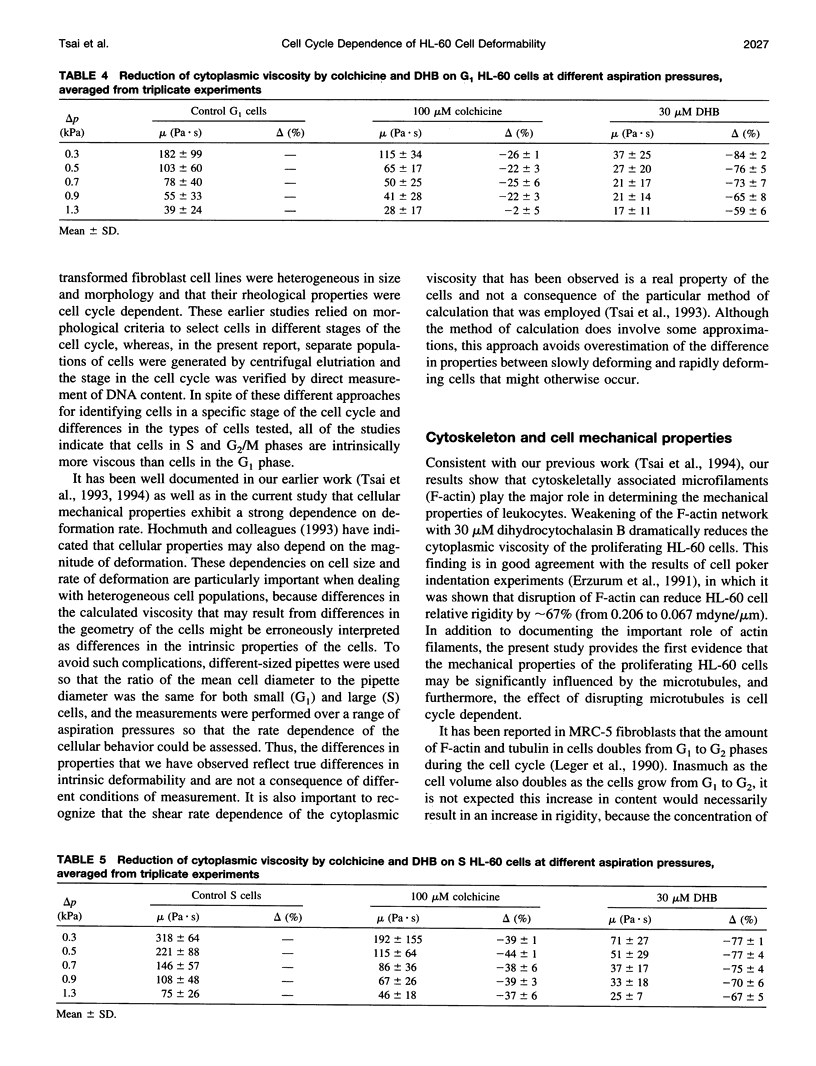
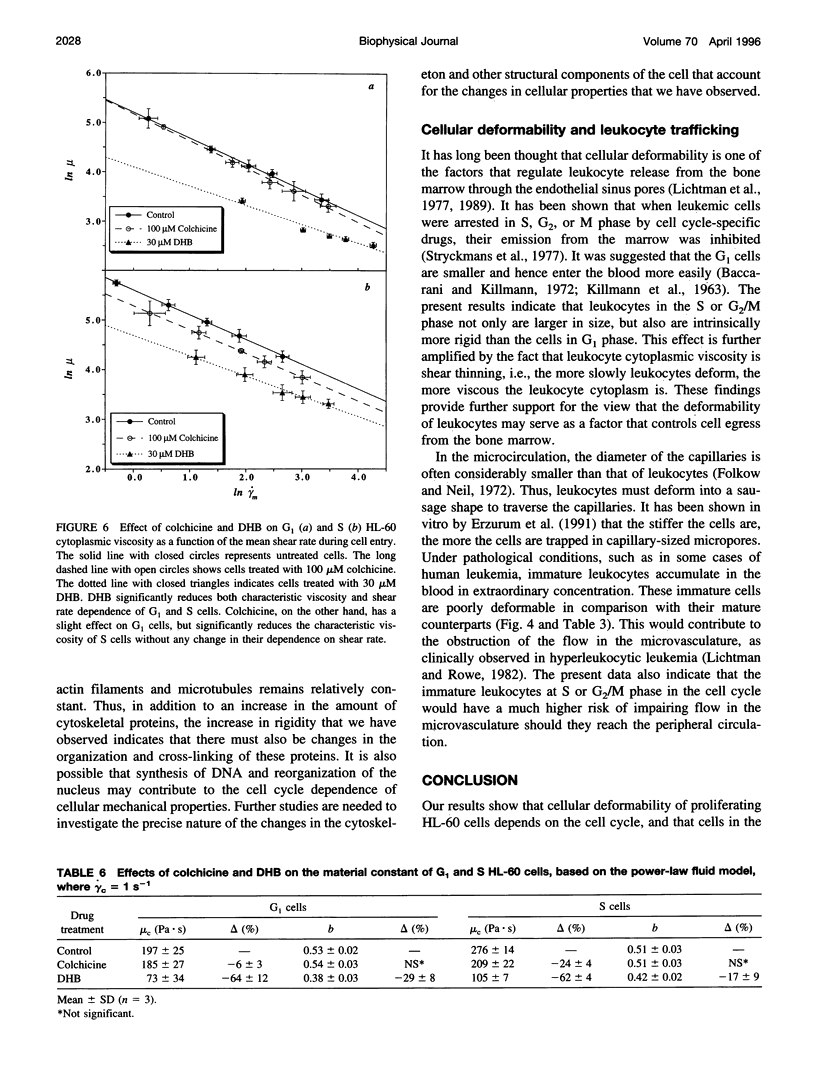
In this study, the role of cytoskeleton in HL-60 deformability during the cell cycle was investigated. G1, S, and G2/M cell fractions were separated by centrifugal elutriation. Cell deformability was evaluated by pipette aspiration. Tested at the same aspiration pressures, S cells were found to be less deformable than G1 cells. Moreover, HL-60 cells exhibited power-law fluid behavior: mu = mu c(gamma m/ gamma c)-b, where mu is cytoplasmic viscosity, gamma m is mean shear rate, mu c is the characteristic viscosity at the characteristic shear rate gamma c, and b is a material constant. At a given shear rate, S cells (mu c = 276 +/- 14 Pa.s, b = 0.51 +/- 0.03) were more viscous than G1 cells (mu c = 197 +/- 25, b = 0.53 +/- 0.02). To evaluate the relative importance of different cytoskeletal components in these cell cycle-dependent properties, HL-60 cells were treated with 30 microM dihydrocytochalasin B (DHB) to disrupt F-actin or 100 microM colchicine to collapse microtubules. DHB dramatically softened both G1 and S cells, which reduced the material constants mu c by approximately 65% and b by 20-30%. Colchicine had a limited effect on G1 cells but significantly reduced mu c of S cells (approximately 25%). Thus, F-actin plays the predominate role in determining cell mechanical properties, but disruption of microtubules may also influence the behavior of proliferating cells in a cell cycle-dependent fashion.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson K. W., Li W. I., Cezeaux J., Zimmer S. In vitro studies of deformation and adhesion properties of transformed cells. Cell Biophys. 1991 Apr;18(2):81–97. doi: 10.1007/BF02989808. [DOI] [PubMed] [Google Scholar]
- Baccarani M., Killmann S. A. Cytokinetic studies in chronic myeloid leukaemia: evidence for early presence of abnormal myeloblasts. Scand J Haematol. 1972;9(3):283–292. doi: 10.1111/j.1600-0609.1972.tb00941.x. [DOI] [PubMed] [Google Scholar]
