Abstract
The current model does not account adequately for the characteristics of miniature endplate currents (MEPCs). We do not understand their relatively slow rise, the shape of their rise, their variable and sometimes prolonged decay, and the correlation between amplitude and decay time. If we assume that ACh is released from the vesicle through a pore and that the vesicle enlarges as it takes on additional transmitter, the predictions are more like MEPCs. However, previous measurements showed that after quantal size was increased the vesicles in the terminal were not enlarged. This need not be a problem, because some of the ACh is added to vesicles positioned at the active zones, a process known as second-stage loading. By using the false transmitter precursor monoethylcholine we provide additional evidence for second-stage loading. The distribution of quantal sizes at the junction usually does not follow a normal probability distribution; it is skewed to the right. The skew can be accounted for by a model incorporating second-stage loading in which the vesicles are released randomly, without regard to their ACh content. If the vesicles increase in size when they contain more transmitter, only vesicles at the active zone need swell.
Full text
PDF

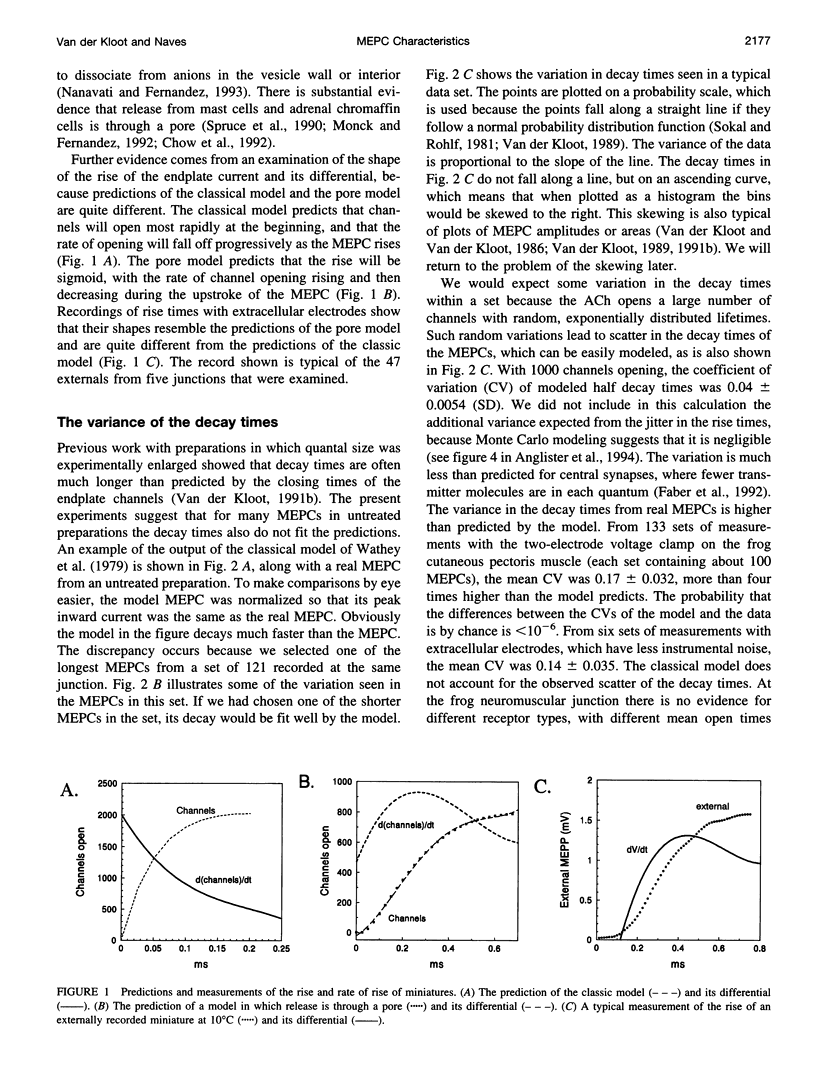
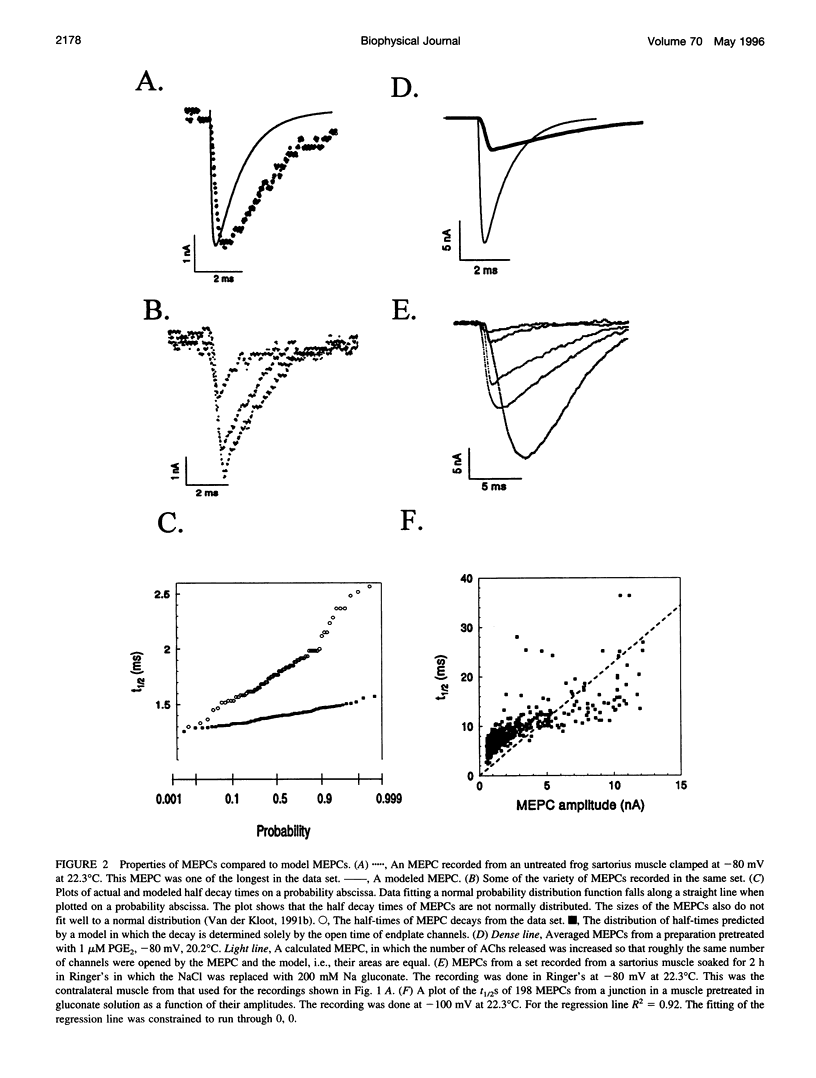
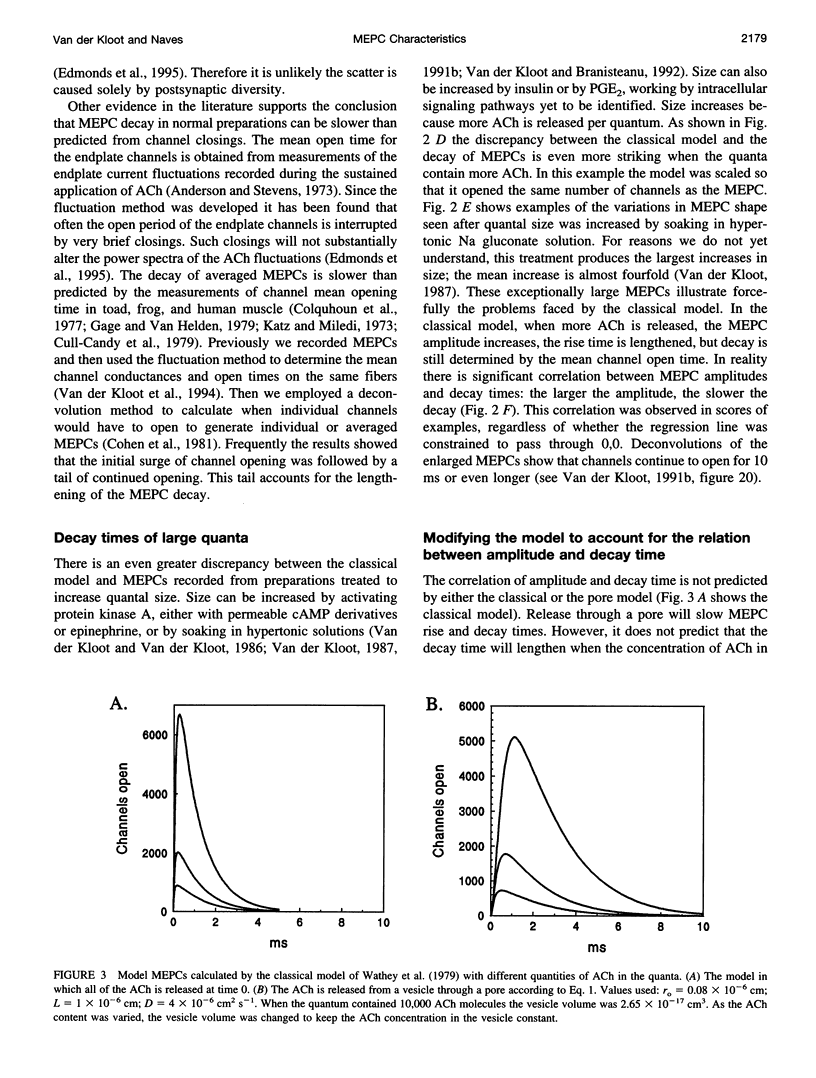

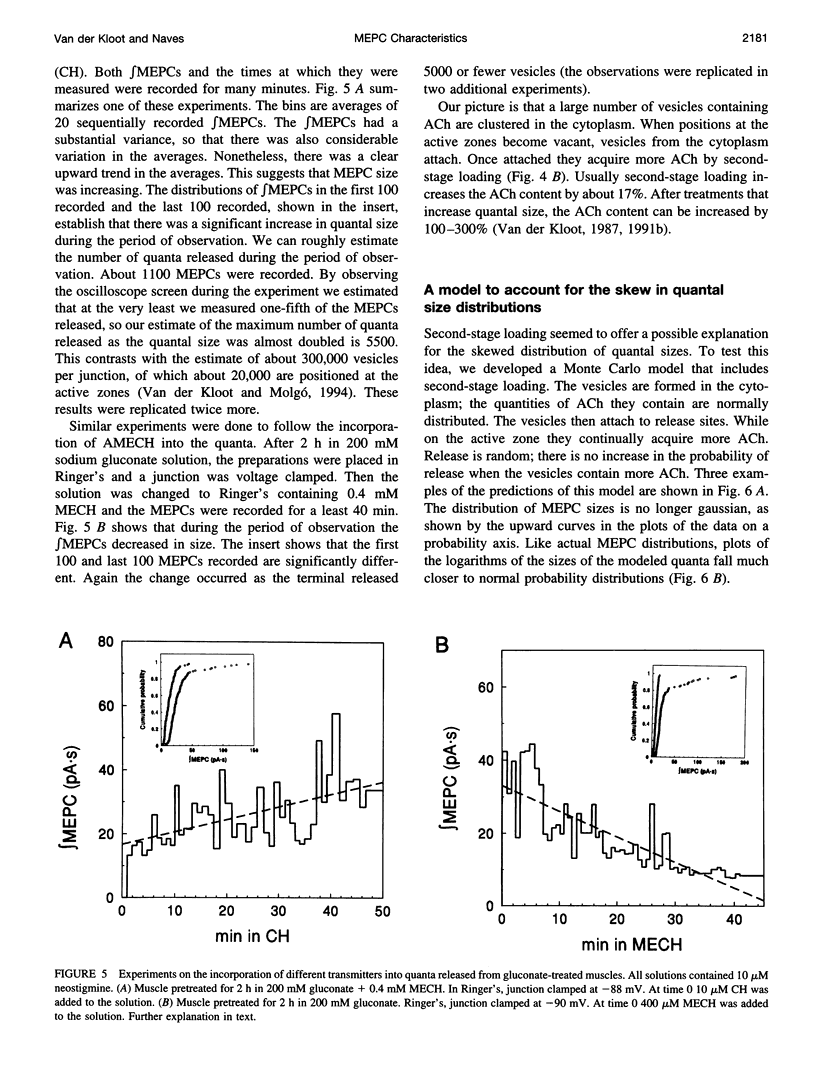


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almers W., Breckenridge L. J., Iwata A., Lee A. K., Spruce A. E., Tse F. W. Millisecond studies of single membrane fusion events. Ann N Y Acad Sci. 1991;635:318–327. doi: 10.1111/j.1749-6632.1991.tb36502.x. [DOI] [PubMed] [Google Scholar]
- Anderson C. R., Stevens C. F. Voltage clamp analysis of acetylcholine produced end-plate current fluctuations at frog neuromuscular junction. J Physiol. 1973 Dec;235(3):655–691. doi: 10.1113/jphysiol.1973.sp010410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson D. C., King S. C., Parsons S. M. Inhibition of [3H]acetylcholine active transport by tetraphenylborate and other anions. Mol Pharmacol. 1983 Jul;24(1):55–59. [PubMed] [Google Scholar]
