Abstract
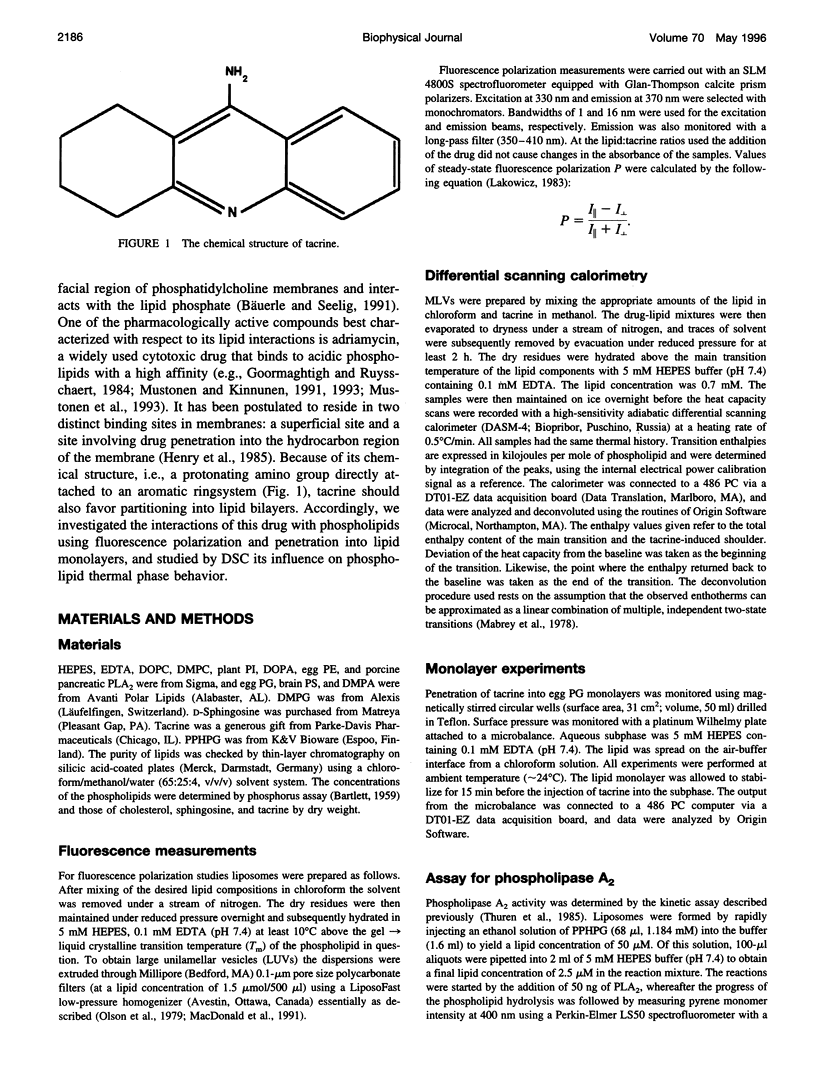
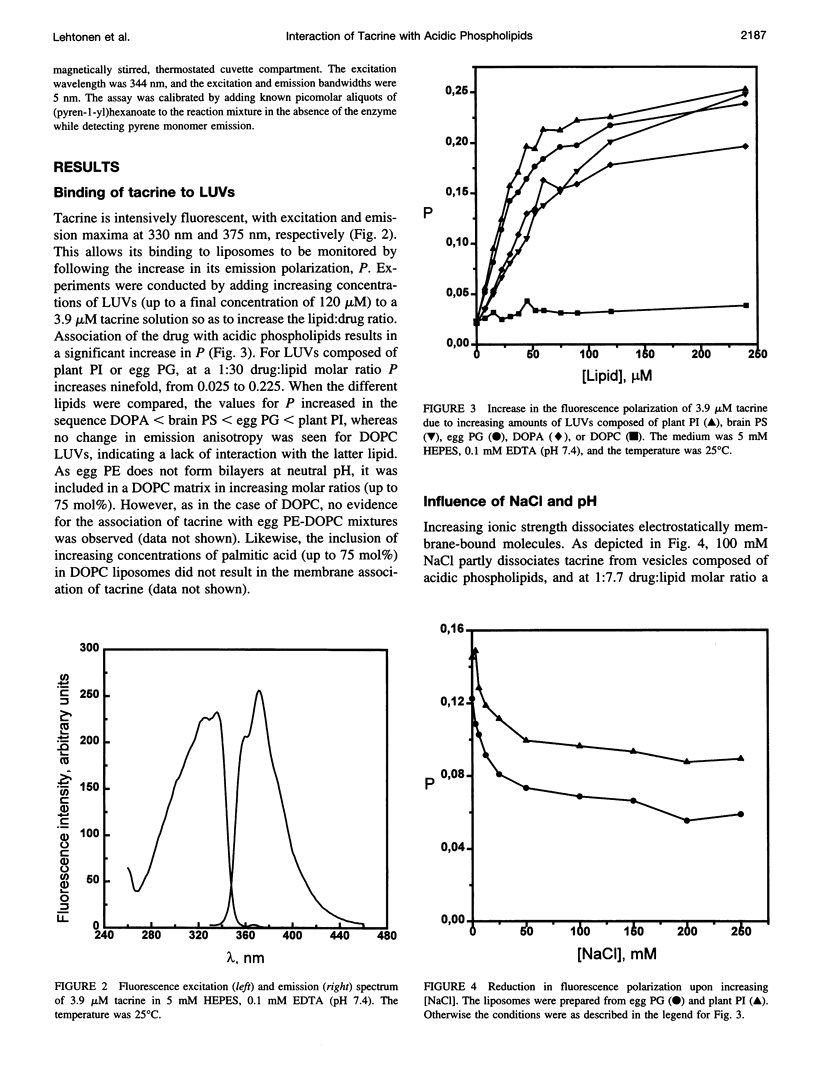
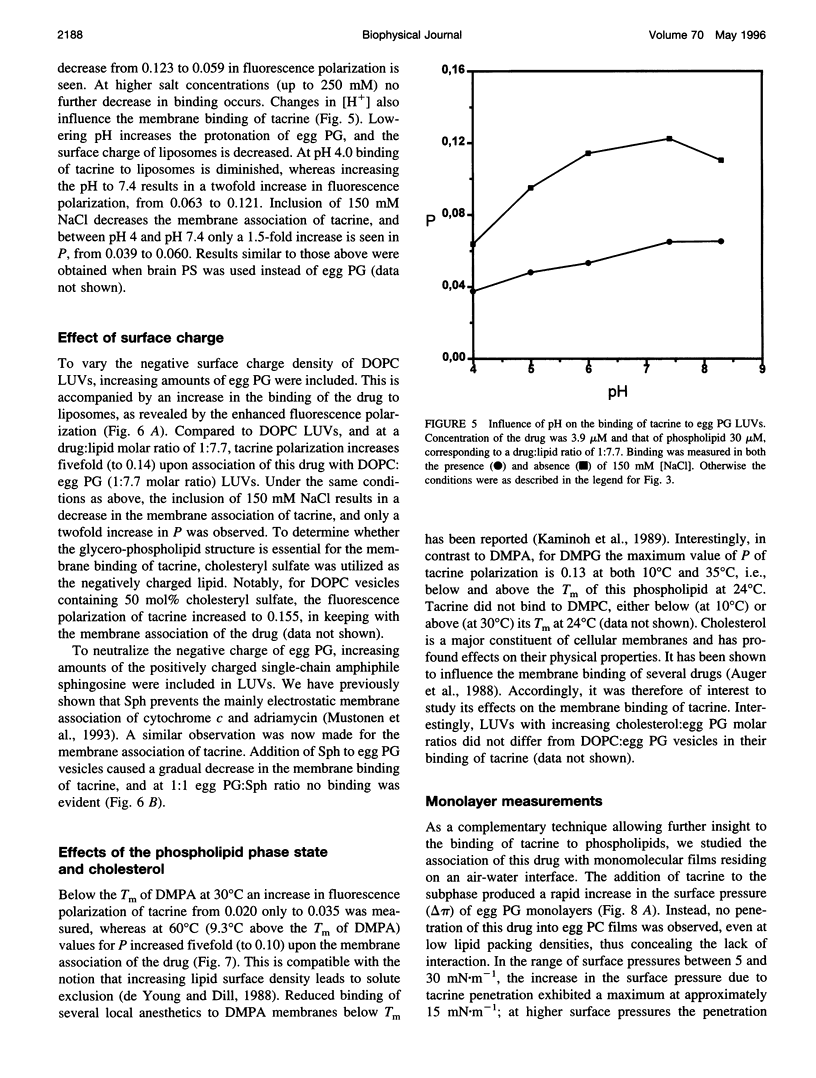
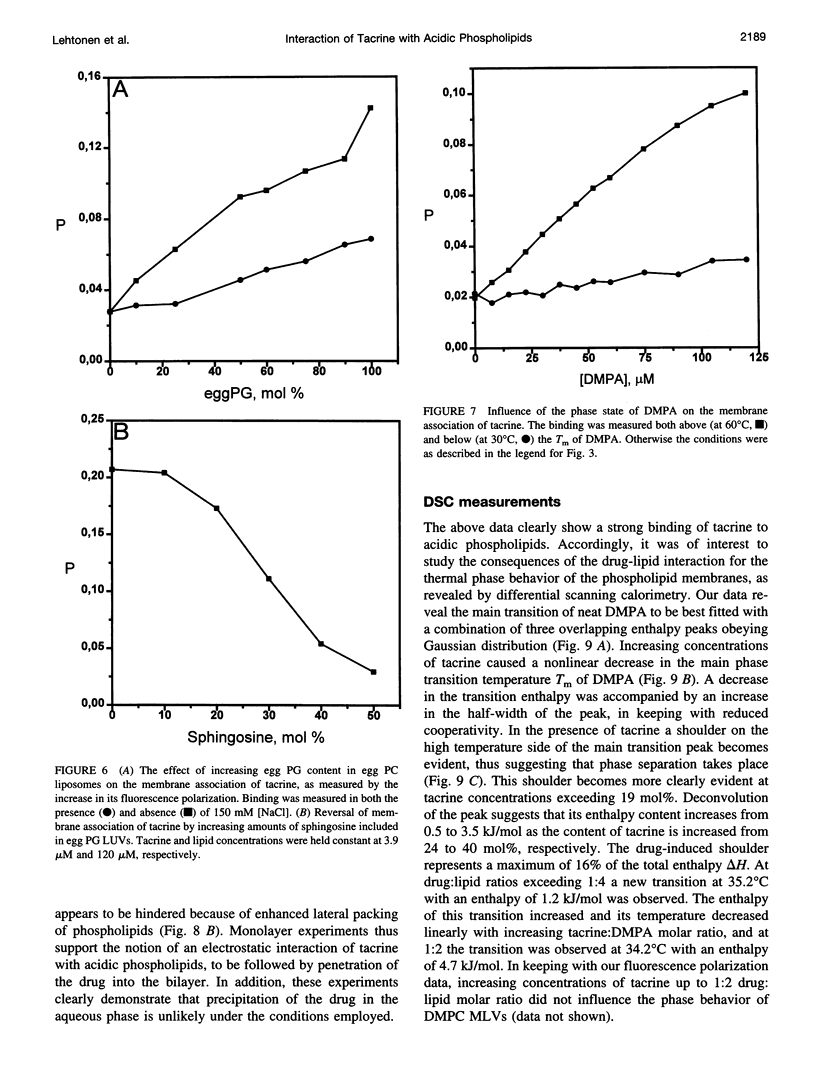
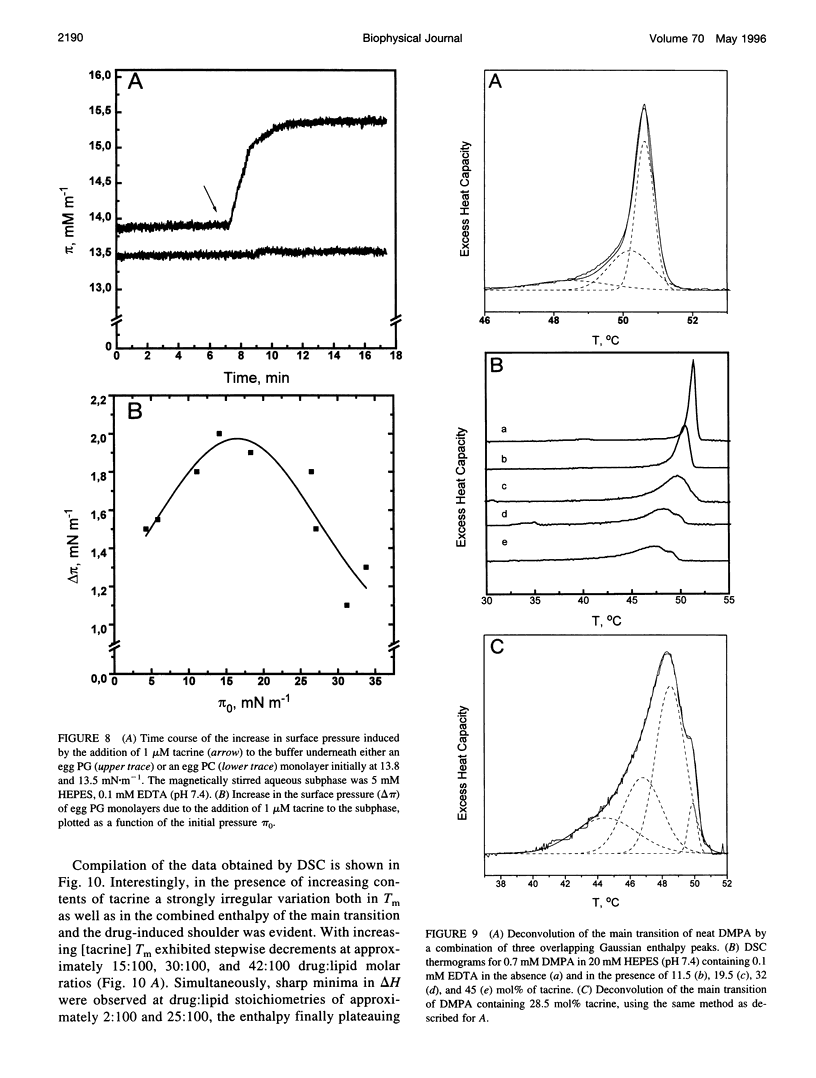
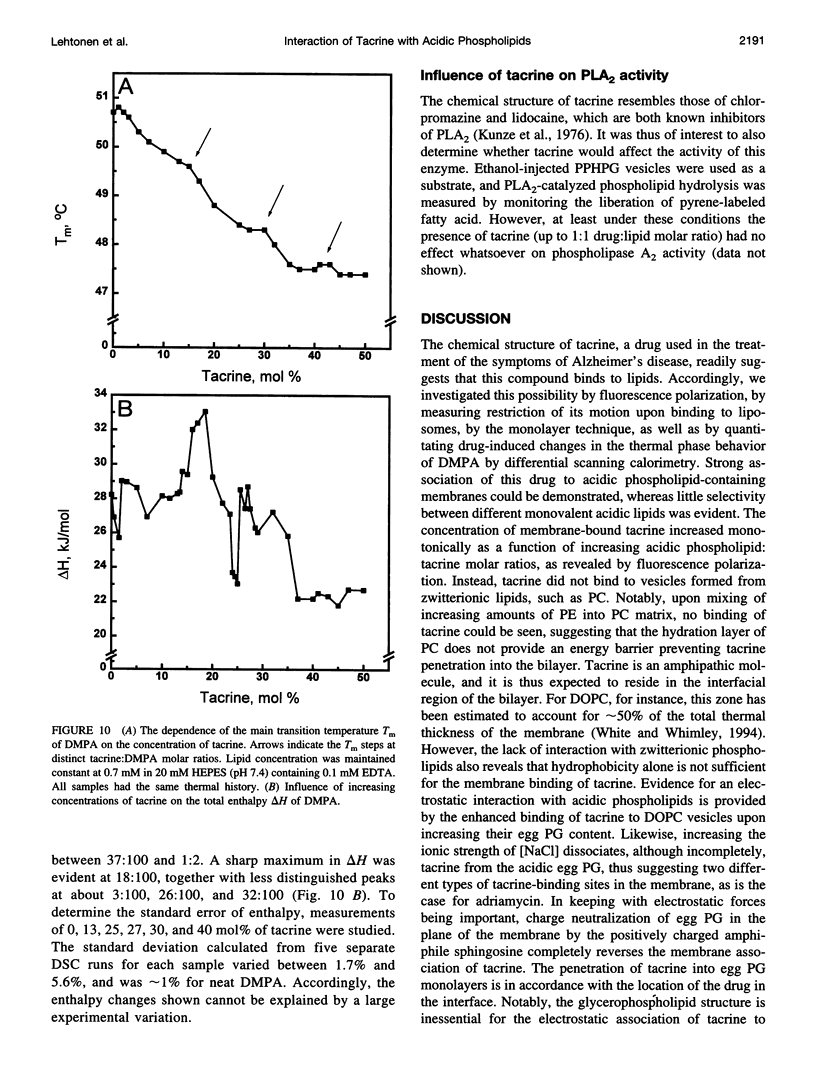
Tacrine (1,2,3,4-tetrahydro-9-acridinamine monohydrate) is an inhibitor of acetylcholinesterase currently used in the treatment of the symptoms of Alzheimer's disease. The present study demonstrates preferential binding of this drug to acidic phospholipids, as revealed by fluorescence polarization, penetration into lipid monolayers, and effects on the thermal phase behavior of dimyristoyl phosphatidic acid (DMPA). A fivefold enhancement in the polarization of tacrine emission is evident above the main phase transition temperature (T(m)) of DMPA vesicles, whereas below T(m) only a 0.75-fold increase is observed. In contrast, the binding of tacrine to another acidic phospholipid, dimyristoylphosphatidylglycerol, did not exhibit strong dependence on T(m). In accordance with the electrostatic nature of the membrane association of tacrine, the extent of binding was augmented with increasing contents of egg PG in phosphatidylcholine liposomes. Furthermore, [NaCl] > 50 mM dissociates tacrine (albeit incompletely) from the liposomes composed of acidic phospholipids. Inclusion of the cationic amphiphile sphingosine in egg PG vesicles decreased the membrane association of tacrine until at 1:1 sphingosine: egg PG stoichiometry binding was no longer evident. Tacrine also penetrated into egg PG but not into egg PC monolayers. Together with broadening of the main transition and causing a shoulder on its high temperature side, the binding of tacrine to DMPA liposomes results in a concentration-dependent reduction both in the combined enthalpy delta H of the above overlapping endotherms and the main transition temperature T(m). Interestingly, these changes in the thermal phase behavior of DMPA as a function of the content of the drug in vesicles were strongly nonlinear. More