Abstract
Stilbene synthase (STS) and chalcone synthase (CHS) are plant-specific polyketide synthases that play key roles in the stilbenoid and flavonoid biosyntheses, respectively. We have recently isolated from Pinus densiflora three STS cDNAs (PDSTS1, PDSTS2, and PDSTS3) and one CHS cDNA (PDCHSX). We then heterologously expressed these cDNAs in Escherichia coli and characterized their properties. An unusual STS isozyme, PDSTS3, lacks the common C-terminal extension of STS because of a frame-shift mutation and shows the highest pinosylvin-forming activity among the STSs tested. Pinosylvin was shown to be a potent inhibitor of PDCHSX (Ki = 6 μM) as well as PDSTS2 (Ki = 13 μM), which presumably maintains the balance between the stilbenoid and flavonoid biosyntheses. PDSTS3 was insensitive to product inhibition. We identified PDSTS3 in the pine seedlings as well as full-length STS. The data provide evidence that PDSTS3 is involved in the potential regulation of the stilbenoid and flavonoid biosynthetic pathways in pine trees.
Many stress-induced phenylpropanoids are classified as phytoalexins (1). They have different biological activities and provide an important source of new pharmaceutical and agrochemical agents. Japanese pines (Pinus densiflora and Pinus thunbergii) have been under severe biotic stress over the past 10 decades from pine-wilt diseases, mainly caused by exotic pine-wood nematodes (2). In the genus Pinus, the stilbenoids, pinosylvin and its monomethyl ether, are key metabolites that can kill nematodes (3). Stilbenoid phytoalexin formation is controlled by stilbene synthase (STS), which comprises a small gene family in most species examined. The multiplicity of gene family members increases by gene duplications and diverges by base substitution and insertion/deletion mutations, leading to functional divergence. Such gene diversity may be selected as a result of biotic stress. Unfortunately, little is known about how general features of enzyme diversity may be of benefit against biotic stress.
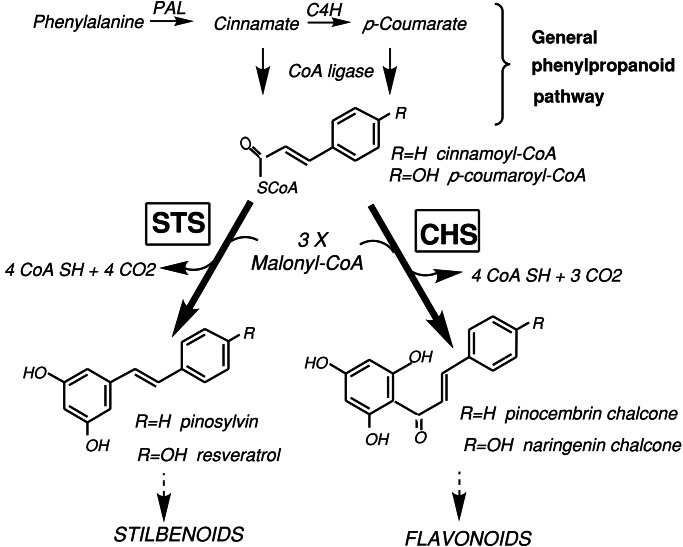
STS appears to have evolved from chalcone synthase (CHS) during land plant evolution (4). STS and CHS belong to a polyketide synthase (PKS) superfamily and share more than 65% amino acid homology. Unlike the bacterial PKSs, which are encoded by large gene clusters (5, 6), both STS and CHS function as unimodular PKSs with a single active site, forming relatively small homodimers (7). STS and CHS use common substrates, three malonyl-CoAs and one cinnamoyl-CoA/p-coumaroyl-CoA, forming their products with similar reaction mechanisms (8, 9). STS are classified into either a p-coumaroyl-CoA-specific type such as resveratrol synthase (EC 2.3.1.95) or a cinnamoyl-CoA-specific type such as pinosylvin synthase (EC 2.3.1.146) (Fig. 1). For example, the latter type has been reported in Scotch pine (Pinus sylvestris) (10) and Eastern white pine (Pinus strobus) (11), whereas the former type has been found in peanut (Arachis hypogaea) (12) and grape (Vitis vinifera) (13). The CHSs from many plant sources (nearly 150 CHSs) have been extensively studied, and some of them were defined as naringenin-chalcone synthases (EC 2.3.1.74). Recently, the crystal structure of CHS from alfalfa was resolved (14). The mechanism of action of other PKSs has also been the subject of considerable attention (15).
Figure 1.
Pathways for stilbenoid and flavonoid biosyntheses. Stilbene synthase (STS) and chalcone synthase (CHS), respectively, lead to stilbenoid and flavonoid biosynthesis from a cinnamoyl-CoA/p-coumaroyl-CoA with three malonyl-CoAs. PAL, phenylalanine ammonia-lyase; C4H, cinnamate 4-hydroxylase.
