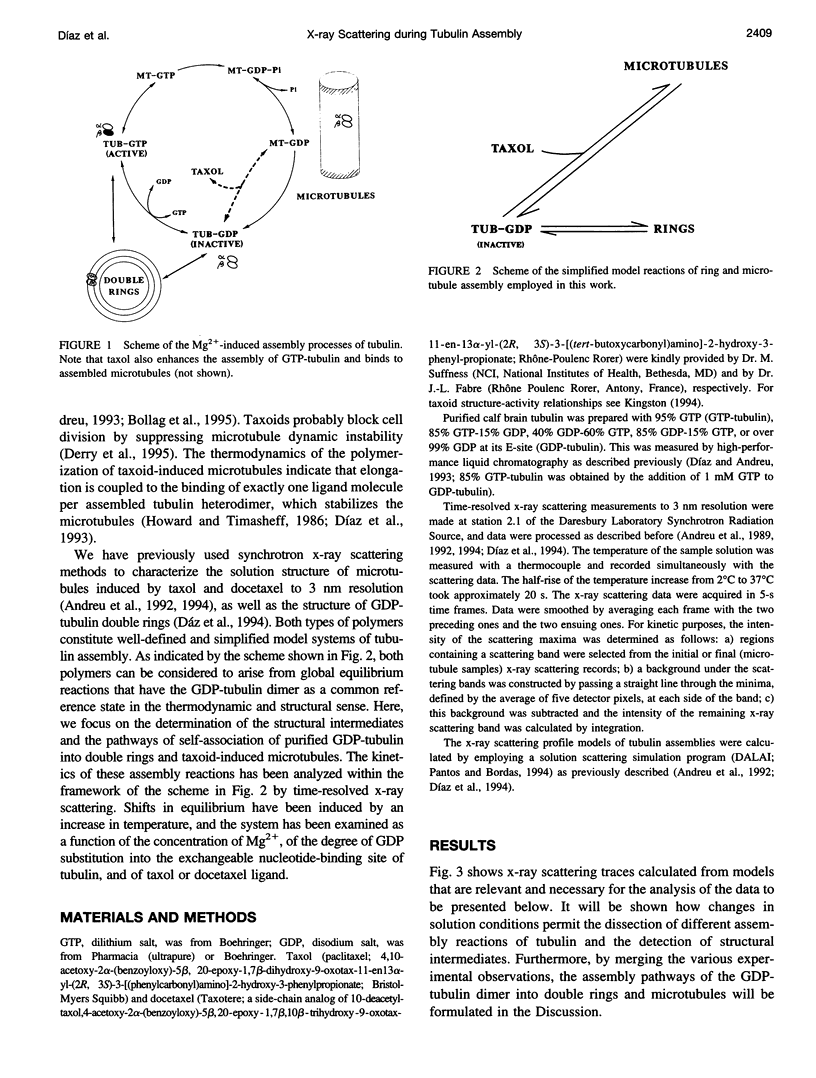