specifically, upon increasing [tacrine], T(m) exhibited stepwise decrements. Simultaneously, sharp minima in delta H were observed at drug:lipid stoichiometries of approximately 2:100 and 25:100, whereas a sharp maximum in delta H was evident at 18:100. The above results are in keeping with tacrine causing phase separation processes in the bilayer and may also relate to microscopic drug-induced ordering processes within the membrane.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adem A. Putative mechanisms of action of tacrine in Alzheimer's disease. Acta Neurol Scand Suppl. 1992;139:69–74. doi: 10.1111/j.1600-0404.1992.tb04458.x. [DOI] [PubMed] [Google Scholar]
- Arispe N., Pollard H. B., Rojas E. Giant multilevel cation channels formed by Alzheimer disease amyloid beta-protein [A beta P-(1-40)] in bilayer membranes. Proc Natl Acad Sci U S A. 1993 Nov 15;90(22):10573–10577. doi: 10.1073/pnas.90.22.10573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auger M., Jarrell H. C., Smith I. C. Interactions of the local anesthetic tetracaine with membranes containing phosphatidylcholine and cholesterol: a 2H NMR study. Biochemistry. 1988 Jun 28;27(13):4660–4667. doi: 10.1021/bi00413a012. [DOI] [PubMed] [Google Scholar]
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Barthel D., Zschoernig O., Lange K., Lenk R., Arnold K. Interaction of electrically charged drug molecules with phospholipid membranes. Biochim Biophys Acta. 1988 Nov 22;945(2):361–366. doi: 10.1016/0005-2736(88)90498-1. [DOI] [PubMed] [Google Scholar]
- Berclaz T., Geoffroy M. Spin-labeling study of phosphatidylcholine-cardiolipin binary mixtures. Biochemistry. 1984 Aug 28;23(18):4033–4039. doi: 10.1021/bi00313a004. [DOI] [PubMed] [Google Scholar]
- Berclaz T., McConnell H. M. Phase Equilibria in binary mixtures of dimyristoylphosphatidylcholine and cardiolipin. Biochemistry. 1981 Nov 10;20(23):6635–6640. doi: 10.1021/bi00526a018. [DOI] [PubMed] [Google Scholar]
