Abstract
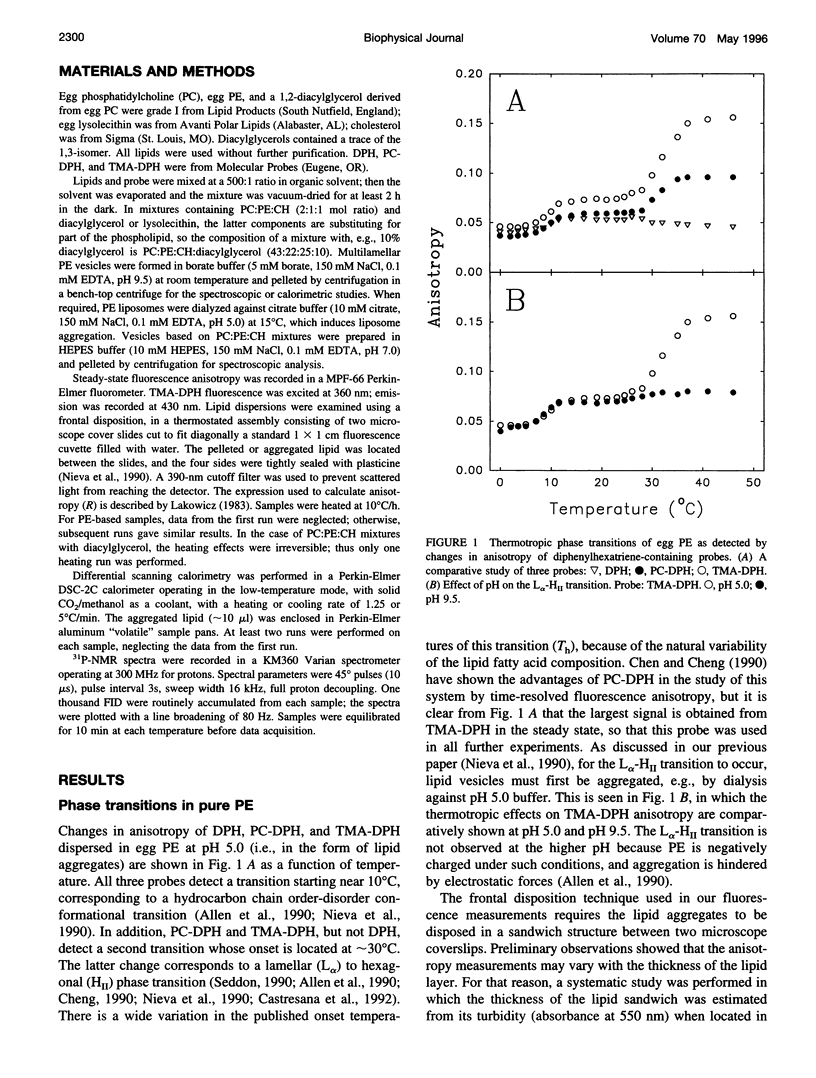
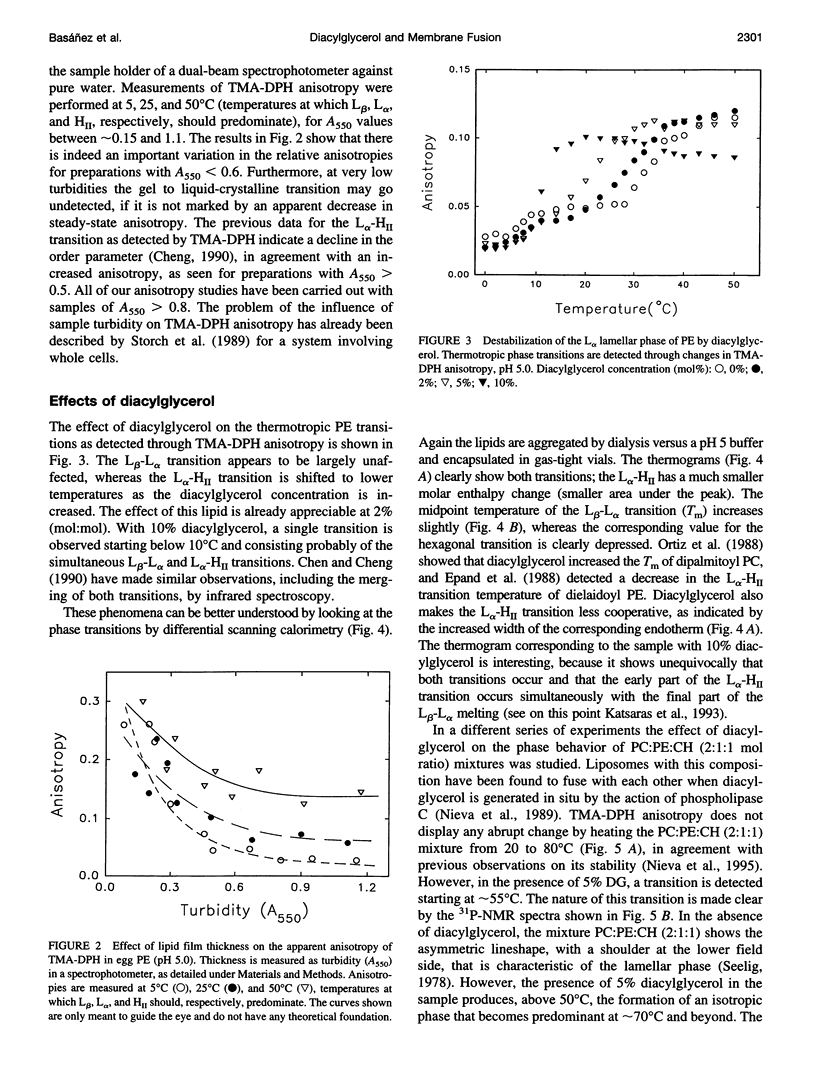
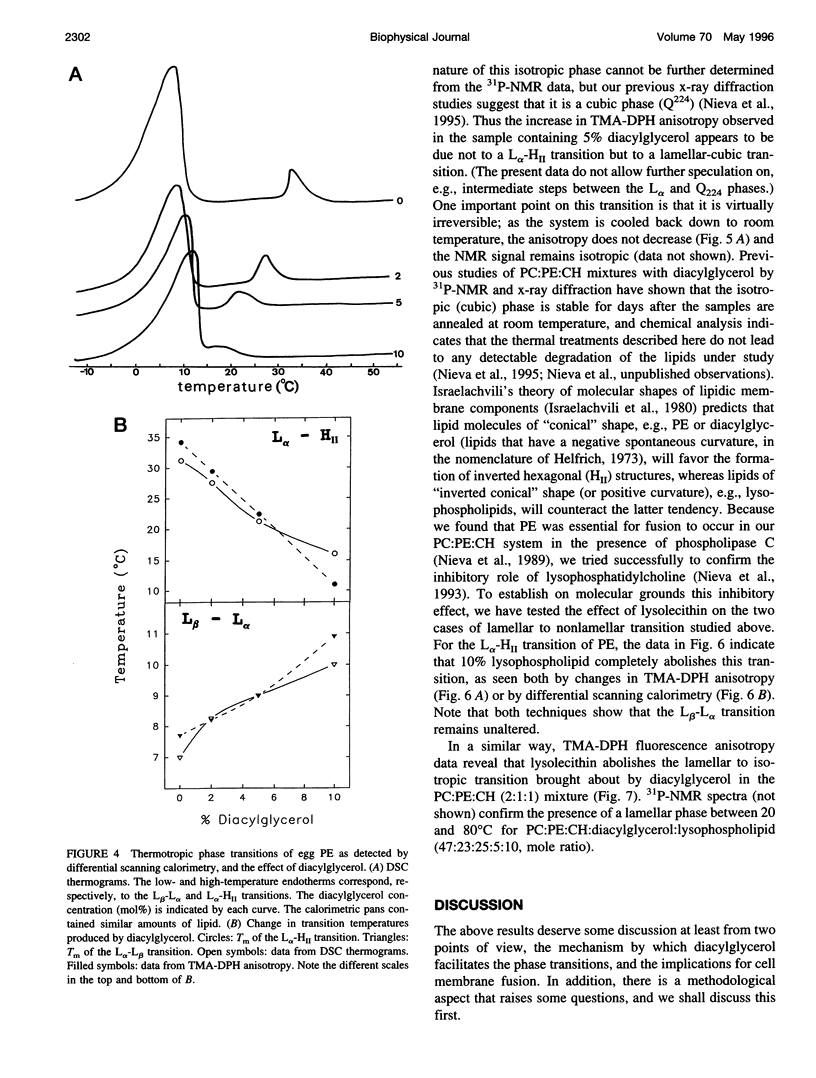
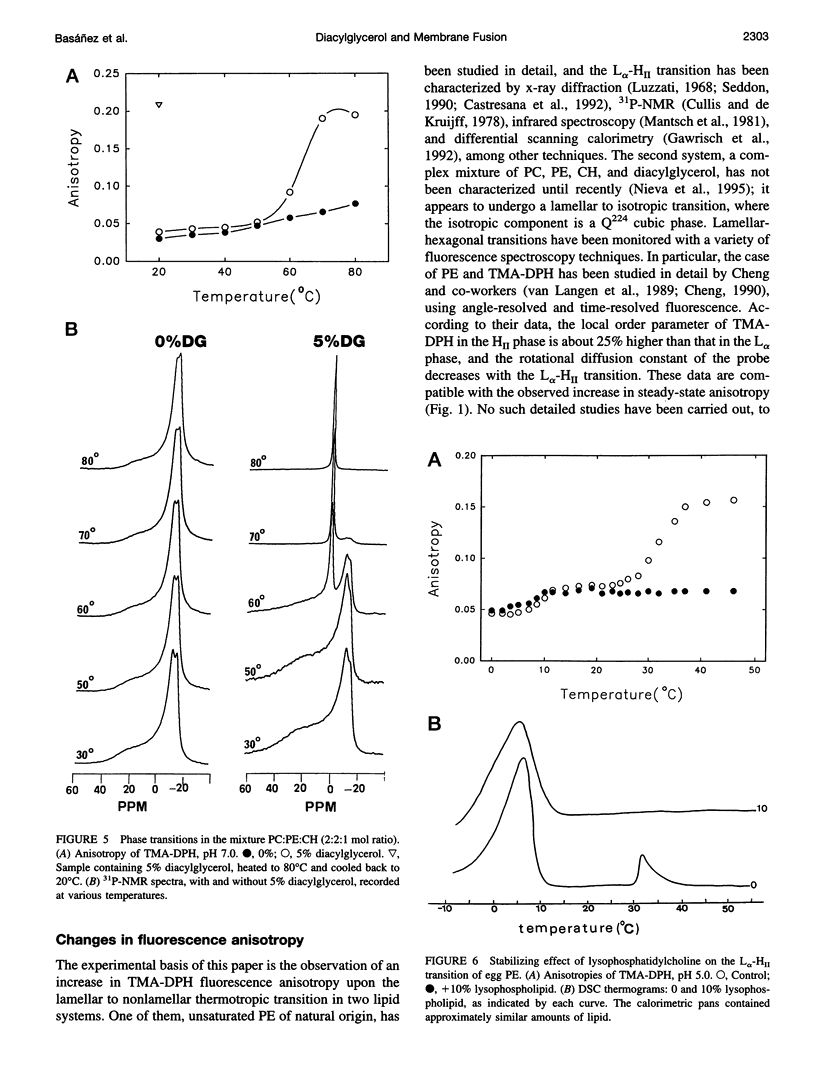
Changes in steady-state fluorescence anisotropy of 1 -(4-trimethylaminophenyl)-6-phenyl-1,3,5-hexatriene TMA-DPH) are applied to the detection of lamellar-hexagonal transitions in egg phosphatidylethanolamine. Even low (2 mole%) proportions of diacylglycerol decrease the hexagonal transition temperature considerably, as confirmed by differential scanning calorimetry. Diacylglycerol is also found to promote a lamellar to "isotropic" (Q(224) cubic) transition in mixtures of phosphatidylcholine: phosphatidylethanolamine:cholesterol. This nonreversible transition is also observed by (31)P nuclear magnetic resonance and detected as a large increase in TMA-DPH steady-state anisotropy. The same technique reveals as well that lysophosphatidylcholine counteracts the effect of diacylglycerol and stabilizes the lamellar phase in both transitions. Diacylglycerol and lysophosphatidylcholine are known to respectively promote and inhibit membrane fusion in a variety of systems. These data are interpreted in support of the hypothesis of a highly bent structural fusion intermediate ("stalk"). They also show the interest of lipid-phase studies in predicting and rationalizing membrane fusion mechanisms.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen T. M., Hong K., Papahadjopoulos D. Membrane contact, fusion, and hexagonal (HII) transitions in phosphatidylethanolamine liposomes. Biochemistry. 1990 Mar 27;29(12):2976–2985. doi: 10.1021/bi00464a013. [DOI] [PubMed] [Google Scholar]
- Ambrosini A., Bertoli E., Tanfani F., Wozniak M., Zolese G. The effect of N-acyl ethanolamines on phosphatidylethanolamine phase transitions studied by laurdan generalised polarisation. Chem Phys Lipids. 1994 Aug 8;72(2):127–134. doi: 10.1016/0009-3084(94)90096-5. [DOI] [PubMed] [Google Scholar]
- Burger K. N., Nieva J. L., Alonso A., Verkleij A. J. Phospholipase C activity-induced fusion of pure lipid model membranes. A freeze fracture study. Biochim Biophys Acta. 1991 Sep 30;1068(2):249–253. doi: 10.1016/0005-2736(91)90216-u. [DOI] [PubMed] [Google Scholar]