- Brosing J. W., Keng P. C., Mulcahy R. T. Survival characteristics of normal differentiated rat thyroid cells maintained in vitro. Radiat Res. 1986 Feb;105(2):138–146. [PubMed] [Google Scholar]
- Collins S. J., Gallo R. C., Gallagher R. E. Continuous growth and differentiation of human myeloid leukaemic cells in suspension culture. Nature. 1977 Nov 24;270(5635):347–349. doi: 10.1038/270347a0. [DOI] [PubMed] [Google Scholar]
- Collins S. J. The HL-60 promyelocytic leukemia cell line: proliferation, differentiation, and cellular oncogene expression. Blood. 1987 Nov;70(5):1233–1244. [PubMed] [Google Scholar]
- Erzurum S. C., Downey G. P., Doherty D. E., Schwab B., 3rd, Elson E. L., Worthen G. S. Mechanisms of lipopolysaccharide-induced neutrophil retention. Relative contributions of adhesive and cellular mechanical properties. J Immunol. 1992 Jul 1;149(1):154–162. [PubMed] [Google Scholar]
- Erzurum S. C., Kus M. L., Bohse C., Elson E. L., Worthen G. S. Mechanical properties of HL60 cells: role of stimulation and differentiation in retention in capillary-sized pores. Am J Respir Cell Mol Biol. 1991 Sep;5(3):230–241. doi: 10.1165/ajrcmb/5.3.230. [DOI] [PubMed] [Google Scholar]
- Fried J., Perez A. G., Clarkson B. D. Flow cytofluorometric analysis of cell cycle distributions using propidium iodide. Properties of the method and mathematical analysis of the data. J Cell Biol. 1976 Oct;71(1):172–181. doi: 10.1083/jcb.71.1.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galbraith P. R., Abu-Zahra H. T. Granulopoiesis in chronic granulocytic leukaemia. Br J Haematol. 1972 Feb;22(2):135–143. doi: 10.1111/j.1365-2141.1972.tb08795.x. [DOI] [PubMed] [Google Scholar]
- Harris A. G., Skalak T. C. Leukocyte cytoskeletal structure determines capillary plugging and network resistance. Am J Physiol. 1993 Nov;265(5 Pt 2):H1670–H1675. doi: 10.1152/ajpheart.1993.265.5.H1670. [DOI] [PubMed] [Google Scholar]
- Hochmuth R. M., Ting-Beall H. P., Beaty B. B., Needham D., Tran-Son-Tay R. Viscosity of passive human neutrophils undergoing small deformations. Biophys J. 1993 May;64(5):1596–1601. doi: 10.1016/S0006-3495(93)81530-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KILLMANN S. A., CRONKITE E. P., ROBERTSON J. S., FLIEDNER T. M., BOND V. P. Estimation of phases of the life cycle of leukemic cells from labeling in human beings in vivo with tritiated thymidine. Lab Invest. 1963 Jul;12:671–684. [PubMed] [Google Scholar]