- Bäuerle H. D., Seelig J. Interaction of charged and uncharged calcium channel antagonists with phospholipid membranes. Binding equilibrium, binding enthalpy, and membrane location. Biochemistry. 1991 Jul 23;30(29):7203–7211. doi: 10.1021/bi00243a023. [DOI] [PubMed] [Google Scholar]
- Callaghan R., Stafford A., Epand R. M. Increased accumulation of drugs in a multidrug resistant cell line by alteration of membrane biophysical properties. Biochim Biophys Acta. 1993 Feb 17;1175(3):277–282. doi: 10.1016/0167-4889(93)90217-d. [DOI] [PubMed] [Google Scholar]
- Canaves J. M., Ferragut J. A., Gonzalez-Ros J. M. Verapamil prevents the effects of daunomycin on the thermotropic phase transition of model lipid bilayers. Biochem J. 1991 Oct 15;279(Pt 2):413–418. doi: 10.1042/bj2790413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong P. L. Evidence for regular distribution of sterols in liquid crystalline phosphatidylcholine bilayers. Proc Natl Acad Sci U S A. 1994 Oct 11;91(21):10069–10073. doi: 10.1073/pnas.91.21.10069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong P. L., Tang D., Sugar I. P. Exploration of physical principles underlying lipid regular distribution: effects of pressure, temperature, and radius of curvature on E/M dips in pyrene-labeled PC/DMPC binary mixtures. Biophys J. 1994 Jun;66(6):2029–2038. doi: 10.1016/S0006-3495(94)80996-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornea R. L., Thomas D. D. Effects of membrane thickness on the molecular dynamics and enzymatic activity of reconstituted Ca-ATPase. Biochemistry. 1994 Mar 15;33(10):2912–2920. doi: 10.1021/bi00176a022. [DOI] [PubMed] [Google Scholar]
- De Young L. R., Dill K. A. Solute partitioning into lipid bilayer membranes. Biochemistry. 1988 Jul 12;27(14):5281–5289. doi: 10.1021/bi00414a050. [DOI] [PubMed] [Google Scholar]
- Dell'Antone P., Bragadin M., Zatta P. Anticholinesterasic drugs: tacrine but not physostigmine, accumulates in acidic compartments of the cells. Biochim Biophys Acta. 1995 Apr 24;1270(2-3):137–141. doi: 10.1016/0925-4439(94)00079-6. [DOI] [PubMed] [Google Scholar]
