Abstract
The intrinsic or spontaneous radius of curvature, R(o), of lipid monolayer assemblies is expressed in terms of a lipid molecular packing parameter, V/AI, for various geometries. It is shown that the equivalent lipid length, 1, in inverted hexagonal (HII) phases, defined by a cylindrical shell of equal total lipid volume, yields an expression for R o identical to that for inverted cylindrical micelles (or, equivalently, HII phases in the presence of excess hydrocarbon). This identity is used to obtain values of the effective packing parameter for various phosphatidylethanolamines. The temperature dependence of the intrinsic radius of curvature is predicted to be negative and to be considerably greater than that for the lipid length in nearly all cases. The thermal expansion coefficient is not constant but is found to vary, depending on the value of the lipid packing parameter. A possible addition rule is constructed for the intrinsic radius of curvature of lipid mixtures, based on the linear additivity of the effective molecular volumes, V, and molecular areas, A. This relation is found to hold for mixtures of dioleoyl phosphatidylcholine (DOPC) with dioleoyl phosphatidylethanolamine, and a value of R(o) of > or = 9 A (V/AI = 1.08) is obtained for DOPC. The energetics of the intrinsic curvature and lamellar-nonlamellar transitions are also discussed within the framework of the model.
Full text
PDF

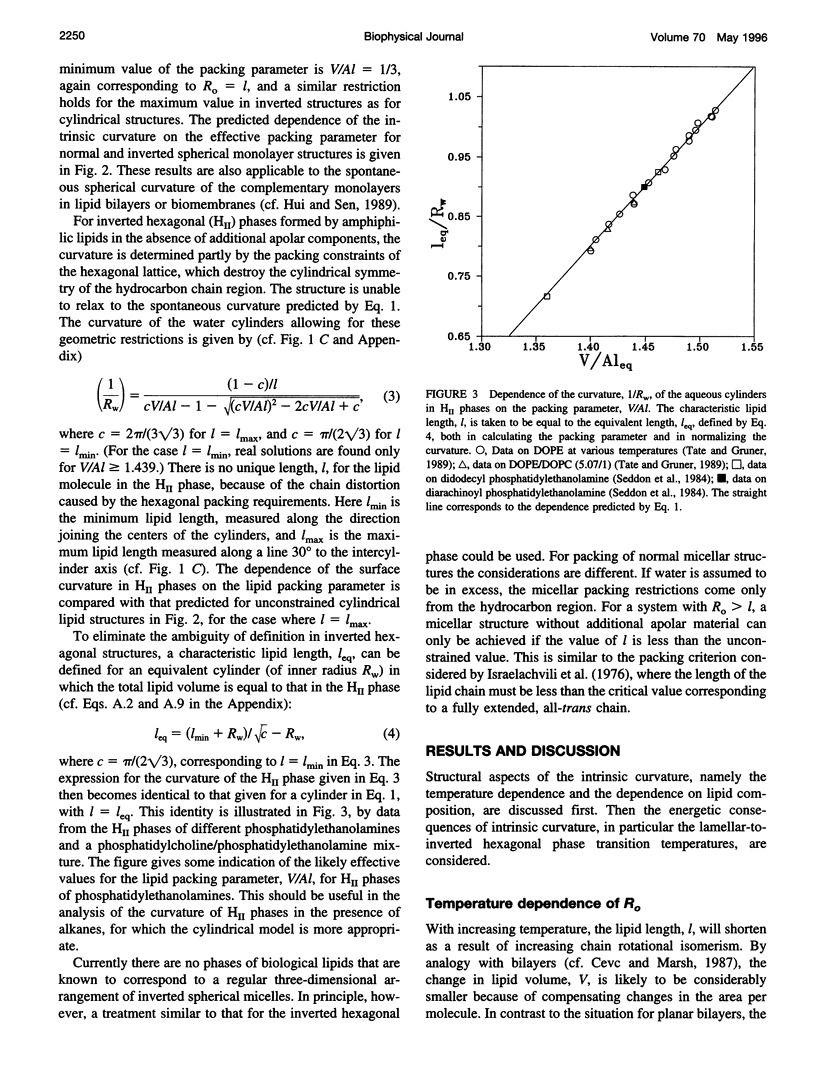


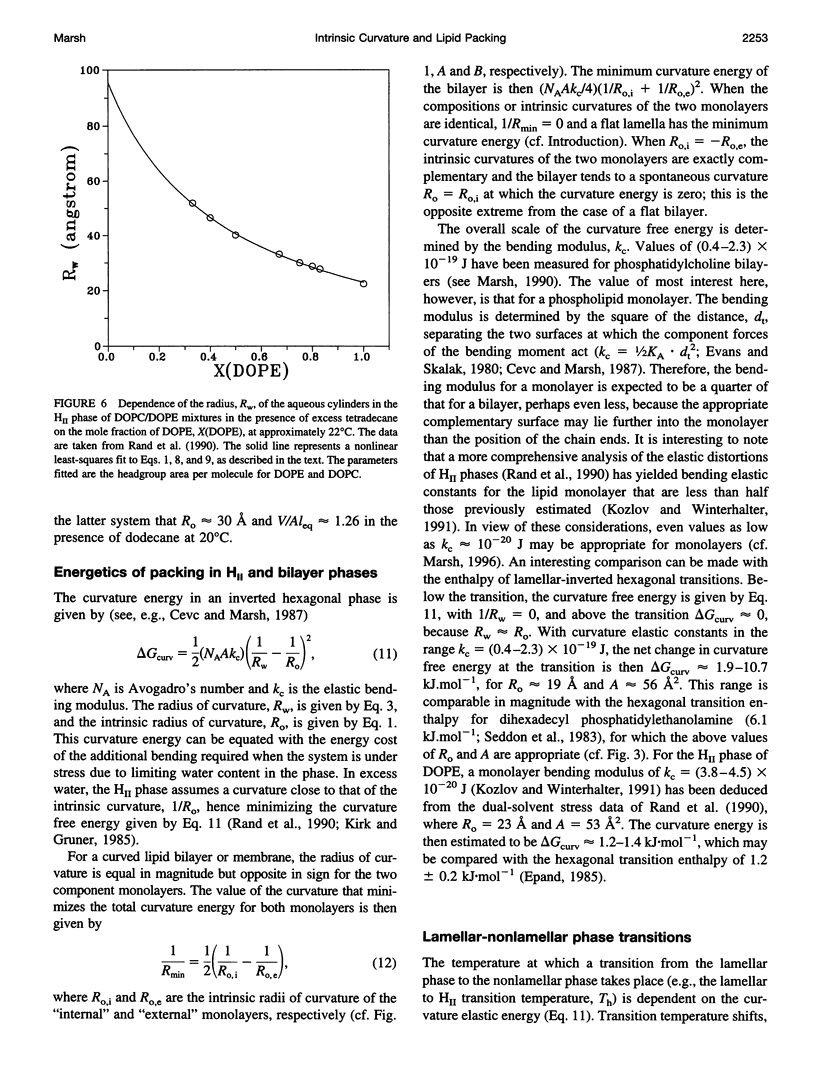


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Gruner S. M. Intrinsic curvature hypothesis for biomembrane lipid composition: a role for nonbilayer lipids. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3665–3669. doi: 10.1073/pnas.82.11.3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruner S. M., Tate M. W., Kirk G. L., So P. T., Turner D. C., Keane D. T., Tilcock C. P., Cullis P. R. X-ray diffraction study of the polymorphic behavior of N-methylated dioleoylphosphatidylethanolamine. Biochemistry. 1988 Apr 19;27(8):2853–2866. doi: 10.1021/bi00408a029. [DOI] [PubMed] [Google Scholar]
- Helfrich W. Blocked lipid exchange in bilayers and its possible influence on the shape of vesicles. Z Naturforsch C. 1974 Sep-Oct;29C(9-10):510–515. doi: 10.1515/znc-1974-9-1010. [DOI] [PubMed] [Google Scholar]
