Abstract
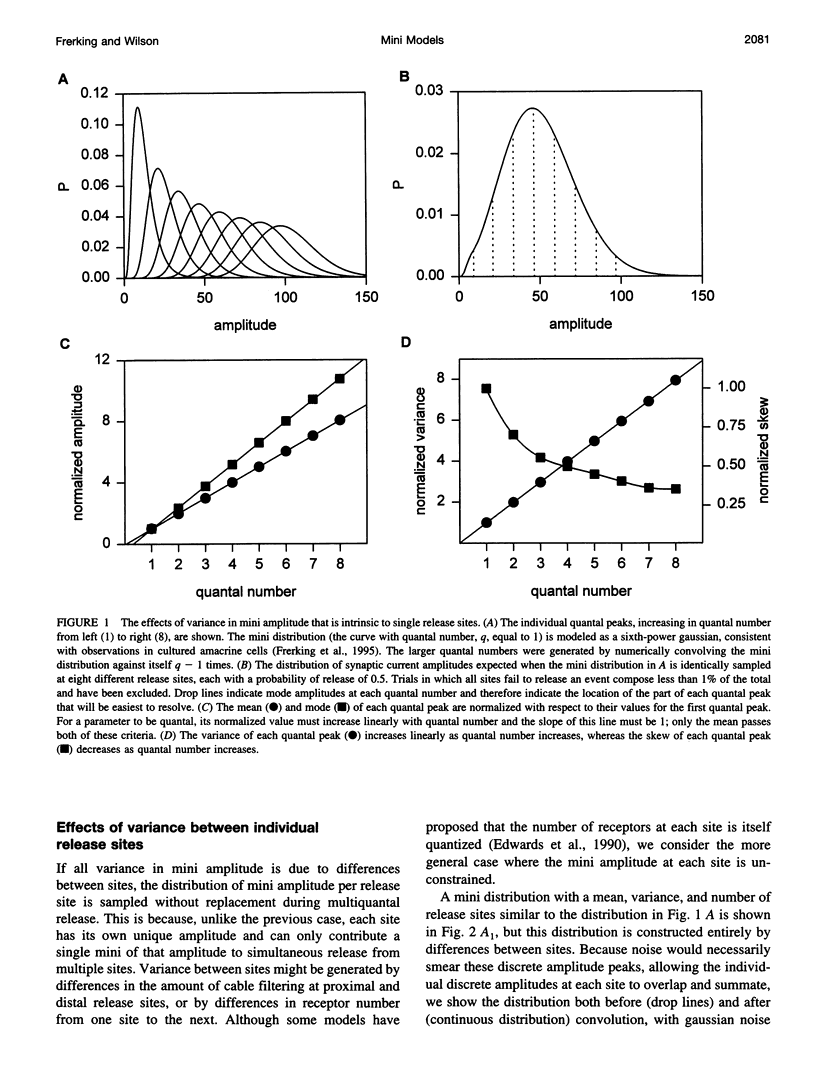
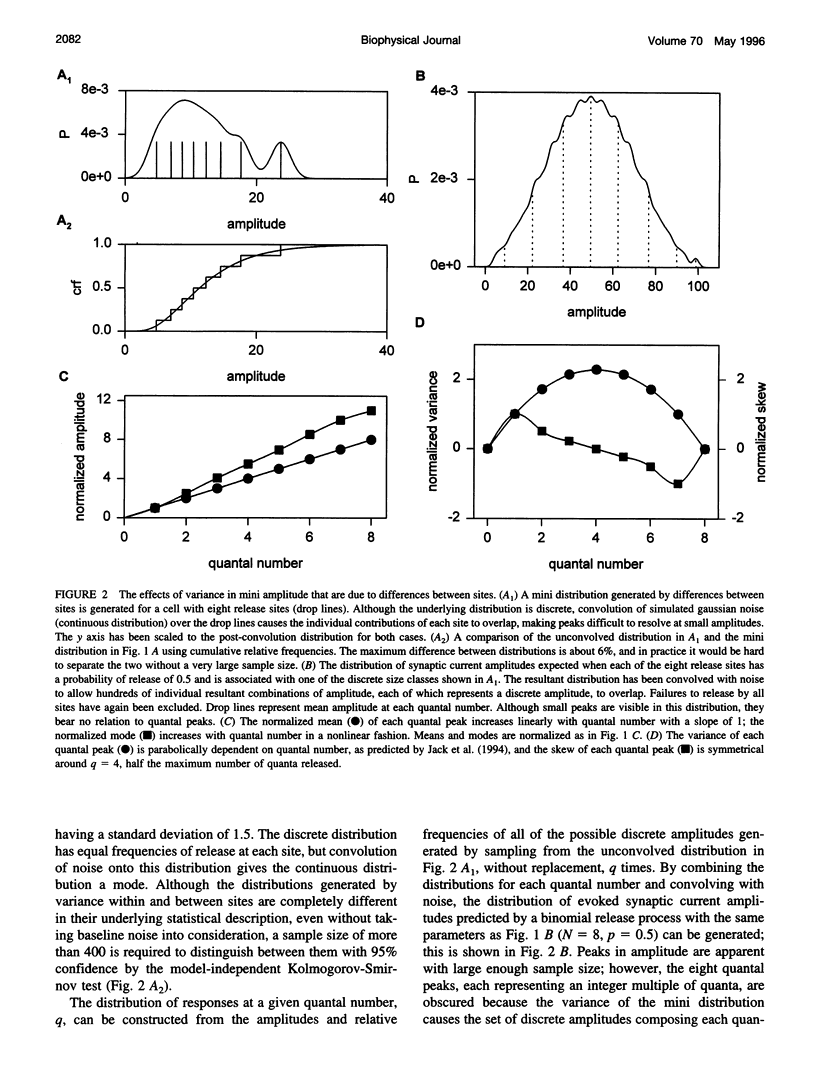
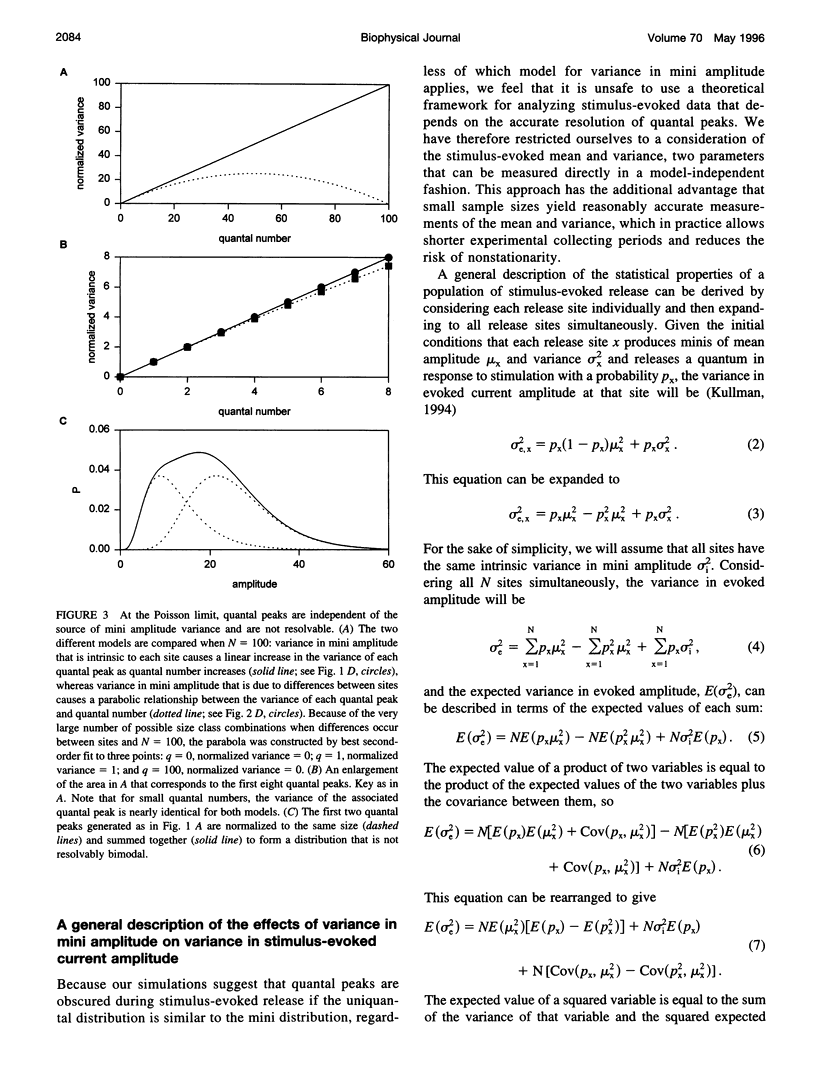
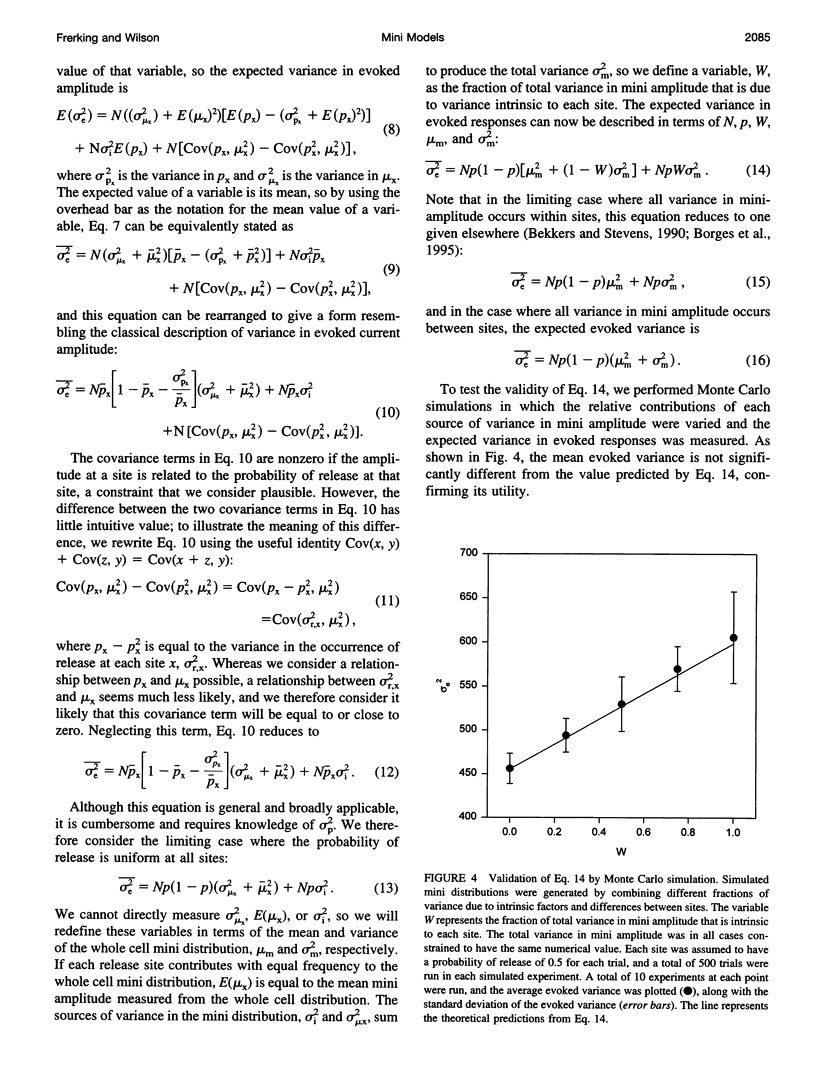
The strength of synaptic connections between two neurons is characterized by the number of release sites (N) on the presynaptic cell, the probability (p) of transmitter release at those sites in response to a stimulus, and the average size (A) of the postsynaptic response from each site. Quantal analysis can determine N, p, and A, but the large variance in the amplitudes of minis at central synapses is predicted to obscure quantal peaks and render quantal analysis unusable. Recently it has been suggested that the variance in mini amplitude is generated by differences between release sites, rather than by quantum-to-quantum fluctuations at identical sites, and that this form of variance in mini amplitude reduces the amount of variance expected in quantal peaks. Using simulations, we examine the possibility of resolving quantal peaks assuming either form of variance in mini amplitude. We find that individual quantal peaks are resolvable in neither case, provided that the uniquantal distribution is similar to the mini distribution. Because this lack of resolution compromises the utility of quantal analysis, we develop a general description that can solve N and p, given the statistical parameters of the mini distribution and the evoked distribution. We find that this description is relatively insensitive to the source of variance in mini amplitude.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOYD I. A., MARTIN A. R. Spontaneous subthreshold activity at mammalian neural muscular junctions. J Physiol. 1956 Apr 27;132(1):61–73. doi: 10.1113/jphysiol.1956.sp005502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOYD I. A., MARTIN A. R. The end-plate potential in mammalian muscle. J Physiol. 1956 Apr 27;132(1):74–91. doi: 10.1113/jphysiol.1956.sp005503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekkers J. M., Richerson G. B., Stevens C. F. Origin of variability in quantal size in cultured hippocampal neurons and hippocampal slices. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5359–5362. doi: 10.1073/pnas.87.14.5359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekkers J. M., Stevens C. F. Excitatory and inhibitory autaptic currents in isolated hippocampal neurons maintained in cell culture. