Abstract
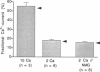
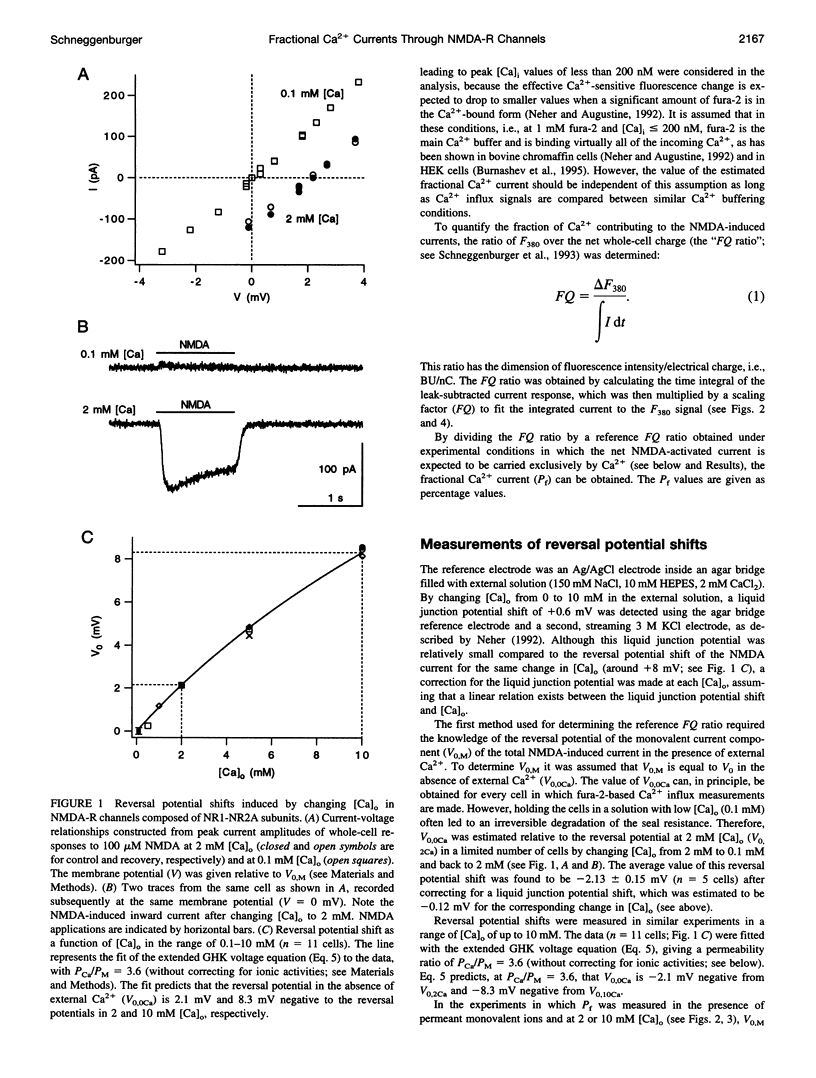
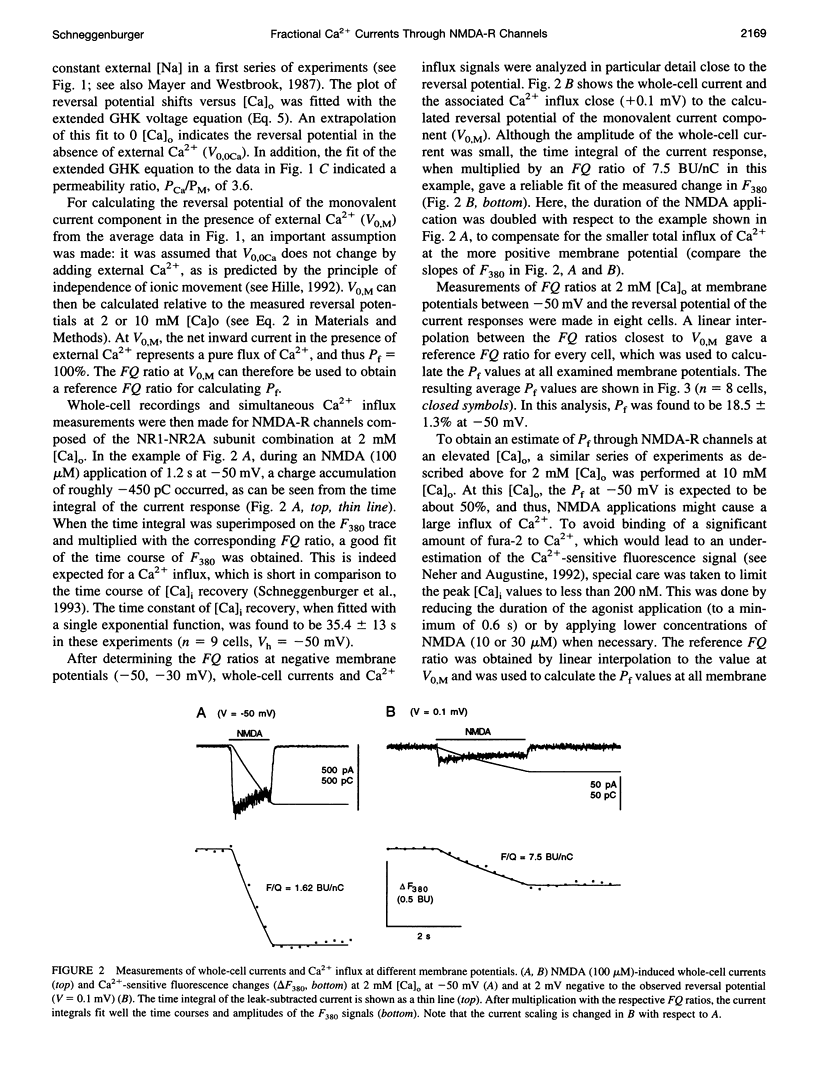
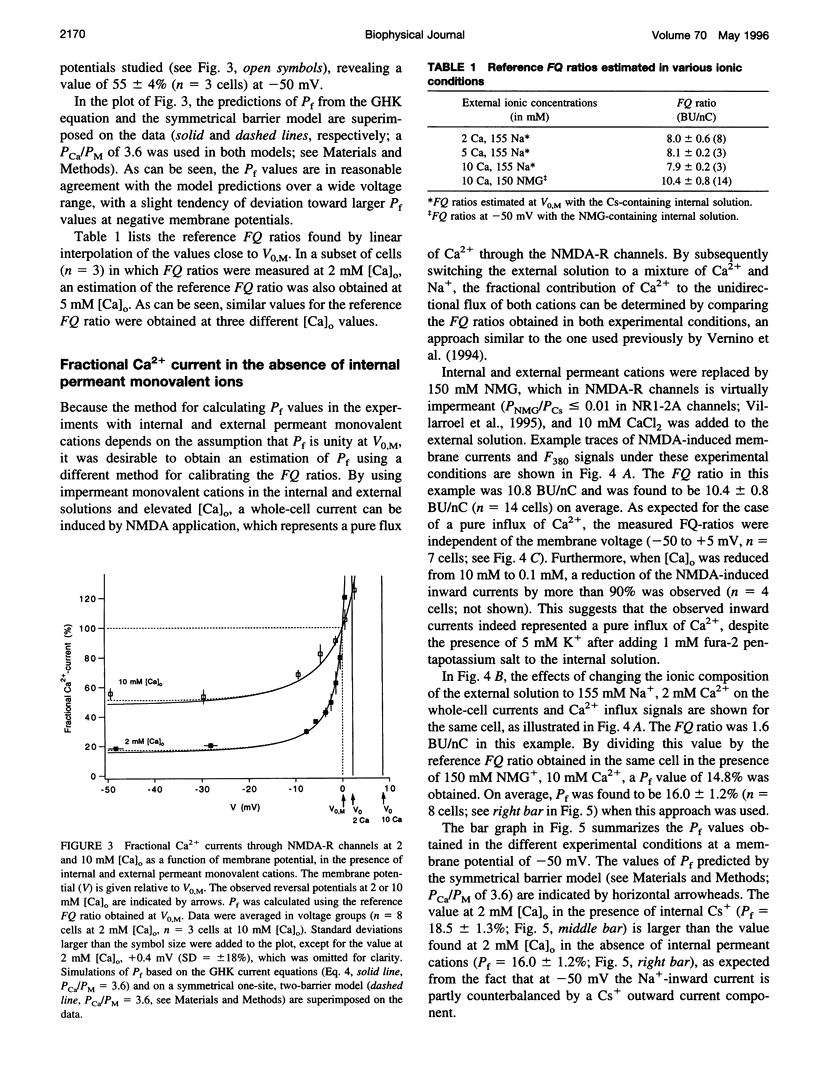
The Ca(2+) permeability of N-methyl-D-aspartate receptor (NMDA-R) channels was studied in human embryonic kidney cells transfected with the NR1-NR2A subunit combination. To determine the fractional Ca(2+) current (P(f)), measurements of fura-2-based Ca(2+) influx and whole-cell currents were made in symmetrical monovalent ion concentrations at membrane potentials between -50 mV and the reversal potential. The ratios of Ca(2+) flux over net whole-cell charge at 2, 5, and 10 mM external Ca(2+) concentrations ([Ca](o)) were identical at a membrane potential close to the reversal potential of the monovalent current component. Assuming unity of P(f) at this potential, the percentage of current carried by Ca(2+) was found to be 18.5 +/- 1.3% at 2 mM [Ca](o) and -50 mV. This value, which is higher than the ones reported previously, was confirmed in independent experiments in which a pure flux of Ca(2+) through NMDA-R channels was used to calibrate the Ca(2+) influx signals. The measured values of fractional Ca(2+) currents, which agree with the predictions of the Goldman-Hodgkin-Katz equations, are also compatible with a two-barrier model for ion permeation, in which the differences between the energy barriers for Ca(2+) and monovalent ions are similar on the external and internal membrane sides.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ascher P., Nowak L. The role of divalent cations in the N-methyl-D-aspartate responses of mouse central neurones in culture. J Physiol. 1988 May;399:247–266. doi: 10.1113/jphysiol.1988.sp017078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss T. V., Collingridge G. L. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993 Jan 7;361(6407):31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Burnashev N., Schoepfer R., Monyer H., Ruppersberg J. P., Günther W., Seeburg P. H., Sakmann B. Control by asparagine residues of calcium permeability and magnesium blockade in the NMDA receptor. Science. 1992 Sep 4;257(5075):1415–1419. doi: 10.1126/science.1382314. [DOI] [PubMed] [Google Scholar]