- Castresana J., Nieva J. L., Rivas E., Alonso A. Partial dehydration of phosphatidylethanolamine phosphate groups during hexagonal phase formation, as seen by i.r. spectroscopy. Biochem J. 1992 Mar 1;282(Pt 2):467–470. doi: 10.1042/bj2820467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S. Y., Cheng K. H. Infrared and time-resolved fluorescence spectroscopic studies of the polymorphic phase behavior of phosphatidylethanolamine/diacylglycerol lipid mixtures. Chem Phys Lipids. 1990 Dec;56(2-3):149–158. doi: 10.1016/0009-3084(90)90097-b. [DOI] [PubMed] [Google Scholar]
- Cheng K. H. Headgroup hydration and motional order of lipids in lamellar liquid crystalline and inverted hexagonal phases of unsaturated phosphatidylethanolamine--a time-resolved fluorescence study. Chem Phys Lipids. 1990 Mar;53(2-3):191–202. doi: 10.1016/0009-3084(90)90045-s. [DOI] [PubMed] [Google Scholar]
- Cheng K. H., Lepock J. R., Hui S. W., Yeagle P. L. The role of cholesterol in the activity of reconstituted Ca-ATPase vesicles containing unsaturated phosphatidylethanolamine. J Biol Chem. 1986 Apr 15;261(11):5081–5087. [PubMed] [Google Scholar]
- Chernomordik L. V., Melikyan G. B., Chizmadzhev Y. A. Biomembrane fusion: a new concept derived from model studies using two interacting planar lipid bilayers. Biochim Biophys Acta. 1987 Oct 5;906(3):309–352. doi: 10.1016/0304-4157(87)90016-5. [DOI] [PubMed] [Google Scholar]
- Chernomordik L. V., Vogel S. S., Sokoloff A., Onaran H. O., Leikina E. A., Zimmerberg J. Lysolipids reversibly inhibit Ca(2+)-, GTP- and pH-dependent fusion of biological membranes. FEBS Lett. 1993 Feb 22;318(1):71–76. doi: 10.1016/0014-5793(93)81330-3. [DOI] [PubMed] [Google Scholar]
- Chernomordik L. V., Zimmerberg J. Bending membranes to the task: structural intermediates in bilayer fusion. Curr Opin Struct Biol. 1995 Aug;5(4):541–547. doi: 10.1016/0959-440x(95)80041-7. [DOI] [PubMed] [Google Scholar]