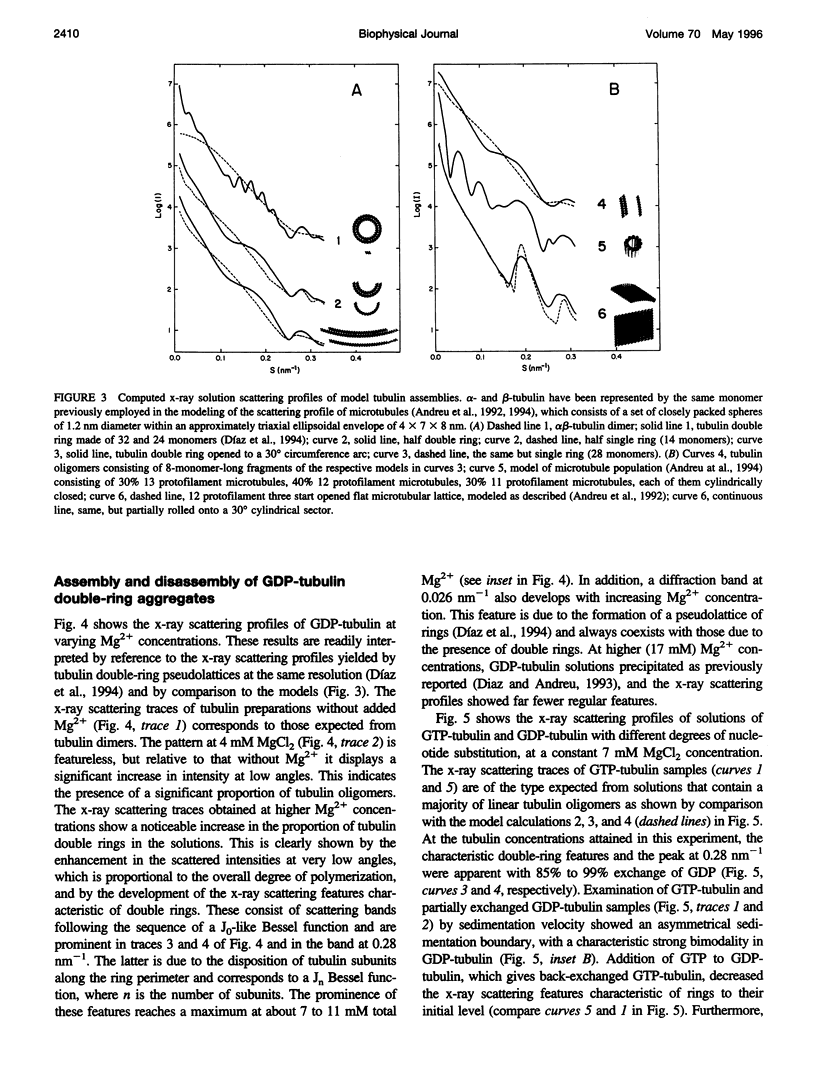
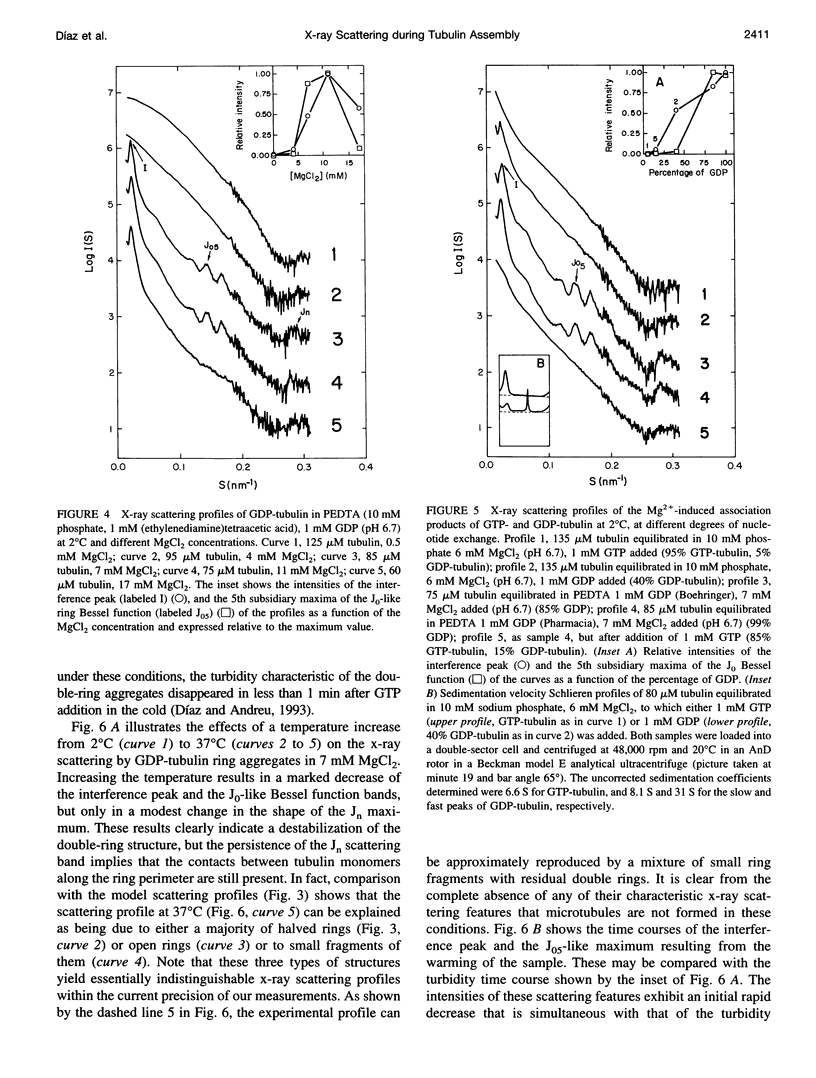