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7834–7838. doi: 10.1073/pnas.88.17.7834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekkers J. M., Stevens C. F. NMDA and non-NMDA receptors are co-localized at individual excitatory synapses in cultured rat hippocampus. Nature. 1989 Sep 21;341(6239):230–233. doi: 10.1038/341230a0. [DOI] [PubMed] [Google Scholar]
- Bekkers J. M., Stevens C. F. Presynaptic mechanism for long-term potentiation in the hippocampus. Nature. 1990 Aug 23;346(6286):724–729. doi: 10.1038/346724a0. [DOI] [PubMed] [Google Scholar]
- Bekkers J. M., Stevens C. F. Quantal analysis of EPSCs recorded from small numbers of synapses in hippocampal cultures. J Neurophysiol. 1995 Mar;73(3):1145–1156. doi: 10.1152/jn.1995.73.3.1145. [DOI] [PubMed] [Google Scholar]
- Bekkers J. M., Stevens C. F. The nature of quantal transmission at central excitatory synapses. Adv Second Messenger Phosphoprotein Res. 1994;29:261–273. doi: 10.1016/s1040-7952(06)80020-0. [DOI] [PubMed] [Google Scholar]
- Borges S., Gleason E., Turelli M., Wilson M. The kinetics of quantal transmitter release from retinal amacrine cells. Proc Natl Acad Sci U S A. 1995 Jul 18;92(15):6896–6900. doi: 10.1073/pnas.92.15.6896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Quantal components of the end-plate potential. J Physiol. 1954 Jun 28;124(3):560–573. doi: 10.1113/jphysiol.1954.sp005129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Koninck Y., Mody I. Noise analysis of miniature IPSCs in adult rat brain slices: properties and modulation of synaptic GABAA receptor channels. J Neurophysiol. 1994 Apr;71(4):1318–1335. doi: 10.1152/jn.1994.71.4.1318. [DOI] [PubMed] [Google Scholar]
- Edwards F. A., Konnerth A., Sakmann B. Quantal analysis of inhibitory synaptic transmission in the dentate gyrus of rat hippocampal slices: a patch-clamp study. J Physiol. 1990 Nov;430:213–249. doi: 10.1113/jphysiol.1990.sp018289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards F. A. LTP--a structural model to explain the inconsistencies. Trends Neurosci. 1995 Jun;18(6):250–255. doi: 10.1016/0166-2236(95)80003-k. [DOI] [PubMed] [Google Scholar]