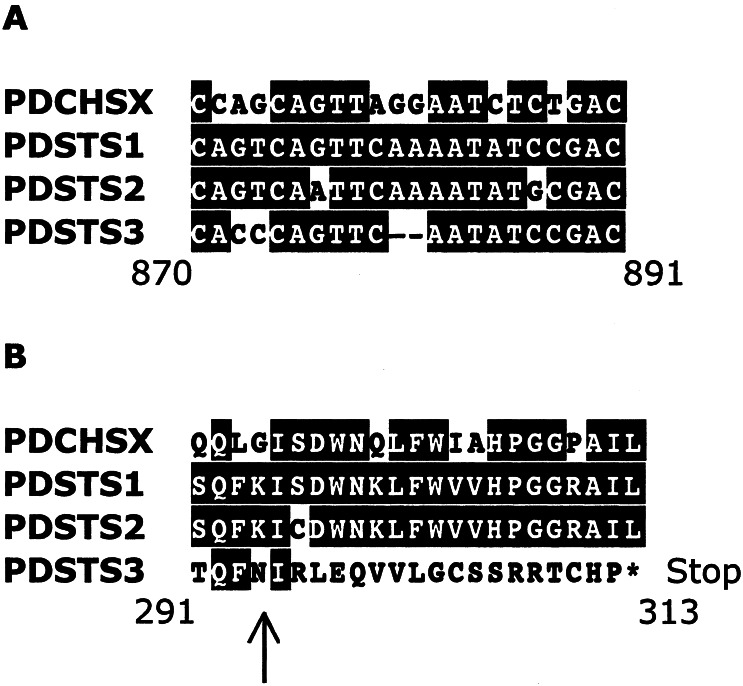
We have recently isolated a structurally unusual STS cDNA, PDSTS3, from the Japanese red pine, P. densiflora (16). PDSTS3 has no common C-terminal extension of STS because of a frame-shift mutation (Fig. 2). Our goal in this article is to compare the enzymological properties of PDSTS3 to those of the other two STSs (PDSTS1, PDSTS2) and one CHS (PDCHSX) from P. densiflora. Is PDSTS3 a functional enzyme, and, if so, is it enzymologically identical to the other STSs; otherwise, does it have new functions? Here we report the biochemical properties of PDSTS3 as well as PDSTS1, PDSTS2, and PDCHSX, and their functional diversity. The biochemical properties of PDSTS3 implicate dynamic regulation of stilbenoid phytoalexin accumulation and flavonoid biosynthesis.
Figure 2.
A frame-shift mutation of PDSTS3. (A) Nucleotide deletion in PDSTS3 cDNA. The two nucleotide deletions are indicated by the hyphens. (B) Alignment of the deduced amino acid sequences for PDCHSX, PDSTS1, PDSTS2, and PDSTS3. The frameshift mutation in PDSTS3 changes the amino acid sequence from N293 indicated by the arrow to the newly occurred stop codon indicated by the asterisk.
Materials and Methods
Chemicals.
[2-14C]Malonyl-CoA (2.04 GBq/mmol) was purchased from Amersham Pharmacia. Cinnamoyl-CoA and p-coumaroyl-CoA were chemically synthesized (17). Pinosylvin was chemically synthesized by a modified Witig reaction (18). Resveratrol was purchased from Sigma. Pinocembrin was purchased from Indofine Chemical (Somerville, NJ). Naringenin was purchased from Tokyo Kasei (Tokyo).
Isolation of STSs and CHS cDNA Clones.
Three P. densiflora STS cDNAs (PDSTS1, PDSTS2, and PDSTS3) and chalcone synthase cDNA (PDCHSX) were obtained in a previous study (16). For the PSSTS2 clone from P. strobus, the total RNA was prepared from P. strobus seedlings elicited according to the reported method (11). The first-strand cDNA was synthesized and PCR-amplified with the primer pairs designed from the published P. strobus PSSTS2 sequences (11). The product was identified as PSSTS2 by sequencing both strands (19) on an ABI PRISM 377 DNA sequencer (Applied Biosystems).
Construction of Plasmids and Expression of Recombinant STSs and CHS in Escherichia coli.
The STSs and CHS cDNAs were subcloned into a pET32Xa/LIC vector (Novagen) and reconfirmed by sequencing both strands. The recombinant STSs and CHS are expressed as the fusion proteins with thioredoxin, His-tag, and S-tag at the N terminus. E. coli strain Origami B (DE3) was cultured in Luria–Bertani medium containing 100 μg/ml carbenicillin at 37°C on a shaker at 200 rpm until the OD600 reached 0.6. After the culture was cooled on ice, 0.4 mM isopropyl β-d-thiogalactoside (IPTG) was added to induce protein expression, and the culture was held at 15°C on a shaker at 200 rpm for 20 h. Cells were harvested by centrifugation, washed, and suspended in 50 mM Tris⋅HCl (pH 8.0).
Purification of Recombinant STSs and CHS.