- Chernomordik L., Chanturiya A., Green J., Zimmerberg J. The hemifusion intermediate and its conversion to complete fusion: regulation by membrane composition. Biophys J. 1995 Sep;69(3):922–929. doi: 10.1016/S0006-3495(95)79966-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernomordik L., Kozlov M. M., Zimmerberg J. Lipids in biological membrane fusion. J Membr Biol. 1995 Jul;146(1):1–14. doi: 10.1007/BF00232676. [DOI] [PubMed] [Google Scholar]
- Cullis P. R., de Kruijff B. The polymorphic phase behaviour of phosphatidylethanolamines of natural and synthetic origin. A 31P NMR study. Biochim Biophys Acta. 1978 Oct 19;513(1):31–42. doi: 10.1016/0005-2736(78)90109-8. [DOI] [PubMed] [Google Scholar]
- Das S., Rand R. P. Modification by diacylglycerol of the structure and interaction of various phospholipid bilayer membranes. Biochemistry. 1986 May 20;25(10):2882–2889. doi: 10.1021/bi00358a022. [DOI] [PubMed] [Google Scholar]
- Epand R. M., Epand R. F., Lancaster C. R. Modulation of the bilayer to hexagonal phase transition of phosphatidylethanolamines by acylglycerols. Biochim Biophys Acta. 1988 Nov 22;945(2):161–166. doi: 10.1016/0005-2736(88)90478-6. [DOI] [PubMed] [Google Scholar]
- Epand R. M., Stafford A., Wang J., Epand R. F. Zwitterionic amphiphiles that raise the bilayer to hexagonal phase transition temperature inhibit protein kinase C. The exception that proves the rule. FEBS Lett. 1992 Jun 15;304(2-3):245–248. doi: 10.1016/0014-5793(92)80629-u. [DOI] [PubMed] [Google Scholar]
- Gawrisch K., Parsegian V. A., Hajduk D. A., Tate M. W., Graner S. M., Fuller N. L., Rand R. P. Energetics of a hexagonal-lamellar-hexagonal-phase transition sequence in dioleoylphosphatidylethanolamine membranes. Biochemistry. 1992 Mar 24;31(11):2856–2864. doi: 10.1021/bi00126a003. [DOI] [PubMed] [Google Scholar]
- Goldberg E. M., Lester D. S., Borchardt D. B., Zidovetzki R. Effects of diacylglycerols and Ca2+ on structure of phosphatidylcholine/phosphatidylserine bilayers. Biophys J. 1994 Feb;66(2 Pt 1):382–393. doi: 10.1016/s0006-3495(94)80788-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goñi F. M., Nieva J. L., Basañez G., Fidelio G., Alonso A. Phospholipase-C-promoted liposome fusion. Biochem Soc Trans. 1994 Aug;22(3):839–844. doi: 10.1042/bst0220839. [DOI] [PubMed] [Google Scholar]