- Keng P. C., Li C. K., Wheeler K. T. Synchronization of 9L rat brain tumor cells by centrifugal elutriation. Cell Biophys. 1980 Sep;2(3):191–206. doi: 10.1007/BF02790449. [DOI] [PubMed] [Google Scholar]
- Lichtman M. A., Rowe J. M. Hyperleukocytic leukemias: rheological, clinical, and therapeutic considerations. Blood. 1982 Aug;60(2):279–283. [PubMed] [Google Scholar]
- Lichtman M. A. The relationship of excessive white cell accumulation to vascular insufficiency in patients with leukemia. Kroc Found Ser. 1984;16:295–306. [PubMed] [Google Scholar]
- Léger I., Giroud F., Brugal G. Quantitative analysis of cytoskeletal proteins throughout the cell cycle of the MRC-5 fibroblastic cell line. Anal Quant Cytol Histol. 1990 Oct;12(5):321–326. [PubMed] [Google Scholar]
- MAUER A. M., JARROLD T. GRANULOCYTE KINETIC STUDIES IN PATIENTS WITH PROLIFERATIVE DISORDERS OF THE BONE MARROW. Blood. 1963 Aug;22:125–138. [PubMed] [Google Scholar]
- Palis J., King B., Keng P. Separation of spontaneously differentiating and cell cycle-specific populations of HL-60 cells. Leuk Res. 1988;12(4):339–344. doi: 10.1016/0145-2126(88)90049-5. [DOI] [PubMed] [Google Scholar]
- Tsai M. A., Frank R. S., Waugh R. E. Passive mechanical behavior of human neutrophils: effect of cytochalasin B. Biophys J. 1994 Jun;66(6):2166–2172. doi: 10.1016/S0006-3495(94)81012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai M. A., Frank R. S., Waugh R. E. Passive mechanical behavior of human neutrophils: power-law fluid. Biophys J. 1993 Nov;65(5):2078–2088. doi: 10.1016/S0006-3495(93)81238-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worthen G. S., Schwab B., 3rd, Elson E. L., Downey G. P. Mechanics of stimulated neutrophils: cell stiffening induces retention in capillaries. Science. 1989 Jul 14;245(4914):183–186. doi: 10.1126/science.2749255. [DOI] [PubMed] [Google Scholar]