For extraction of the recombinant proteins except that of PDSTS3, the recombinant proteins were released from the E. coli cytoplasm under osmotic stress (20). The recombinant protein was affinity purified by using S-protein agarose (Novagen). The recombinant protein with no N-terminal tag was rescued from the agarose by factor Xa digestion. The factor Xa coexisting in the sample was then removed by Xarrest agarose (Novagen). In recombinant PDSTS3, the cells harboring pET32-PDSTS3 were pelleted, harvested, and resuspended in 20 mM sodium phosphate, pH 7.4/500 mM NaCl/60 mM imidazole/10% (vol/vol) glycerol. After sonication and centrifugation, the supernatant was passed through a Ni2+-nitrilotriacetate (NTA) column, the column was washed with 10 bed volumes of lysis buffer, and the recombinant PDSTS3 was then eluted with lysis buffer containing 250 mM imidazole. The purified recombinant protein was desalted and buffer-exchanged through a prebuffered NICK Spin Column (Sephadex G50 DNA Grade; Amersham Pharmacia). The protein was quantified by using a Coomassie blue protein assay reagent kit (Pierce) with BSA as the standard.
STS and CHS Assays.
The STS and CHS activities were determined by measuring the conversion of [2-14C]malonyl-CoA into reaction products. The reaction mixture contained recombinant STSs or CHS (<10 pmol), 15 μM malonyl-CoA (0.25 kBq), and 20 μM cinnamoyl-CoA in 100 μl of reaction buffer. The reaction buffer consisted of 20 mM Hepes buffer (pH 7.0), 5 mM EDTA, and 0.3 mM DTT. The mixture was incubated at 30°C for 20 min. The products were extracted twice with ethyl acetate. The extracts were evaporated to dryness and redissolved in methanol, and the products were separated on Whatman LK6DF silica TLC plates. The plates were developed with an organic layer of water-saturated diisopropyl ether. The radiograms on an imaging plate (BAS-IP SR 2025; Fuji) were analyzed by BAS-1800 (Fuji). The enzyme assays were repeated twice with an appropriate control assay.
Determination of Inhibition Constant (Ki).
For Ki determination, various concentrations (1 μM to 500 μM) of either pinosylvin or pinocembrin, dissolved in 50% ethyl cellosolve (4 μl), were incubated at 30°C with the respective enzyme in the reaction buffer (96 μl), which contained different concentrations of cinnamoyl-CoA and 15 μM malonyl-CoA. The precise concentration range was selected to produce a 20–80% inhibition of the initial activity. Ki was estimated from a double-reciprocal Lineweaver–Burk plot.
Preparation of Anti-STS Antibody.
The recombinant PDSTS2 antigen was injected into a rabbit at 2-week intervals. The polyclonal antiserum, which had an antibody titer of 10−6 by ELISA analysis, was obtained 63 days after the first injection. The antiserum was used for immunoprecipitation together with an anti-rabbit IgG-peroxidase conjugate and protein-A agarose (Amersham Pharmacia).
Extraction of Crude Enzymes from Pine Seedlings.
One gram of the seedlings was ground with liquid nitrogen in a mortar. The homogenate was extracted with 1 ml of 100 mM Hepes buffer (pH 7.0) containing 3 mM DTT, 20% (vol/vol) glycerol, and 1% (wt/vol) BSA, and was squeezed through Miracloth (Calbiochem) and transferred to a new 1.5-ml tube. The extracts were passed through a prebuffered NICK Spin Column (Sephadex G50 DNA grade). The enzyme assays were performed as described above, except that the 15 μM [2-14C]malonyl-CoA (2.5 kBq) and the pine crude enzymes (1.4 mg) were used in the reaction mixture.
Results
Expression and Purification of Recombinant STS and CHS.
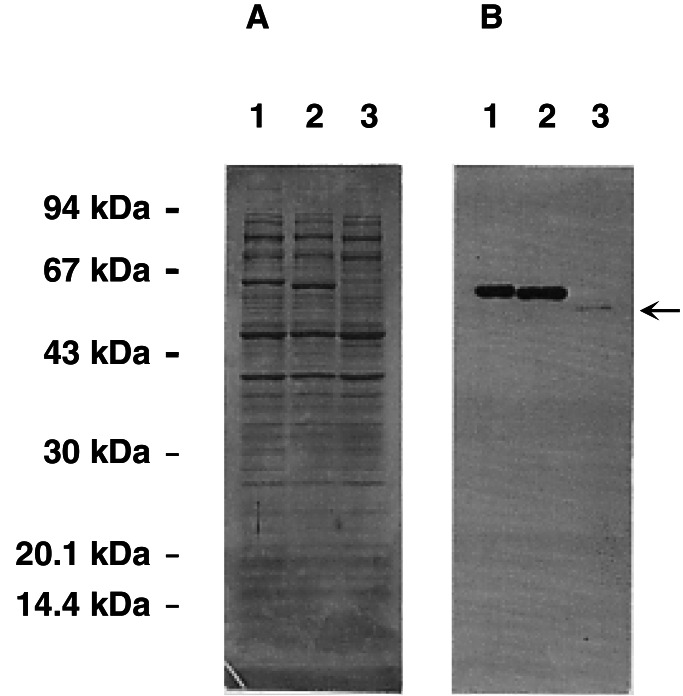
The three STSs (PDSTS1, PDSTS2, and PDSTS3), a CHS (PDCHSX) from P. densiflora, and PSSTS2 from P. strobus were expressed in E. coli. Four of the five recombinant proteins, PDCHSX, PDSTS1, PDSTS2, and PSSTS2, were obtained in sufficient amounts (PDCHSX ≫ PDSTS2 > PSSTS2, PDSTS1) for purification. The recombinant PDSTS3 showed poor solubility; the amount recovered was less than 1/10 compared with PDSTS2 (Fig. 3), and most of the portions were presented as inclusion bodies. A lower temperature and varying isopropyl β-d-thiogalactoside (IPTG) concentrations did not improve the solubility. We found that inclusion bodies were especially abundant after the removal of the N-terminal tag from the PDSTS3 fusion protein. Thus we characterized the recombinant PDSTS3 as a fusion form (approximately 52 kDa), whereas the other recombinant proteins were characterized by using intact proteins (approximately 42 kDa). We have tested the pH dependencies and time course activities of both PDSTS2 and PDCHSX in the presence and absence of the tag protein, which suggested that the tag-protein had no effect on the true STS and CHS functions. In addition, to confirm that a natural frame-shift mutation is present in PDSTS3, we have subcloned PDSTS3 cDNA into the pET32 vector not as the truncated form but as the original form (base pairs 88–1264 in GenBank accession no. AB030140). The truncated PDSTS3 protein subsequently occurred (Fig. 3B), which provided evidence that PDSTS3 was made by a natural frame-shift mutation.