- FATT P., KATZ B. Spontaneous subthreshold activity at motor nerve endings. J Physiol. 1952 May;117(1):109–128. [PMC free article] [PubMed] [Google Scholar]
- Faber D. S., Korn H. Applicability of the coefficient of variation method for analyzing synaptic plasticity. Biophys J. 1991 Nov;60(5):1288–1294. doi: 10.1016/S0006-3495(91)82162-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber D. S., Young W. S., Legendre P., Korn H. Intrinsic quantal variability due to stochastic properties of receptor-transmitter interactions. Science. 1992 Nov 27;258(5087):1494–1498. doi: 10.1126/science.1279813. [DOI] [PubMed] [Google Scholar]
- Frerking M., Borges S., Wilson M. Variation in GABA mini amplitude is the consequence of variation in transmitter concentration. Neuron. 1995 Oct;15(4):885–895. doi: 10.1016/0896-6273(95)90179-5. [DOI] [PubMed] [Google Scholar]
- Hessler N. A., Shirke A. M., Malinow R. The probability of transmitter release at a mammalian central synapse. Nature. 1993 Dec 9;366(6455):569–572. doi: 10.1038/366569a0. [DOI] [PubMed] [Google Scholar]
- Isaacson J. S., Walmsley B. Counting quanta: direct measurements of transmitter release at a central synapse. Neuron. 1995 Oct;15(4):875–884. doi: 10.1016/0896-6273(95)90178-7. [DOI] [PubMed] [Google Scholar]
- Jack J. J., Redman S. J., Wong K. The components of synaptic potentials evoked in cat spinal motoneurones by impulses in single group Ia afferents. J Physiol. 1981 Dec;321:65–96. doi: 10.1113/jphysiol.1981.sp013972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas P., Major G., Sakmann B. Quantal components of unitary EPSCs at the mossy fibre synapse on CA3 pyramidal cells of rat hippocampus. J Physiol. 1993 Dec;472:615–663. doi: 10.1113/jphysiol.1993.sp019965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn H., Bausela F., Charpier S., Faber D. S. Synaptic noise and multiquantal release at dendritic synapses. J Neurophysiol. 1993 Sep;70(3):1249–1254. doi: 10.1152/jn.1993.70.3.1249. [DOI] [PubMed] [Google Scholar]
- Korn H., Faber D. S. Quantal analysis and synaptic efficacy in the CNS. Trends Neurosci. 1991 Oct;14(10):439–445. doi: 10.1016/0166-2236(91)90042-s. [DOI] [PubMed] [Google Scholar]