- Gulik A., Luzzati V., DeRosa M., Gambacorta A. Tetraether lipid components from a thermoacidophilic archaebacterium. Chemical structure and physical polymorphism. J Mol Biol. 1988 May 20;201(2):429–435. doi: 10.1016/0022-2836(88)90149-0. [DOI] [PubMed] [Google Scholar]
- Han X., Gross R. W. Nonmonotonic alterations in the fluorescence anisotropy of polar head group labeled fluorophores during the lamellar to hexagonal phase transition of phospholipids. Biophys J. 1992 Aug;63(2):309–316. doi: 10.1016/S0006-3495(92)81616-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hein M., Post A., Galla H. J. Implications of a non-lamellar lipid phase for the tight junction stability. Part I: Influence of basic amino acids, pH and protamine on the bilayer-hexagonal II phase behaviour of PS-containing PE membranes. Chem Phys Lipids. 1992 Dec;63(3):213–221. doi: 10.1016/0009-3084(92)90037-p. [DOI] [PubMed] [Google Scholar]
- Helfrich W. Elastic properties of lipid bilayers: theory and possible experiments. Z Naturforsch C. 1973 Nov-Dec;28(11):693–703. doi: 10.1515/znc-1973-11-1209. [DOI] [PubMed] [Google Scholar]
- Hong K., Baldwin P. A., Allen T. M., Papahadjopoulos D. Fluorometric detection of the bilayer-to-hexagonal phase transition in liposomes. Biochemistry. 1988 May 31;27(11):3947–3955. doi: 10.1021/bi00411a009. [DOI] [PubMed] [Google Scholar]
- Israelachvili J. N., Marcelja S., Horn R. G. Physical principles of membrane organization. Q Rev Biophys. 1980 May;13(2):121–200. doi: 10.1017/s0033583500001645. [DOI] [PubMed] [Google Scholar]
- Katsaras J., Jeffrey K. R., Yang D. S., Epand R. M. Direct evidence for the partial dehydration of phosphatidylethanolamine bilayers on approaching the hexagonal phase. Biochemistry. 1993 Oct 12;32(40):10700–10707. doi: 10.1021/bi00091a021. [DOI] [PubMed] [Google Scholar]
- Koynova R., Caffrey M. Phases and phase transitions of the hydrated phosphatidylethanolamines. Chem Phys Lipids. 1994 Jan;69(1):1–34. doi: 10.1016/0009-3084(94)90024-8. [DOI] [PubMed] [Google Scholar]