- Eibl H., Woolley P. Electrostatic interactions at charged lipid membranes. Hydrogen bonds in lipid membrane surfaces. Biophys Chem. 1979 Nov;10(3-4):261–271. doi: 10.1016/0301-4622(79)85015-2. [DOI] [PubMed] [Google Scholar]
- Escriba P. V., Ferrer-Montiel A. V., Ferragut J. A., Gonzalez-Ros J. M. Role of membrane lipids in the interaction of daunomycin with plasma membranes from tumor cells: implications in drug-resistance phenomena. Biochemistry. 1990 Aug 7;29(31):7275–7282. doi: 10.1021/bi00483a017. [DOI] [PubMed] [Google Scholar]
- Goormaghtigh E., Ruysschaert J. M. Anthracycline glycoside-membrane interactions. Biochim Biophys Acta. 1984 Sep 3;779(3):271–288. doi: 10.1016/0304-4157(84)90013-3. [DOI] [PubMed] [Google Scholar]
- Henry N., Fantine E. O., Bolard J., Garnier-Suillerot A. Interaction of adriamycin with negatively charged model membranes: evidence of two types of binding sites. Biochemistry. 1985 Dec 3;24(25):7085–7092. doi: 10.1021/bi00346a010. [DOI] [PubMed] [Google Scholar]
- Herbette L. G., Chester D. W., Rhodes D. G. Structural analysis of drug molecules in biological membranes. Biophys J. 1986 Jan;49(1):91–94. doi: 10.1016/S0006-3495(86)83605-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbette L., Katz A. M., Sturtevant J. M. Comparisons of the interaction of propranolol and timolol with model and biological membrane systems. Mol Pharmacol. 1983 Sep;24(2):259–269. [PubMed] [Google Scholar]
- Ito S., Werth D. K., Richert N. D., Pastan I. Vinculin phosphorylation by the src kinase. Interaction of vinculin with phospholipid vesicles. J Biol Chem. 1983 Dec 10;258(23):14626–14631. [PubMed] [Google Scholar]
- Kaminoh Y., Kamaya H., Ueda I. Differential affinity of charged local anesthetics to solid-gel and liquid-crystalline states of dimyristoylphosphatidic acid vesicle membranes. Biochim Biophys Acta. 1989 Dec 11;987(1):63–68. doi: 10.1016/0005-2736(89)90455-0. [DOI] [PubMed] [Google Scholar]