- Anglister L., Stiles J. R., Salpeter M. M. Acetylcholinesterase density and turnover number at frog neuromuscular junctions, with modeling of their role in synaptic function. Neuron. 1994 Apr;12(4):783–794. doi: 10.1016/0896-6273(94)90331-x. [DOI] [PubMed] [Google Scholar]
- Bartol T. M., Jr, Land B. R., Salpeter E. E., Salpeter M. M. Monte Carlo simulation of miniature endplate current generation in the vertebrate neuromuscular junction. Biophys J. 1991 Jun;59(6):1290–1307. doi: 10.1016/S0006-3495(91)82344-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekkers J. M., Richerson G. B., Stevens C. F. Origin of variability in quantal size in cultured hippocampal neurons and hippocampal slices. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5359–5362. doi: 10.1073/pnas.87.14.5359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow R. H., von Rüden L., Neher E. Delay in vesicle fusion revealed by electrochemical monitoring of single secretory events in adrenal chromaffin cells. Nature. 1992 Mar 5;356(6364):60–63. doi: 10.1038/356060a0. [DOI] [PubMed] [Google Scholar]
- Cohen I., van der Kloot W., Attwell D. The timing of channel opening during miniature end-plate currents. Brain Res. 1981 Oct 26;223(1):185–189. doi: 10.1016/0006-8993(81)90821-0. [DOI] [PubMed] [Google Scholar]
- Colasante C., Pécot-Dechavassine M. Cd(2+)-and K(+)-evoked ACh release induce different synaptophysin and synaptobrevin immunolabelling at the frog neuromuscular junction. J Neurocytol. 1995 Aug;24(8):547–558. doi: 10.1007/BF01257371. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Large W. A., Rang H. P. An analysis of the action of a false transmitter at the neuromuscular junction. J Physiol. 1977 Apr;266(2):361–395. doi: 10.1113/jphysiol.1977.sp011772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper R. L., Stewart B. A., Wojtowicz J. M., Wang S., Atwood H. L. Quantal measurement and analysis methods compared for crayfish and Drosophila neuromuscular junctions, and rat hippocampus. J Neurosci Methods. 1995 Sep-Oct;61(1-2):67–78. doi: 10.1016/0165-0270(95)00024-o. [DOI] [PubMed] [Google Scholar]