- Korn H., Mallet A., Triller A., Faber D. S. Transmission at a central inhibitory synapse. II. Quantal description of release, with a physical correlate for binomial n. J Neurophysiol. 1982 Sep;48(3):679–707. doi: 10.1152/jn.1982.48.3.679. [DOI] [PubMed] [Google Scholar]
- Korn H., Triller A., Mallet A., Faber D. S. Fluctuating responses at a central synapse: n of binomial fit predicts number of stained presynaptic boutons. Science. 1981 Aug 21;213(4510):898–901. doi: 10.1126/science.6266015. [DOI] [PubMed] [Google Scholar]
- Kullmann D. M. Amplitude fluctuations of dual-component EPSCs in hippocampal pyramidal cells: implications for long-term potentiation. Neuron. 1994 May;12(5):1111–1120. doi: 10.1016/0896-6273(94)90318-2. [DOI] [PubMed] [Google Scholar]
- Kullmann D. M., Nicoll R. A. Long-term potentiation is associated with increases in quantal content and quantal amplitude. Nature. 1992 May 21;357(6375):240–244. doi: 10.1038/357240a0. [DOI] [PubMed] [Google Scholar]
- Kullmann D. M. Quantal analysis using maximum entropy noise deconvolution. J Neurosci Methods. 1992 Aug;44(1):47–57. doi: 10.1016/0165-0270(92)90113-r. [DOI] [PubMed] [Google Scholar]
- Kullmann D. M. Quantal variability of excitatory transmission in the hippocampus: implications for the opening probability of fast glutamate-gated channels. Proc Biol Sci. 1993 Jul 22;253(1336):107–116. doi: 10.1098/rspb.1993.0088. [DOI] [PubMed] [Google Scholar]
- LILEY A. W. The quantal components of the mammalian end-plate potential. J Physiol. 1956 Sep 27;133(3):571–587. doi: 10.1113/jphysiol.1956.sp005610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkman A., Hannay T., Stratford K., Jack J. Presynaptic release probability influences the locus of long-term potentiation. Nature. 1992 Nov 5;360(6399):70–73. doi: 10.1038/360070a0. [DOI] [PubMed] [Google Scholar]
- Larkman A., Stratford K., Jack J. Quantal analysis of excitatory synaptic action and depression in hippocampal slices. Nature. 1991 Mar 28;350(6316):344–347. doi: 10.1038/350344a0. [DOI] [PubMed] [Google Scholar]
- Liu G., Tsien R. W. Properties of synaptic transmission at single hippocampal synaptic boutons. Nature. 1995 Jun 1;375(6530):404–408. doi: 10.1038/375404a0. [DOI] [PubMed] [Google Scholar]
- Malgaroli A., Tsien R. W. Glutamate-induced long-term potentiation of the frequency of miniature synaptic currents in cultured hippocampal neurons. Nature. 1992 May 14;357(6374):134–139. doi: 10.1038/357134a0. [DOI] [PubMed] [Google Scholar]