- Kunze H., Nahas N., Traynor J. R., Wurl M. Effects of local anaesthetics on phospholipases. Biochim Biophys Acta. 1976 Jul 20;441(1):93–102. doi: 10.1016/0005-2760(76)90284-8. [DOI] [PubMed] [Google Scholar]
- Lieber M. R., Lange Y., Weinstein R. S., Steck T. L. Interaction of chlorpromazine with the human erythrocyte membrane. J Biol Chem. 1984 Jul 25;259(14):9225–9234. [PubMed] [Google Scholar]
- Luxnat M., Galla H. J. Partition of chlorpromazine into lipid bilayer membranes: the effect of membrane structure and composition. Biochim Biophys Acta. 1986 Apr 14;856(2):274–282. doi: 10.1016/0005-2736(86)90037-4. [DOI] [PubMed] [Google Scholar]
- Mabrey S., Mateo P. L., Sturtevant J. M. High-sensitivity scanning calorimetric study of mixtures of cholesterol with dimyristoyl- and dipalmitoylphosphatidylcholines. Biochemistry. 1978 Jun 13;17(12):2464–2468. doi: 10.1021/bi00605a034. [DOI] [PubMed] [Google Scholar]
- MacDonald R. C., MacDonald R. I., Menco B. P., Takeshita K., Subbarao N. K., Hu L. R. Small-volume extrusion apparatus for preparation of large, unilamellar vesicles. Biochim Biophys Acta. 1991 Jan 30;1061(2):297–303. doi: 10.1016/0005-2736(91)90295-j. [DOI] [PubMed] [Google Scholar]
- Marotta C. A., Majocha R. E., Tate B. Molecular and cellular biology of Alzheimer amyloid. J Mol Neurosci. 1992;3(3):111–125. doi: 10.1007/BF02919403. [DOI] [PubMed] [Google Scholar]
- Mustonen P., Kinnunen P. K. Activation of phospholipase A2 by adriamycin in vitro. Role of drug-lipid interactions. J Biol Chem. 1991 Apr 5;266(10):6302–6307. [PubMed] [Google Scholar]
- Mustonen P., Kinnunen P. K. On the reversal by deoxyribonucleic acid of the binding of adriamycin to cardiolipin-containing liposomes. J Biol Chem. 1993 Jan 15;268(2):1074–1080. [PubMed] [Google Scholar]
- Mustonen P., Lehtonen J., Kõiv A., Kinnunen P. K. Effects of sphingosine on peripheral membrane interactions: comparison of adriamycin, cytochrome c, and phospholipase A2. Biochemistry. 1993 May 25;32(20):5373–5380. doi: 10.1021/bi00071a012. [DOI] [PubMed] [Google Scholar]