- Burnashev N., Zhou Z., Neher E., Sakmann B. Fractional calcium currents through recombinant GluR channels of the NMDA, AMPA and kainate receptor subtypes. J Physiol. 1995 Jun 1;485(Pt 2):403–418. doi: 10.1113/jphysiol.1995.sp020738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garaschuk O., Schneggenburger R., Schirra C., Tempia F., Konnerth A. Fractional Ca2+ currents through somatic and dendritic glutamate receptor channels of rat hippocampal CA1 pyramidal neurones. J Physiol. 1996 Mar 15;491(Pt 3):757–772. doi: 10.1113/jphysiol.1996.sp021255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hollmann M., Boulter J., Maron C., Beasley L., Sullivan J., Pecht G., Heinemann S. Zinc potentiates agonist-induced currents at certain splice variants of the NMDA receptor. Neuron. 1993 May;10(5):943–954. doi: 10.1016/0896-6273(93)90209-a. [DOI] [PubMed] [Google Scholar]
- Iino M., Ozawa S., Tsuzuki K. Permeation of calcium through excitatory amino acid receptor channels in cultured rat hippocampal neurones. J Physiol. 1990 May;424:151–165. doi: 10.1113/jphysiol.1990.sp018060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahr C. E., Stevens C. F. Calcium permeability of the N-methyl-D-aspartate receptor channel in hippocampal neurons in culture. Proc Natl Acad Sci U S A. 1993 Dec 15;90(24):11573–11577. doi: 10.1073/pnas.90.24.11573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan L. Y., Jan Y. N. L-glutamate as an excitatory transmitter at the Drosophila larval neuromuscular junction. J Physiol. 1976 Oct;262(1):215–236. doi: 10.1113/jphysiol.1976.sp011593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh D. S., Geiger J. R., Jonas P., Sakmann B. Ca(2+)-permeable AMPA and NMDA receptor channels in basket cells of rat hippocampal dentate gyrus. J Physiol. 1995 Jun 1;485(Pt 2):383–402. doi: 10.1113/jphysiol.1995.sp020737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konnerth A., Lux H. D., Morad M. Proton-induced transformation of calcium channel in chick dorsal root ganglion cells. J Physiol. 1987 May;386:603–633. doi: 10.1113/jphysiol.1987.sp016553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis C. A. Ion-concentration dependence of the reversal potential and the single channel conductance of ion channels at the frog neuromuscular junction. J Physiol. 1979 Jan;286:417–445. doi: 10.1113/jphysiol.1979.sp012629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F., Stevens C. F. Both open and closed NMDA receptor channels desensitize. J Neurosci. 1994 Apr;14(4):2153–2160. doi: 10.1523/JNEUROSCI.14-04-02153.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDermott A. B., Mayer M. L., Westbrook G. L., Smith S. J., Barker J. L. NMDA-receptor activation increases cytoplasmic calcium concentration in cultured spinal cord neurones. 1986 May 29-Jun 4Nature. 321(6069):519–522. doi: 10.1038/321519a0. [DOI] [PubMed] [Google Scholar]