- Helfrich W. Elastic properties of lipid bilayers: theory and possible experiments. Z Naturforsch C. 1973 Nov-Dec;28(11):693–703. doi: 10.1515/znc-1973-11-1209. [DOI] [PubMed] [Google Scholar]
- Hui S. W., Sen A. Effects of lipid packing on polymorphic phase behavior and membrane properties. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5825–5829. doi: 10.1073/pnas.86.15.5825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller S. L., Bezrukov S. M., Gruner S. M., Tate M. W., Vodyanoy I., Parsegian V. A. Probability of alamethicin conductance states varies with nonlamellar tendency of bilayer phospholipids. Biophys J. 1993 Jul;65(1):23–27. doi: 10.1016/S0006-3495(93)81040-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh D. Analysis of the chainlength dependence of lipid phase transition temperatures: main and pretransitions of phosphatidylcholines; main and non-lamellar transitions of phosphatidylethanolamines. Biochim Biophys Acta. 1991 Feb 11;1062(1):1–6. doi: 10.1016/0005-2736(91)90326-4. [DOI] [PubMed] [Google Scholar]
- Marsh D. Components of the lateral pressure in lipid bilayers deduced from HII phase dimensions. Biochim Biophys Acta. 1996 Mar 13;1279(2):119–123. doi: 10.1016/0005-2736(95)00296-0. [DOI] [PubMed] [Google Scholar]
- Rand R. P., Fuller N. L., Gruner S. M., Parsegian V. A. Membrane curvature, lipid segregation, and structural transitions for phospholipids under dual-solvent stress. Biochemistry. 1990 Jan 9;29(1):76–87. doi: 10.1021/bi00453a010. [DOI] [PubMed] [Google Scholar]
- Seddon J. M., Cevc G., Kaye R. D., Marsh D. X-ray diffraction study of the polymorphism of hydrated diacyl- and dialkylphosphatidylethanolamines. Biochemistry. 1984 Jun 5;23(12):2634–2644. doi: 10.1021/bi00307a015. [DOI] [PubMed] [Google Scholar]
- Seddon J. M., Cevc G., Marsh D. Calorimetric studies of the gel-fluid (L beta-L alpha) and lamellar-inverted hexagonal (L alpha-HII) phase transitions in dialkyl- and diacylphosphatidylethanolamines. Biochemistry. 1983 Mar 1;22(5):1280–1289. doi: 10.1021/bi00274a045. [DOI] [PubMed] [Google Scholar]
- Tate M. W., Gruner S. M. Temperature dependence of the structural dimensions of the inverted hexagonal (HII) phase of phosphatidylethanolamine-containing membranes. Biochemistry. 1989 May 16;28(10):4245–4253. doi: 10.1021/bi00436a019. [DOI] [PubMed] [Google Scholar]