- Cull-Candy S. G., Miledi R., Trautmann A. End-plate currents and acetylcholine noise at normal and myasthenic human end-plates. J Physiol. 1979 Feb;287:247–265. doi: 10.1113/jphysiol.1979.sp012657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty P., Hawgood B. J., Smith I. C. Changes in miniature end-plate potentials after brief nervous stimulation at the frog neuromuscular junction. J Physiol. 1984 Nov;356:349–358. doi: 10.1113/jphysiol.1984.sp015469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty P., Hawgood B. J., Smith I. C. Changes in miniature end-plate potentials due to moderate hypertonicity at the frog neuromuscular junction. J Physiol. 1986 Jul;376:1–11. doi: 10.1113/jphysiol.1986.sp016138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer T. M. The rising phase of the miniature endplate current at the frog neuromuscular junction. Biochim Biophys Acta. 1981 Aug 6;646(1):51–60. doi: 10.1016/0005-2736(81)90271-6. [DOI] [PubMed] [Google Scholar]
- Edmonds B., Gibb A. J., Colquhoun D. Mechanisms of activation of muscle nicotinic acetylcholine receptors and the time course of endplate currents. Annu Rev Physiol. 1995;57:469–493. doi: 10.1146/annurev.ph.57.030195.002345. [DOI] [PubMed] [Google Scholar]
- Edwards F. A. Patch-clamping in brain slices: synaptic transmission from ATP to long-term potentiation. J Neurosci Methods. 1995 Jun;59(1):59–65. doi: 10.1016/0165-0270(94)00194-l. [DOI] [PubMed] [Google Scholar]
- Faber D. S., Young W. S., Legendre P., Korn H. Intrinsic quantal variability due to stochastic properties of receptor-transmitter interactions. Science. 1992 Nov 27;258(5087):1494–1498. doi: 10.1126/science.1279813. [DOI] [PubMed] [Google Scholar]