- Mustonen P., Virtanen J. A., Somerharju P. J., Kinnunen P. K. Binding of cytochrome c to liposomes as revealed by the quenching of fluorescence from pyrene-labeled phospholipids. Biochemistry. 1987 Jun 2;26(11):2991–2997. doi: 10.1021/bi00385a006. [DOI] [PubMed] [Google Scholar]
- Olson F., Hunt C. A., Szoka F. C., Vail W. J., Papahadjopoulos D. Preparation of liposomes of defined size distribution by extrusion through polycarbonate membranes. Biochim Biophys Acta. 1979 Oct 19;557(1):9–23. doi: 10.1016/0005-2736(79)90085-3. [DOI] [PubMed] [Google Scholar]
- Recktenwald D. J., McConnell H. M. Phase equilibria in binary mixtures of phosphatidylcholine and cholesterol. Biochemistry. 1981 Jul 21;20(15):4505–4510. doi: 10.1021/bi00518a042. [DOI] [PubMed] [Google Scholar]
- Rhodes D. G., Newton R., Butler R., Herbette L. Equilibrium and kinetic studies of the interactions of salmeterol with membrane bilayers. Mol Pharmacol. 1992 Oct;42(4):596–602. [PubMed] [Google Scholar]
- Rhodes D. G., Sarmiento J. G., Herbette L. G. Kinetics of binding of membrane-active drugs to receptor sites. Diffusion-limited rates for a membrane bilayer approach of 1,4-dihydropyridine calcium channel antagonists to their active site. Mol Pharmacol. 1985 Jun;27(6):612–623. [PubMed] [Google Scholar]
- Rubenstein J. L., Owicki J. C., McConnell H. M. Dynamic properties of binary mixtures of phosphatidylcholines and cholesterol. Biochemistry. 1980 Feb 5;19(3):569–573. doi: 10.1021/bi00544a027. [DOI] [PubMed] [Google Scholar]
- Rubenstein J. L., Smith B. A., McConnell H. M. Lateral diffusion in binary mixtures of cholesterol and phosphatidylcholines. Proc Natl Acad Sci U S A. 1979 Jan;76(1):15–18. doi: 10.1073/pnas.76.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent D. F., Schwyzer R. Membrane lipid phase as catalyst for peptide-receptor interactions. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5774–5778. doi: 10.1073/pnas.83.16.5774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seelig A., Allegrini P. R., Seelig J. Partitioning of local anesthetics into membranes: surface charge effects monitored by the phospholipid head-group. Biochim Biophys Acta. 1988 Apr 7;939(2):267–276. doi: 10.1016/0005-2736(88)90070-3. [DOI] [PubMed] [Google Scholar]