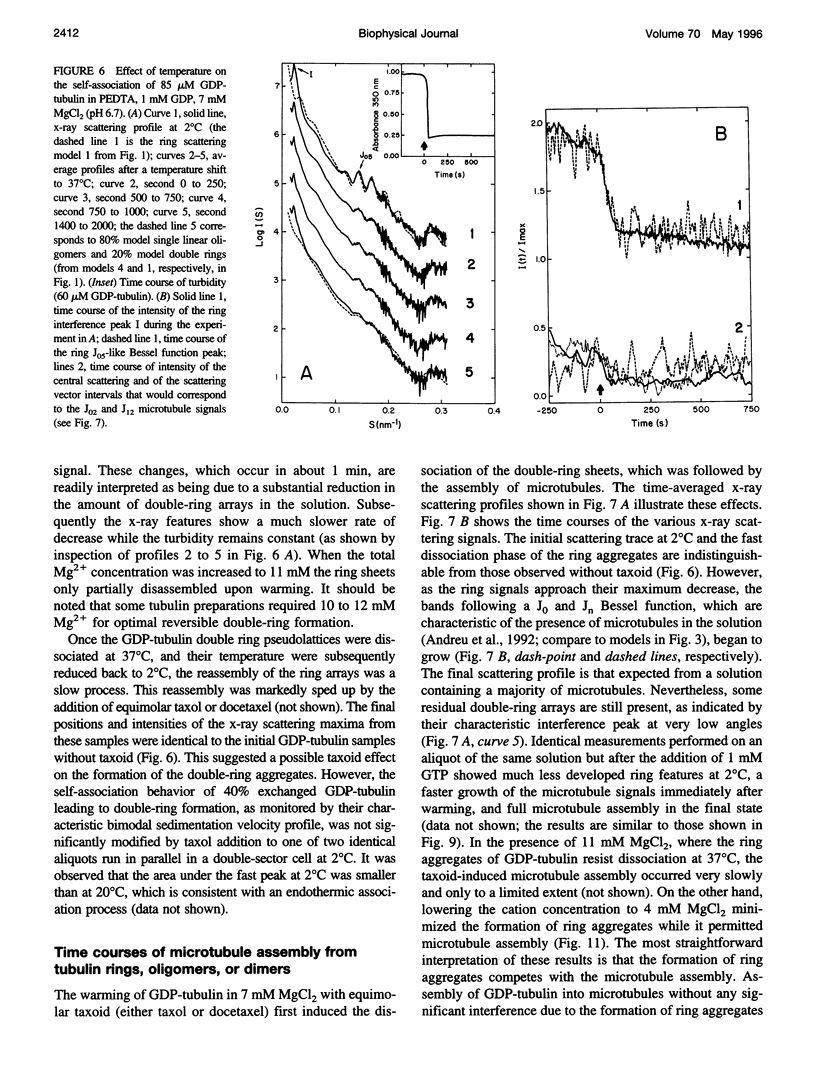