Figure 3.
Production of soluble PDSTSs in E. coli. (A) The same amounts of the soluble proteins (3 μg) were separated by SDS/PAGE and stained. Lane 1, PDSTS1; lane 2, PDSTS2; lane 3, PDSTS3. (B) Western blot analysis. The soluble fusion proteins were crossreacted with the S-protein alkaline phosphatase conjugate. The band of PDSTS3 is indicated by the arrow. PDSTS1 and PDSTS2, 60 kDa; PDSTS3, 52 kDa.
Enzyme Activity and Kinetic Analysis of Recombinant STSs.
The purified recombinant STSs were assayed for their enzyme activities with cinnamoyl-CoA and [2-14C]malonyl-CoA. The main reaction product was pinosylvin; however, the formation of a small amount of at least three radioactive products was also found. These were probably noncyclized derailment products derived from partially formed polyketide chains (21). A GC-MS analysis confirmed that the main product was pinosylvin.
The recombinant PDSTS1 activity was very low and barely detectable between pH 6.0 and 8.0. Therefore we were unable to evaluate the kinetic parameters. The steady-state kinetic parameters were determined in the purified recombinant PDSTS2, PDSTS3, and P. strobus PSSTS2. We used the recombinant P. strobus PSSTS2 as a control, because its kinetics has already been reported (11). Our Km value for recombinant P. strobus PSSTS2 was in good agreement with the reported value (Table 1). The steady-state kinetic analysis showed that the recombinant PDSTS2 preferred cinnamoyl-CoA to p-coumaroyl-CoA, as did recombinant P. strobus PSSTS2 (Table 1). However, unlike P. strobus PSSTS2, the recombinant PDSTS2 accepted p-coumaroyl-CoA to a considerable extent. The high specificity for p-coumaroyl-CoA is unexpected because resveratrol and its derivatives have not yet been reported to occur in P. densiflora. A mixture of equimolar cinnamoyl-CoA and p-coumaroyl-CoA incubated with the recombinant PDSTS2 resulted in the dominant formation of pinosylvin. Thus we defined the recombinant PDSTS2 to be pinosylvin synthase.
Table 1.
Kinetic parameters of pine recombinant CHS and STSs
| Enzyme | Km, μM | kcat, 10−3 min−1 | kcat/Km, s−1 ·M−1 |
|---|---|---|---|
| Cinnamoyl-CoA | |||
| PDCHSX | 1.1 | 193 | 2,926 |
| PDSTS2 | 3.8 | 228 | 1,002 |
| PDSTS3 | 0.4 | 285 | 11,879 |
| PSSTS2 | 4.0 | 873 | 3,639 |
| p-Coumaroyl-CoA | |||
| PDCHSX | 2.9 | 365 | 2,097 |
| PDSTS2 | 5.3 | 228 | 718 |
| PDSTS3 | 0.9 | 264 | 4,883 |
| PSSTS2 | 11.1 | 412 | 618 |
| Malonyl-CoA | |||
| PDCHSX | 3.1 | 246 | 1,323 |
| PDSTS2 | 6.0 | 456 | 1,269 |
| PDSTS3 | 24.0 | 534 | 372 |
Despite the absence of a common STS C terminus, the recombinant PDSTS3 had a catalytic activity comparable to that of the recombinant P. strobus PSSTS2. The optimum pH was pH 7.0 for cinnamoyl-CoA, and pH 8.0 for p-coumaroyl-CoA as well as PDSTS2. A steady-state kinetic analysis showed that the recombinant PDSTS3 exhibited a preference for cinnamoyl-CoA (Km = 0.4 μM) over p-coumaroyl-CoA (Km = 0.9 μM) and was identified as pinosylvin synthase. The high Km value (24 μM) for malonyl-CoA may reflect the absence of the C-terminal segment, which plays an important role in malonyl-CoA decarboxylation activity (22). The catalytic efficiency (kcat/Km) of the recombinant PDSTS3 for cinnamoyl-CoA was 12-fold higher than that of the recombinant PDSTS2 and 3-fold higher than that of the recombinant P. strobus PSSTS2 (Table 1).