- Kozlov M. M., Leikin S., Rand R. P. Bending, hydration and interstitial energies quantitatively account for the hexagonal-lamellar-hexagonal reentrant phase transition in dioleoylphosphatidylethanolamine. Biophys J. 1994 Oct;67(4):1603–1611. doi: 10.1016/S0006-3495(94)80633-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landh T. From entangled membranes to eclectic morphologies: cubic membranes as subcellular space organizers. FEBS Lett. 1995 Aug 1;369(1):13–17. doi: 10.1016/0014-5793(95)00660-2. [DOI] [PubMed] [Google Scholar]
- Luk A. S., Kaler E. W., Lee S. P. Phospholipase C-induced aggregation and fusion of cholesterol-lecithin small unilamellar vesicles. Biochemistry. 1993 Jul 13;32(27):6965–6973. doi: 10.1021/bi00078a022. [DOI] [PubMed] [Google Scholar]
- Luzzati V., Vargas R., Mariani P., Gulik A., Delacroix H. Cubic phases of lipid-containing systems. Elements of a theory and biological connotations. J Mol Biol. 1993 Jan 20;229(2):540–551. doi: 10.1006/jmbi.1993.1053. [DOI] [PubMed] [Google Scholar]
- López-García F., Villalaín J., Gómez-Fernández J. C., Quinn P. J. The phase behavior of mixed aqueous dispersions of dipalmitoyl derivatives of phosphatidylcholine and diacylglycerol. Biophys J. 1994 Jun;66(6):1991–2004. doi: 10.1016/S0006-3495(94)80992-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantsch H. H., Martin A., Cameron D. G. Characterization by infrared spectroscopy of the bilayer to nonbilayer phase transition of phosphatidylethanolamines. Biochemistry. 1981 May 26;20(11):3138–3145. doi: 10.1021/bi00514a024. [DOI] [PubMed] [Google Scholar]
- Nieva J. L., Alonso A., Basáez G., Goñi F. M., Gulik A., Vargas R., Luzzati V. Topological properties of two cubic phases of a phospholipid:cholesterol:diacylglycerol aqueous system and their possible implications in the phospholipase C-induced liposome fusion. FEBS Lett. 1995 Jul 10;368(1):143–147. doi: 10.1016/0014-5793(95)00631-i. [DOI] [PubMed] [Google Scholar]
- Nieva J. L., Castresana J., Alonso A. The lamellar to hexagonal phase transition in phosphatidylethanolamine liposomes: a fluorescence anisotropy study. Biochem Biophys Res Commun. 1990 May 16;168(3):987–992. doi: 10.1016/0006-291x(90)91126-d. [DOI] [PubMed] [Google Scholar]