- Malinow R., Tsien R. W. Presynaptic enhancement shown by whole-cell recordings of long-term potentiation in hippocampal slices. Nature. 1990 Jul 12;346(6280):177–180. doi: 10.1038/346177a0. [DOI] [PubMed] [Google Scholar]
- Manabe T., Nicoll R. A. Long-term potentiation: evidence against an increase in transmitter release probability in the CA1 region of the hippocampus. Science. 1994 Sep 23;265(5180):1888–1892. doi: 10.1126/science.7916483. [DOI] [PubMed] [Google Scholar]
- Manabe T., Renner P., Nicoll R. A. Postsynaptic contribution to long-term potentiation revealed by the analysis of miniature synaptic currents. Nature. 1992 Jan 2;355(6355):50–55. doi: 10.1038/355050a0. [DOI] [PubMed] [Google Scholar]
- Murphy T. H., Baraban J. M., Wier W. G. Mapping miniature synaptic currents to single synapses using calcium imaging reveals heterogeneity in postsynaptic output. Neuron. 1995 Jul;15(1):159–168. doi: 10.1016/0896-6273(95)90073-x. [DOI] [PubMed] [Google Scholar]
- Oliet S. H., Malenka R. C., Nicoll R. A. Bidirectional control of quantal size by synaptic activity in the hippocampus. Science. 1996 Mar 1;271(5253):1294–1297. doi: 10.1126/science.271.5253.1294. [DOI] [PubMed] [Google Scholar]
- Otis T. S., Mody I. Modulation of decay kinetics and frequency of GABAA receptor-mediated spontaneous inhibitory postsynaptic currents in hippocampal neurons. Neuroscience. 1992 Jul;49(1):13–32. doi: 10.1016/0306-4522(92)90073-b. [DOI] [PubMed] [Google Scholar]
- Redman S., Walmsley B. Amplitude fluctuations in synaptic potentials evoked in cat spinal motoneurones at identified group Ia synapses. J Physiol. 1983 Oct;343:135–145. doi: 10.1113/jphysiol.1983.sp014885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rekling J. C. Effects of met-enkephalin on GABAergic spontaneous miniature IPSPs in organotypic slice cultures of the rat hippocampus. J Neurosci. 1993 May;13(5):1954–1964. doi: 10.1523/JNEUROSCI.13-05-01954.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ropert N., Miles R., Korn H. Characteristics of miniature inhibitory postsynaptic currents in CA1 pyramidal neurones of rat hippocampus. J Physiol. 1990 Sep;428:707–722. doi: 10.1113/jphysiol.1990.sp018236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenmund C., Clements J. D., Westbrook G. L. Nonuniform probability of glutamate release at a hippocampal synapse. Science. 1993 Oct 29;262(5134):754–757. doi: 10.1126/science.7901909. [DOI] [PubMed] [Google Scholar]