Additionally, unlike the enzymes involved in the lignin pathway, the recombinant STSs tested in this study showed remarkably low catalytic efficiency, as did those of the reported recombinant CHS (23, 24). This observation presumably reflected a series of decarboxylation, condensation, and cyclization reactions of STS. This result may explain in part why pinosylvin is a minor component while lignin accumulates in pine trees.
Enzyme Activity and Kinetic Analysis of Recombinant PDCHSX.
The CHS reaction forms chalcones followed by spontaneous cyclization to flavanones, pinocembrin or naringenin, from cinnamoyl-CoA or p-coumaroyl-CoA, respectively. Thus we quantified the spots, which corresponded to either pinocembrin or naringenin, on the autoradiogram. The recombinant PDCHSX had enzyme activity, and the optimum pH was 7.0 for both cinnamoyl-CoA and p-coumaroyl-CoA. The steady-state kinetic analysis showed that the recombinant PDCHSX preferred cinnamoyl-CoA (Km = 1.1 μM) to p-coumaroyl-CoA (Km = 2.9 μM) (Table 1).
Inhibition Studies.
Naringenin, naringenin chalcone, and CoASH are potent inhibitors of CHS (25–27). The recombinant PDSTS2, PDSTS3, and PDCHSX were examined to determine whether their main products, pinosylvin and pinocembrin, might inhibit these activities. An inhibition kinetic analysis showed that pinosylvin and pinocembrin inhibited the STS and CHS activities in a noncompetitive manner, i.e., with increasing inhibitor concentrations, decreased Vmax, and an unchanged Km for cinnamoyl-CoA. This result indicated that pinosylvin and pinocembrin reversibly interacted with both STS and CHS at sites other than the binding site of cinnamoyl-CoA.
Pinosylvin was more than twice as strong an inhibitor for PDCHSX (Ki = 6 μM) as for PDSTS2 (Ki = 13 μM) (Table 2), suggesting that the CHS-catalyzed reaction can be regulated by the pinosylvin biosynthesis in pine trees. Pinosylvin inhibited PDSTS3 with a much higher Ki of 152 μM, which clearly indicated that PDSTS3 was not susceptible to the product inhibition. Like pinosylvin, pinocembrin was also an effective inhibitor of PDCHSX and PDSTS2. Pinocembrin inhibited PDCHSX (Ki = 15 μM) over 2-fold more effectively than PDSTS2 (Ki = 37 μM), but did not effectively inhibit PDSTS3 (Ki = 382 μM) as well as pinosylvin.
Table 2.
Inhibition constant (Ki) of pinosylvin and pinocembrin against purified recombinant PDSTS2, PDSTS3, and PDCHSX
| Recombinant protein |
Ki, μM
|
|
|---|---|---|
| Pinosylvin | Pinocembrin | |
| PDSTS2 | 13 | 37 |
| PDSTS3 | 152 | 382 |
| PDCHSX | 6 | 15 |
Competitive Experiment Between Recombinant STSs and CHS.
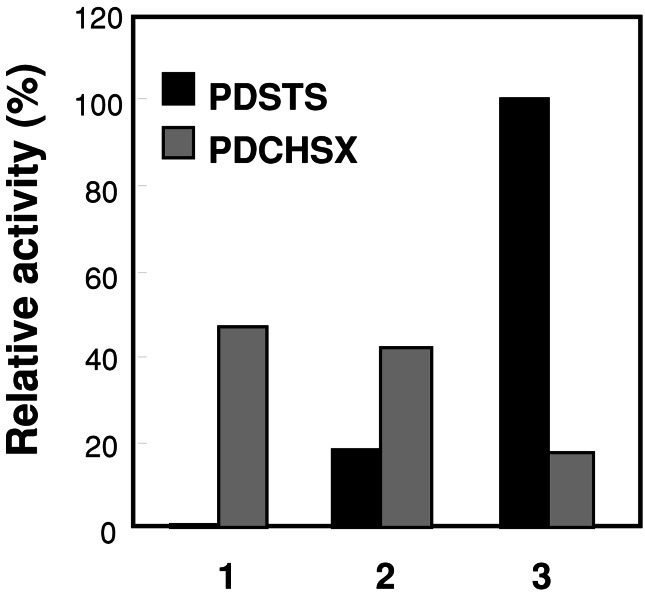
Together with the common substrates in STS and CHS, the findings of the product inhibitions led to the idea that STS activity may inhibit CHS activity in vitro. Ten picomolar amounts of PDCHSX in combination with equimolar amounts of the three STSs were then co-incubated at 30°C for 20 min with saturated amounts of the substrates (110 μM [2-14C]malonyl-CoA and 34 μM cinnamoyl-CoA) in 100-μl reactions. Neither PDSTS1 nor PDSTS2 inhibited the PDCHSX activity (Fig. 4). PDSTS3 produced the highest amounts of pinosylvin among the three STSs and clearly diminished the PDCHSX activity by approximately 50% compared with PDSTS1 or PDSTS2 (Fig. 4). This result suggested that PDSTS3 could down-regulate the flavonoid biosynthesis.