- Nieva J. L., Goñi F. M., Alonso A. Liposome fusion catalytically induced by phospholipase C. Biochemistry. 1989 Sep 5;28(18):7364–7367. doi: 10.1021/bi00444a032. [DOI] [PubMed] [Google Scholar]
- Nieva J. L., Goñi F. M., Alonso A. Phospholipase C-promoted membrane fusion. Retroinhibition by the end-product diacylglycerol. Biochemistry. 1993 Feb 2;32(4):1054–1058. doi: 10.1021/bi00055a009. [DOI] [PubMed] [Google Scholar]
- Oberhauser A. F., Monck J. R., Fernandez J. M. Events leading to the opening and closing of the exocytotic fusion pore have markedly different temperature dependencies. Kinetic analysis of single fusion events in patch-clamped mouse mast cells. Biophys J. 1992 Mar;61(3):800–809. doi: 10.1016/S0006-3495(92)81884-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz A., Villalaín J., Gómez-Fernández J. C. Interaction of diacylglycerols with phosphatidylcholine vesicles as studied by differential scanning calorimetry and fluorescence probe depolarization. Biochemistry. 1988 Dec 13;27(25):9030–9036. doi: 10.1021/bi00425a022. [DOI] [PubMed] [Google Scholar]
- Rand R. P. Interacting phospholipid bilayers: measured forces and induced structural changes. Annu Rev Biophys Bioeng. 1981;10:277–314. doi: 10.1146/annurev.bb.10.060181.001425. [DOI] [PubMed] [Google Scholar]
- Seddon J. M. Structure of the inverted hexagonal (HII) phase, and non-lamellar phase transitions of lipids. Biochim Biophys Acta. 1990 Feb 28;1031(1):1–69. doi: 10.1016/0304-4157(90)90002-t. [DOI] [PubMed] [Google Scholar]
- Seelig J. 31P nuclear magnetic resonance and the head group structure of phospholipids in membranes. Biochim Biophys Acta. 1978 Jul 31;515(2):105–140. doi: 10.1016/0304-4157(78)90001-1. [DOI] [PubMed] [Google Scholar]
- Siegel D. P., Banschbach J. L. Lamellar/inverted cubic (L alpha/QII) phase transition in N-methylated dioleoylphosphatidylethanolamine. Biochemistry. 1990 Jun 26;29(25):5975–5981. doi: 10.1021/bi00477a014. [DOI] [PubMed] [Google Scholar]