Abstract
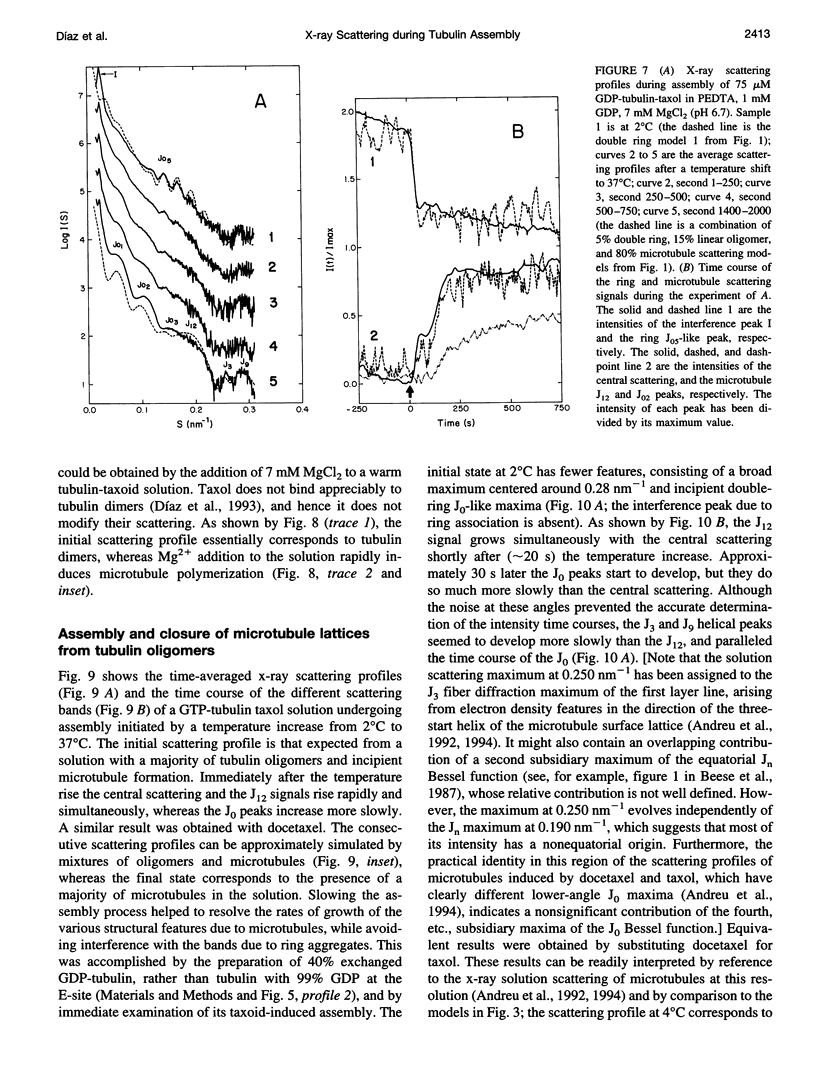