Figure 4.
Activities of PDCHSX that coexisted with three PDSTSs. The purified recombinant PDCHSX (10 pmol) was mixed with equimolar amounts of the purified recombinant. 1, PDSTS1; 2, PDSTS2; 3, PDSTS3. The activities were measured twice as described in the text.
Occurrence of PDSTS3 in Pine Extracts.
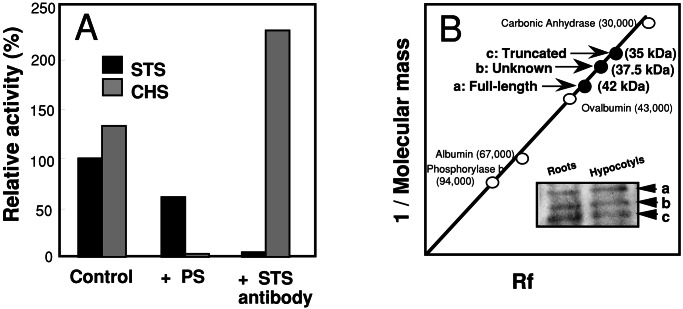
The pine crude enzyme extracts simultaneously formed similar amounts of pinosylvin and pinocembrin in vitro (Fig. 5A). To demonstrate the activity of native PDSTS3 in the pine, we exploited the insensitivity to product inhibition (Table 2). According to the Lineweaver–Burk plot of inhibition kinetics (data not shown), at 200 μM pinosylvin in the reaction, PDSTS3 would show 43% activity of the original, whereas PDSTS2 and PDCHSX would show only 8% and 2% of the activities of the original, respectively. To test this hypothesis, we preincubated crude enzyme extracts from the pine seedlings with 200 μM pinosylvin. Although little or no CHS activity was detected, about 50% STS activity was still found (Fig. 5A). This result suggested that PDSTS3 does occur in the pine.
Figure 5.
Occurrence of PDSTS3 in pine. (A) STS and CHS activities in pine extracts preincubated with either 200 μM pinosylvin (PS) or anti-STS antibody. The enzyme assays were performed as described in the text. (B) Immunodetection of naturally occurring STS in pine seedlings.
In addition, we demonstrated that our anti-STS antibody crossreacted with STS and did not crossreact with CHS because little or no STS activity was found when the crude enzyme extracts were preincubated with the antibody, whereas increased CHS activity was found (Fig. 5A). The increased CHS activity was presumably ascribed to little or no inhibition by pinosylvin. We used the antibody to immunodetect naturally occurring STS in pine. At least three STS bands were observed on a Western blot. The three bands were estimated to be 42 kDa, 37.5 kDa, and 35 kDa in size, corresponding to the full-length STS such as PDSTS1 and PDSTS2, an unknown STS, and PDSTS3, respectively (Fig. 5B). This result indicated that not only the full-length STS but also PDSTS3 and another truncated STS were likely to occur in the pine.
Discussion
The C terminus of both STS and CHS is important for their catalytic activities because it contains some highly conserved amino acid residues (22, 28, 29). Other CHS-like enzymes, including the 2-pyrone synthases (30), bibenzyl synthases (31), acridone synthases (32), and the rppA CHS-like protein (33), also contain such amino acids in the C terminus. However, the C-terminus-truncated PDSTS3 showed an extremely high potential for pinosylvin formation. Furthermore, neither pinosylvin nor pinocembrin inhibited the PDSTS3 activity in vitro, whereas they effectively inhibited PDSTS2 and PDCHSX (Table 2). PDSTS3 has provided strong evidence for functional divergence of this enzyme. A high concentration of pinosylvin is harmful for conifers (34), therefore PDSTS3 may lead to cell death if substantial amounts of pinosylvin accumulate with little product inhibition. Such stilbenoid formation might operate in the pine heartwood formation. On the other hand, the PDSTS2 does not accumulate a high level of the stilbenoid because of the product inhibition (Table 2). The PDSTS2 may serve to produce slow phytoalexin formation in response to various biotic and abiotic stresses. The rapid and slow responses of our STSs may reflect different adaptations against biotic stresses.