- Gage P. W., Van Helden D. Effects of permeant monovalent cations on end-plate channels. J Physiol. 1979 Mar;288:509–528. [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. The binding of acetylcholine to receptors and its removal from the synaptic cleft. J Physiol. 1973 Jun;231(3):549–574. doi: 10.1113/jphysiol.1973.sp010248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Large W. A., Rang H. P. Factors affecting the rate of incorporation of a false transmitter into mammalian motor nerve terminals. J Physiol. 1978 Dec;285:1–24. doi: 10.1113/jphysiol.1978.sp012553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen B. W., Edeson R. O., Lam H. S., Milne R. K. Numerical simulation of miniature endplate currents. Neurosci Lett. 1984 Jul 13;48(1):67–74. doi: 10.1016/0304-3940(84)90290-8. [DOI] [PubMed] [Google Scholar]
- Magleby K. L., Stevens C. F. A quantitative description of end-plate currents. J Physiol. 1972 May;223(1):173–197. doi: 10.1113/jphysiol.1972.sp009840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monck J. R., Fernandez J. M. The exocytotic fusion pore. J Cell Biol. 1992 Dec;119(6):1395–1404. doi: 10.1083/jcb.119.6.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanavati C., Fernandez J. M. The secretory granule matrix: a fast-acting smart polymer. Science. 1993 Feb 12;259(5097):963–965. doi: 10.1126/science.8438154. [DOI] [PubMed] [Google Scholar]
- Nigmatullin N. R., Snetkov V. A., Nikol'skii E. E., Magazanik L. G. Analiz modeli miniatiurnogo toka kontsevoi plastinki. Neirofiziologiia. 1988;20(3):390–398. [PubMed] [Google Scholar]
- Parnas H., Flashner M., Spira M. E. Sequential model to describe the nicotinic synaptic current. Biophys J. 1989 May;55(5):875–884. doi: 10.1016/S0006-3495(89)82886-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prior C., Marshall I. G., Parsons S. M. The pharmacology of vesamicol: an inhibitor of the vesicular acetylcholine transporter. Gen Pharmacol. 1992 Nov;23(6):1017–1022. doi: 10.1016/0306-3623(92)90280-w. [DOI] [PubMed] [Google Scholar]