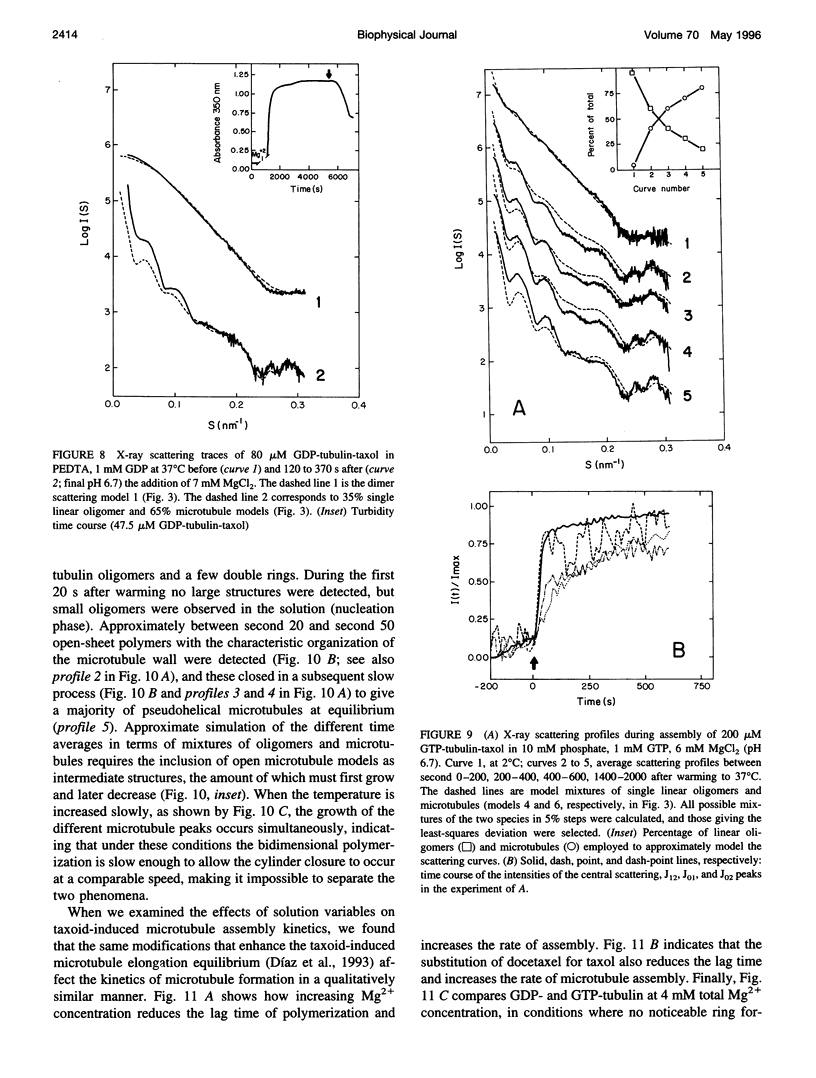
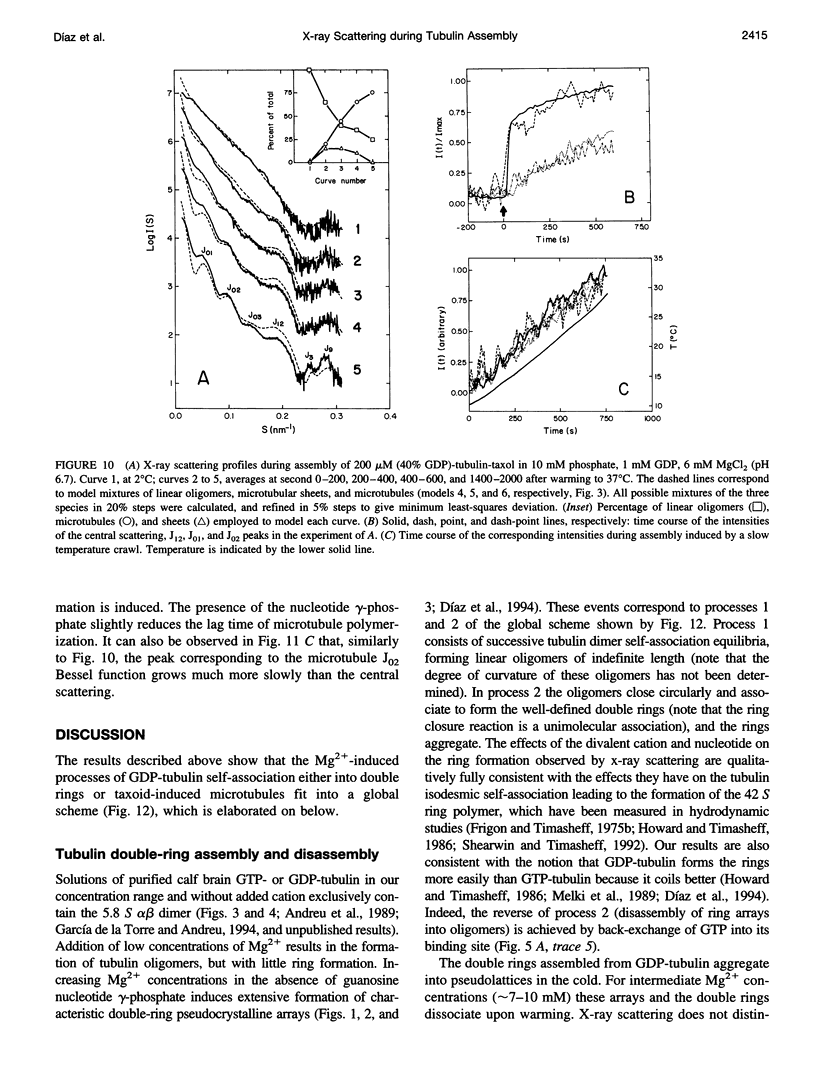