STS and CHS compete through their common precursors (35). A key regulatory aspect of the balance is their catalytic efficiencies (kcat/Km). Our results indicate that product inhibition could be an important factor in maintaining the balance between the stilbenoid and flavonoid biosyntheses. Pinosylvin inhibited both the recombinant PDCHSX and PDSTS2 activities 2.5-fold more effectively than did pinocembrin (Table 2), indicating that the CHS-catalyzed reaction can be regulated by pinosylvin biosynthesis in pine (Fig. 5A). This effect is probably caused by the planar structure of pinosylvin, which could allow easy access to the binding pockets. Because the pinocembrin level was appreciably lower than that of pinosylvin in the heartwood (36), pinocembrin formation is possibly inhibited by pinosylvin as demonstrated in PDCHSX (Table 2). PDSTS3 inhibited PDCHSX activity by producing high amounts of pinosylvin in vitro, whereas PDSTS1 and PDSTS2 did not (Fig. 4). Furthermore, we have detected the translation product for PDSTS3 (35 kDa) in the pine (Fig. 5B). Based on these findings, PDSTS3 may also function for down-regulation of the flavonoid biosynthesis.
The reason why PDSTS3 has this enzymatic property has not yet been clarified. However, the C terminus, which contains ≈20 amino acid residues, was converted into a different sequence by the frame-shift mutation, is presumably responsible. Sequence comparisons indicated that the 3′ untranslated region (3′ UTR) corresponding to the truncated C terminus underwent strong constraints against amino acid substitutions because nonsynonymous substitutions did not accumulate (data not shown). This finding leads us to the conclusion that the frame-shift mutation has recently occurred in PDSTS3. A gene mutation may result in a new function as in the case of PDSTS3, but loss of function is also expected to occur at a substantial rate as in PDSTS1, which shows negligible enzyme activity. Similarly, P. strobus PSSTS1 had only 3–5% of the P. strobus PSSTS2 activity, although they are virtually identical in their primary structures (11). Only a few amino acid substitutions appear to be sufficient for activation and inactivation of the enzyme.
Taken together, it is likely that the STS gene family comprises a mixture of fully active and inactive members in the examined pines. The STS isozymes exhibit distinct catalytic activities with different product inhibition. The diversity of the biochemical properties presumably reflects a process whereby a member of the gene family diverges and evolves new functions. The functional divergence may be a general feature in conifers because of their large genomes. PDSTS3 has increased activity and it has a lost inhibitory function. Furthermore, pinosylvin appears to down-regulate the CHS-catalyzed reaction (Table 2). Together with the occurrence of PDSTS3 in the pine (Fig. 5), it is likely that PDSTS3 is involved in the dynamic regulation of the stilbenoid phytoalexin accumulation and flavonoid biosynthesis. However, further research is needed to elucidate the physiological significance of PDSTS3 in the conifer resistance to biotic stress.
Acknowledgments
We thank Prof. M. Shimada of the Wood Research Institute for his critical discussion of the manuscript. We are also grateful to Dr. T. Umezawa, Dr. T. Hattori, and Ms. K. Etoh of the Wood Research Institute for their kind support in the identification of the enzyme substrates and products. This work was supported by a Grant-in-Aid for Science Research from the Ministry of Education, Science, and Culture of Japan (to H.K.).
Abbreviations
- STS
stilbene synthase
- CHS
chalcone synthase
- PDSTS1
STS1 from P. densiflora
- PDSTS2
STS2 from P. densiflora
- PDSTS3
STS3 from P. densiflora
- PDCHSX
CHSX from P. densiflora
- PSSTS2
STS2 from P. strobus
Footnotes
References
- 1.Dixon R A, Paiva N L. Plant Cell. 1995;7:1085–1097. doi: 10.1105/tpc.7.7.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mamiya Y. In: Pathogenicity of the Pine Wood Nematode. Wingfield M J, editor. St. Paul: Am. Phytopathol. Soc.; 1987. pp. 59–65. [Google Scholar]
- 3.Suga T, Ohta S, Munesada K, Ide N, Kurokawa M, Shimizu M, Ohta E. Phytochemistry. 1993;33:1395–1401. [Google Scholar]