- Rosenberry T. L. Quantitative simulation of endplate currents at neuromuscular junctions based on the reaction of acetylcholine with acetylcholine receptor and acetylcholinesterase. Biophys J. 1979 May;26(2):263–289. doi: 10.1016/S0006-3495(79)85249-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spruce A. E., Breckenridge L. J., Lee A. K., Almers W. Properties of the fusion pore that forms during exocytosis of a mast cell secretory vesicle. Neuron. 1990 May;4(5):643–654. doi: 10.1016/0896-6273(90)90192-i. [DOI] [PubMed] [Google Scholar]
- Tauc L. Non vesicular release of neurotransmitter. Physiol Rev. 1982 Jul;62(3):857–893. doi: 10.1152/physrev.1982.62.3.857. [DOI] [PubMed] [Google Scholar]
- Van der Kloot W., Balezina O. P., Molgó J., Naves L. A. The timing of channel opening during miniature endplate currents at the frog and mouse neuromuscular junctions: effects of fasciculin-2, other anti-cholinesterases and vesamicol. Pflugers Arch. 1994 Sep;428(2):114–126. doi: 10.1007/BF00374848. [DOI] [PubMed] [Google Scholar]
- Van der Kloot W., Brănişteanu D. D. Effects of activators and inhibitors of protein kinase A on increases in quantal size at the frog neuromuscular junction. Pflugers Arch. 1992 Mar;420(3-4):336–341. doi: 10.1007/BF00374467. [DOI] [PubMed] [Google Scholar]
- Van der Kloot W. Down-regulation of quantal size at frog neuromuscular junctions: possible roles for elevated intracellular calcium and for protein kinase C. J Neurobiol. 1991 Mar;22(2):204–214. doi: 10.1002/neu.480220210. [DOI] [PubMed] [Google Scholar]
- Van der Kloot W., Molgó J. Quantal acetylcholine release at the vertebrate neuromuscular junction. Physiol Rev. 1994 Oct;74(4):899–991. doi: 10.1152/physrev.1994.74.4.899. [DOI] [PubMed] [Google Scholar]