- Seelig J., Ganz P. Nonclassical hydrophobic effect in membrane binding equilibria. Biochemistry. 1991 Sep 24;30(38):9354–9359. doi: 10.1021/bi00102a031. [DOI] [PubMed] [Google Scholar]
- Siegfried J. A., Kennedy K. A., Sartorelli A. C., Tritton T. R. The role of membranes in the mechanism of action of the antineoplastic agent adriamycin. Spin-labeling studies with chronically hypoxic and drug-resistant tumor cells. J Biol Chem. 1983 Jan 10;258(1):339–343. [PubMed] [Google Scholar]
- Sisodia S. S., Price D. L. Role of the beta-amyloid protein in Alzheimer's disease. FASEB J. 1995 Mar;9(5):366–370. doi: 10.1096/fasebj.9.5.7896005. [DOI] [PubMed] [Google Scholar]
- Somerharju P. J., Virtanen J. A., Eklund K. K., Vainio P., Kinnunen P. K. 1-Palmitoyl-2-pyrenedecanoyl glycerophospholipids as membrane probes: evidence for regular distribution in liquid-crystalline phosphatidylcholine bilayers. Biochemistry. 1985 May 21;24(11):2773–2781. doi: 10.1021/bi00332a027. [DOI] [PubMed] [Google Scholar]
- Soreghan B., Kosmoski J., Glabe C. Surfactant properties of Alzheimer's A beta peptides and the mechanism of amyloid aggregation. J Biol Chem. 1994 Nov 18;269(46):28551–28554. [PubMed] [Google Scholar]
- Tang D., Chong P. L. E/M dips. Evidence for lipids regularly distributed into hexagonal super-lattices in pyrene-PC/DMPC binary mixtures at specific concentrations. Biophys J. 1992 Oct;63(4):903–910. doi: 10.1016/S0006-3495(92)81672-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang D., Wieb van der Meer B., Chen S. Y. Evidence for a regular distribution of cholesterol in phospholipid bilayers from diphenylhexatriene fluorescence. Biophys J. 1995 May;68(5):1944–1951. doi: 10.1016/S0006-3495(95)80371-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terzi E., Hölzemann G., Seelig J. Alzheimer beta-amyloid peptide 25-35: electrostatic interactions with phospholipid membranes. Biochemistry. 1994 Jun 14;33(23):7434–7441. doi: 10.1021/bi00189a051. [DOI] [PubMed] [Google Scholar]