- 4.Tröpf S, Lanz T, Rensing S A, Schröder J, Schröder G. J Mol Evol. 1994;38:610–618. doi: 10.1007/BF00175881. [DOI] [PubMed] [Google Scholar]
- 5.Kao C M, Katz L, Khosla C. Science. 1994;265:509–512. doi: 10.1126/science.8036492. [DOI] [PubMed] [Google Scholar]
- 6.Pfeifer B A, Admiraal S J, Gramajo H, Cane D E, Khosla C. Science. 2001;291:1790–1792. doi: 10.1126/science.1058092. [DOI] [PubMed] [Google Scholar]
- 7.Tröpf S, Karcher B, Schröder G, Schröder J. J Biol Chem. 1995;270:7922–7928. doi: 10.1074/jbc.270.14.7922. [DOI] [PubMed] [Google Scholar]
- 8.Grisebach H. In: Biosynthesis and Biodegradation of Wood Components. Higuchi T, editor. Orlando, FL: Academic; 1985. pp. 291–324. [Google Scholar]
- 9.Kindl H. In: Biosynthesis and Biodegradation of Wood Components. Higuchi T, editor. Orlando, FL: Academic; 1985. pp. 349–377. [Google Scholar]
- 10.Fliegmann J, Schröder G, Schanz S, Britsch L, Schröder G. Plant Mol Biol. 1992;18:489–503. doi: 10.1007/BF00040665. [DOI] [PubMed] [Google Scholar]
- 11.Raiber S, Schröder G, Schröder J. FEBS Lett. 1995;361:299–302. doi: 10.1016/0014-5793(95)00199-j. [DOI] [PubMed] [Google Scholar]
- 12.Schröder G, Brown J W, Schröder J. Eur J Biochem. 1988;172:160–169. doi: 10.1111/j.1432-1033.1988.tb13868.x. [DOI] [PubMed] [Google Scholar]
- 13.Melchior F, Kindl H. FEBS Lett. 1990;268:17–20. doi: 10.1016/0014-5793(90)80961-h. [DOI] [PubMed] [Google Scholar]
- 14.Ferrer J L, Jez J M, Bowman M E, Dixon R A, Noel J P. Nat Struct Biol. 1999;6:775–784. doi: 10.1038/11553. [DOI] [PubMed] [Google Scholar]
- 15.Jez J M, Austin M B, Ferrer J, Bowman M E, Schröder J, Noel J P. Chem Biol. 2000;7:919–930. doi: 10.1016/s1074-5521(00)00041-7. [DOI] [PubMed] [Google Scholar]
- 16.Kodan A, Kuroda H, Sakai F. J Wood Sci. 2001;47:58–62. [Google Scholar]
- 17.Stöckigt L, Zenk H M. Z Naturforsch. 1975;30c:352–358. doi: 10.1515/znc-1975-5-609. [DOI] [PubMed] [Google Scholar]
- 18.Bachelor F W, Loman A A, Snowdon L R. Can J Chem. 1970;48:1554–1557. [Google Scholar]
- 19.Sanger F, Nicklen S, Coulson A R. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lunn C A, Pigiet V P. J Biol Chem. 1982;257:11424–11430. [PubMed] [Google Scholar]
- 21.Yamaguchi T, Kurosaki F, Suh D Y, Sankawa U, Nishioka M, Akiyama T, Shibuya M, Ebizuka Y. FEBS Lett. 1999;460:457–461. doi: 10.1016/s0014-5793(99)01403-9. [DOI] [PubMed] [Google Scholar]
- 22.Jez J M, Noel J P. J Biol Chem. 2000;275:39640–39646. doi: 10.1074/jbc.M008569200. [DOI] [PubMed] [Google Scholar]
- 23.Jez J M, Ferrer J L, Bowman M E, Dixon R A, Noel J P. Biochemistry. 2000;39:890–902. doi: 10.1021/bi991489f. [DOI] [PubMed] [Google Scholar]
- 24.Abe I, Morita H, Nomura A, Noguchi H. J Am Chem Soc. 2000;122:11242–11243. [Google Scholar]
- 25.Kreuzaler F, Hahlbrock K. Eur J Biochem. 1975;56:205–213. doi: 10.1111/j.1432-1033.1975.tb02223.x. [DOI] [PubMed] [Google Scholar]
- 26.Whitehead I M, Dixon R A. Biochim Biophys Acta. 1983;747:298–303. [Google Scholar]
- 27.Hinderer W, Seitz H U. Arch Biochem Biophys. 1985;240:265–272. doi: 10.1016/0003-9861(85)90032-3. [DOI] [PubMed] [Google Scholar]
- 28.Suh D Y, Fukuma K, Kagami J, Yamazaki Y, Shibuya M, Ebizuka Y, Sankawa U. Biochem J. 2000;350:229–235. [PMC free article] [PubMed] [Google Scholar]
- 29.Suh D Y, Kagami J, Fukuma K, Sankawa U. Biochem Biophys Res Commun. 2000;275:725–730. doi: 10.1006/bbrc.2000.3368. [DOI] [PubMed] [Google Scholar]
- 30.Helariutta Y, Kotilainen M, Elomaa P, Kalkkinen N, Bremer K, Teeri T H, Albert V A. Proc Natl Acad Sci USA. 1996;93:9033–9038. doi: 10.1073/pnas.93.17.9033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Preisig-Muller R, Gnau P, Kindl H. Arch Biochem Biophys. 1995;317:201–207. doi: 10.1006/abbi.1995.1154. [DOI] [PubMed] [Google Scholar]
- 32.Junghanns K T, Kneusel R E, Baumert A, Maier W, Groger D, Matern U. Plant Mol Biol. 1995;27:681–692. doi: 10.1007/BF00020222. [DOI] [PubMed] [Google Scholar]
- 33.Funa N, Ohnishi Y, Fujii I, Shibuya M, Ebizuka Y, Horinouchi S. Nature (London) 1999;400:897–899. doi: 10.1038/23748. [DOI] [PubMed] [Google Scholar]
- 34.Bonello P, Heller W, Sandermann H., Jr New Phytol. 1993;124:653–663. doi: 10.1111/j.1469-8137.1993.tb03855.x. [DOI] [PubMed] [Google Scholar]
- 35.Fischer R, Budde I, Hain R. Plant J. 1997;11:489–498. [Google Scholar]
- 36.Higuchi T, Shimada M, Watanabe K. Mokuzai Gakkaishi. 1967;13:274–279. [Google Scholar]