- Malenka R. C., Nicoll R. A. NMDA-receptor-dependent synaptic plasticity: multiple forms and mechanisms. Trends Neurosci. 1993 Dec;16(12):521–527. doi: 10.1016/0166-2236(93)90197-t. [DOI] [PubMed] [Google Scholar]
- Marshall J., Molloy R., Moss G. W., Howe J. R., Hughes T. E. The jellyfish green fluorescent protein: a new tool for studying ion channel expression and function. Neuron. 1995 Feb;14(2):211–215. doi: 10.1016/0896-6273(95)90279-1. [DOI] [PubMed] [Google Scholar]
- Mayer M. L., MacDermott A. B., Westbrook G. L., Smith S. J., Barker J. L. Agonist- and voltage-gated calcium entry in cultured mouse spinal cord neurons under voltage clamp measured using arsenazo III. J Neurosci. 1987 Oct;7(10):3230–3244. doi: 10.1523/JNEUROSCI.07-10-03230.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L. Permeation and block of N-methyl-D-aspartic acid receptor channels by divalent cations in mouse cultured central neurones. J Physiol. 1987 Dec;394:501–527. doi: 10.1113/jphysiol.1987.sp016883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monyer H., Burnashev N., Laurie D. J., Sakmann B., Seeburg P. H. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994 Mar;12(3):529–540. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- Monyer H., Sprengel R., Schoepfer R., Herb A., Higuchi M., Lomeli H., Burnashev N., Sakmann B., Seeburg P. H. Heteromeric NMDA receptors: molecular and functional distinction of subtypes. Science. 1992 May 22;256(5060):1217–1221. doi: 10.1126/science.256.5060.1217. [DOI] [PubMed] [Google Scholar]
- Moriyoshi K., Masu M., Ishii T., Shigemoto R., Mizuno N., Nakanishi S. Molecular cloning and characterization of the rat NMDA receptor. Nature. 1991 Nov 7;354(6348):31–37. doi: 10.1038/354031a0. [DOI] [PubMed] [Google Scholar]
- Nakazawa K., Hess P. Block by calcium of ATP-activated channels in pheochromocytoma cells. J Gen Physiol. 1993 Mar;101(3):377–392. doi: 10.1085/jgp.101.3.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E., Augustine G. J. Calcium gradients and buffers in bovine chromaffin cells. J Physiol. 1992 May;450:273–301. doi: 10.1113/jphysiol.1992.sp019127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E. Correction for liquid junction potentials in patch clamp experiments. Methods Enzymol. 1992;207:123–131. doi: 10.1016/0076-6879(92)07008-c. [DOI] [PubMed] [Google Scholar]