- Siegel D. P., Banschbach J., Alford D., Ellens H., Lis L. J., Quinn P. J., Yeagle P. L., Bentz J. Physiological levels of diacylglycerols in phospholipid membranes induce membrane fusion and stabilize inverted phases. Biochemistry. 1989 May 2;28(9):3703–3709. doi: 10.1021/bi00435a012. [DOI] [PubMed] [Google Scholar]
- Siegel D. P. Energetics of intermediates in membrane fusion: comparison of stalk and inverted micellar intermediate mechanisms. Biophys J. 1993 Nov;65(5):2124–2140. doi: 10.1016/S0006-3495(93)81256-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel D. P., Green W. J., Talmon Y. The mechanism of lamellar-to-inverted hexagonal phase transitions: a study using temperature-jump cryo-electron microscopy. Biophys J. 1994 Feb;66(2 Pt 1):402–414. doi: 10.1016/s0006-3495(94)80790-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storch J., Shulman S. L., Kleinfeld A. M. Plasma membrane lipid order and composition during adipocyte differentiation of 3T3F442A cells. Studies in intact cells with 1-[4-(trimethylamino)phenyl]-6-phenylhexatriene. J Biol Chem. 1989 Jun 25;264(18):10527–10533. [PubMed] [Google Scholar]
- Walter A., Yeagle P. L., Siegel D. P. Diacylglycerol and hexadecane increase divalent cation-induced lipid mixing rates between phosphatidylserine large unilamellar vesicles. Biophys J. 1994 Feb;66(2 Pt 1):366–376. doi: 10.1016/s0006-3495(94)80786-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao H., Hatta I., Koynova R., Tenchov B. Time-resolved x-ray diffraction and calorimetric studies at low scan rates: II. On the fine structure of the phase transitions in hydrated dipalmitoylphosphatidylethanolamine. Biophys J. 1992 Mar;61(3):683–693. doi: 10.1016/S0006-3495(92)81873-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Langen H., Schrama C. A., van Ginkel G., Ranke G., Levine Y. K. Order and dynamics in the lamellar L alpha and in the hexagonal HII phase. Dioleoylphosphatidylethanolamine studied with angle-resolved fluorescence depolarization. Biophys J. 1989 May;55(5):937–947. doi: 10.1016/S0006-3495(89)82892-9. [DOI] [PMC free article] [PubMed] [Google Scholar]