- Van der Kloot W. Nicotinic agonists antagonize quantal size increases and evoked release at frog neuromuscular junction. J Physiol. 1993 Aug;468:567–589. doi: 10.1113/jphysiol.1993.sp019789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Kloot W. Pretreatment with hypertonic solutions increases quantal size at the frog neuromuscular junction. J Neurophysiol. 1987 May;57(5):1536–1554. doi: 10.1152/jn.1987.57.5.1536. [DOI] [PubMed] [Google Scholar]
- Van der Kloot W. Spontaneous and uniquantal-evoked endplate currents in normal frogs are indistinguishable. J Physiol. 1996 Apr 1;492(Pt 1):155–162. doi: 10.1113/jphysiol.1996.sp021297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Kloot W. Statistical and graphical methods for testing the hypothesis that quanta are made up of subunits. J Neurosci Methods. 1989 Feb;27(1):81–89. doi: 10.1016/0165-0270(89)90054-x. [DOI] [PubMed] [Google Scholar]
- Van der Kloot W. The regulation of quantal size. Prog Neurobiol. 1991;36(2):93–130. doi: 10.1016/0301-0082(91)90019-w. [DOI] [PubMed] [Google Scholar]
- Van der Kloot W. The rise times of miniature endplate currents suggest that acetylcholine may be released over a period of time. Biophys J. 1995 Jul;69(1):148–154. doi: 10.1016/S0006-3495(95)79884-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walmsley B. Interpretation of 'quantal' peaks in distributions of evoked synaptic transmission at central synapses. Proc Biol Sci. 1995 Aug 22;261(1361):245–250. doi: 10.1098/rspb.1995.0144. [DOI] [PubMed] [Google Scholar]
- Wathey J. C., Nass M. M., Lester H. A. Numerical reconstruction of the quantal event at nicotinic synapses. Biophys J. 1979 Jul;27(1):145–164. doi: 10.1016/S0006-3495(79)85208-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu S. P., Van der Kloot W. Increasing quantal size at the mouse neuromuscular junction and the role of choline. J Physiol. 1991 Feb;433:677–704. doi: 10.1113/jphysiol.1991.sp018450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Kloot W., van der Kloot T. E. Catecholamines, insulin and ACTH increase quantal size at the frog neuromuscular junction. Brain Res. 1986 Jun 25;376(2):378–381. doi: 10.1016/0006-8993(86)90203-9. [DOI] [PubMed] [Google Scholar]