- Neher E. The use of fura-2 for estimating Ca buffers and Ca fluxes. Neuropharmacology. 1995 Nov;34(11):1423–1442. doi: 10.1016/0028-3908(95)00144-u. [DOI] [PubMed] [Google Scholar]
- Poenie M. Alteration of intracellular Fura-2 fluorescence by viscosity: a simple correction. Cell Calcium. 1990 Feb-Mar;11(2-3):85–91. doi: 10.1016/0143-4160(90)90062-y. [DOI] [PubMed] [Google Scholar]
- Rogers M., Dani J. A. Comparison of quantitative calcium flux through NMDA, ATP, and ACh receptor channels. Biophys J. 1995 Feb;68(2):501–506. doi: 10.1016/S0006-3495(95)80211-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneggenburger R., Zhou Z., Konnerth A., Neher E. Fractional contribution of calcium to the cation current through glutamate receptor channels. Neuron. 1993 Jul;11(1):133–143. doi: 10.1016/0896-6273(93)90277-x. [DOI] [PubMed] [Google Scholar]
- Spruston N., Jonas P., Sakmann B. Dendritic glutamate receptor channels in rat hippocampal CA3 and CA1 pyramidal neurons. J Physiol. 1995 Jan 15;482(Pt 2):325–352. doi: 10.1113/jphysiol.1995.sp020521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernino S., Rogers M., Radcliffe K. A., Dani J. A. Quantitative measurement of calcium flux through muscle and neuronal nicotinic acetylcholine receptors. J Neurosci. 1994 Sep;14(9):5514–5524. doi: 10.1523/JNEUROSCI.14-09-05514.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villarroel A., Burnashev N., Sakmann B. Dimensions of the narrow portion of a recombinant NMDA receptor channel. Biophys J. 1995 Mar;68(3):866–875. doi: 10.1016/S0006-3495(95)80263-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M., Inoue Y., Sakimura K., Mishina M. Developmental changes in distribution of NMDA receptor channel subunit mRNAs. Neuroreport. 1992 Dec;3(12):1138–1140. doi: 10.1097/00001756-199212000-00027. [DOI] [PubMed] [Google Scholar]
- Zarei M. M., Dani J. A. Ionic permeability characteristics of the N-methyl-D-aspartate receptor channel. J Gen Physiol. 1994 Feb;103(2):231–248. doi: 10.1085/jgp.103.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z., Neher E. Calcium permeability of nicotinic acetylcholine receptor channels in bovine adrenal chromaffin cells. Pflugers Arch. 1993 Dec;425(5-6):511–517. doi: 10.1007/BF00374879. [DOI] [PubMed] [Google Scholar]