- Silver R. A., Traynelis S. F., Cull-Candy S. G. Rapid-time-course miniature and evoked excitatory currents at cerebellar synapses in situ. Nature. 1992 Jan 9;355(6356):163–166. doi: 10.1038/355163a0. [DOI] [PubMed] [Google Scholar]
- Sorra K. E., Harris K. M. Occurrence and three-dimensional structure of multiple synapses between individual radiatum axons and their target pyramidal cells in hippocampal area CA1. J Neurosci. 1993 Sep;13(9):3736–3748. doi: 10.1523/JNEUROSCI.13-09-03736.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang C. M., Margulis M., Shi Q. Y., Fielding A. Saturation of postsynaptic glutamate receptors after quantal release of transmitter. Neuron. 1994 Dec;13(6):1385–1393. doi: 10.1016/0896-6273(94)90423-5. [DOI] [PubMed] [Google Scholar]
- Tong G., Jahr C. E. Block of glutamate transporters potentiates postsynaptic excitation. Neuron. 1994 Nov;13(5):1195–1203. doi: 10.1016/0896-6273(94)90057-4. [DOI] [PubMed] [Google Scholar]
- Ulrich D., Lüscher H. R. Miniature excitatory synaptic currents corrected for dendritic cable properties reveal quantal size and variance. J Neurophysiol. 1993 May;69(5):1769–1773. doi: 10.1152/jn.1993.69.5.1769. [DOI] [PubMed] [Google Scholar]
- Walmsley B., Edwards F. R., Tracey D. J. Nonuniform release probabilities underlie quantal synaptic transmission at a mammalian excitatory central synapse. J Neurophysiol. 1988 Sep;60(3):889–908. doi: 10.1152/jn.1988.60.3.889. [DOI] [PubMed] [Google Scholar]
- Wyllie D. J., Manabe T., Nicoll R. A. A rise in postsynaptic Ca2+ potentiates miniature excitatory postsynaptic currents and AMPA responses in hippocampal neurons. Neuron. 1994 Jan;12(1):127–138. doi: 10.1016/0896-6273(94)90158-9. [DOI] [PubMed] [Google Scholar]
- von Kitzing E., Jonas P., Sakmann B. Quantal analysis of excitatory postsynaptic currents at the hippocampal mossy fiber-CA3 pyramidal cell synapse. Adv Second Messenger Phosphoprotein Res. 1994;29:235–260. doi: 10.1016/s1040-7952(06)80019-4. [DOI] [PubMed] [Google Scholar]