- Thuren T., Virtanen J. A., Lalla M., Kinnunen P. K. Fluorometric assay for phospholipase A2 in serum. Clin Chem. 1985 May;31(5):714–717. [PubMed] [Google Scholar]
- Triton T. R., Yee G. The anticancer agent adriamycin can be actively cytotoxic without entering cells. Science. 1982 Jul 16;217(4556):248–250. doi: 10.1126/science.7089561. [DOI] [PubMed] [Google Scholar]
- Tsai Y. S., Ma S. M., Kamaya H., Ueda I. Fourier transform infrared studies on phospholipid hydration: phosphate-oriented hydrogen bonding and its attenuation by volatile anesthetics. Mol Pharmacol. 1987 Jun;31(6):623–630. [PubMed] [Google Scholar]
- Wadkins R. M., Houghton P. J. The role of drug-lipid interactions in the biological activity of modulators of multi-drug resistance. Biochim Biophys Acta. 1993 Dec 12;1153(2):225–236. doi: 10.1016/0005-2736(93)90409-s. [DOI] [PubMed] [Google Scholar]
- Wright S. E., White J. C. Membrane ordering effects of the anticancer agent VM-26. Biochim Biophys Acta. 1986 Dec 16;863(2):297–304. doi: 10.1016/0005-2736(86)90270-1. [DOI] [PubMed] [Google Scholar]
- Xiao W. B., Nordberg A., Zhang X. Effect of in vivo microdialysis of 1,2,3,4-tetrahydro-9-aminoacridine (THA) on the extracellular concentration of acetylcholine in the striatum of anesthetized rats. J Pharmacol Exp Ther. 1993 May;265(2):759–764. [PubMed] [Google Scholar]
- Yanagisawa K., Odaka A., Suzuki N., Ihara Y. GM1 ganglioside-bound amyloid beta-protein (A beta): a possible form of preamyloid in Alzheimer's disease. Nat Med. 1995 Oct;1(10):1062–1066. doi: 10.1038/nm1095-1062. [DOI] [PubMed] [Google Scholar]
- Young H. S., Skita V., Mason R. P., Herbette L. G. Molecular basis for the inhibition of 1,4-dihydropyridine calcium channel drugs binding to their receptors by a nonspecific site interaction mechanism. Biophys J. 1992 May;61(5):1244–1255. doi: 10.1016/S0006-3495(92)81933-1. [DOI] [PMC free article] [PubMed] [Google Scholar]