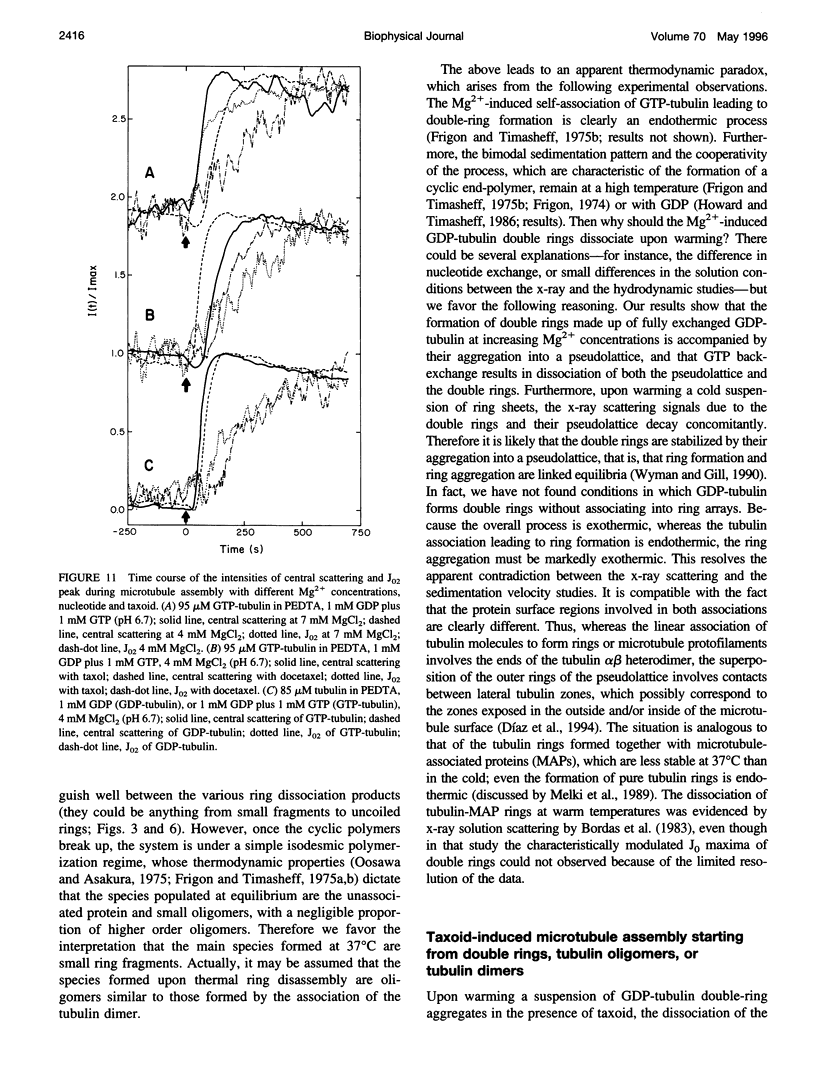
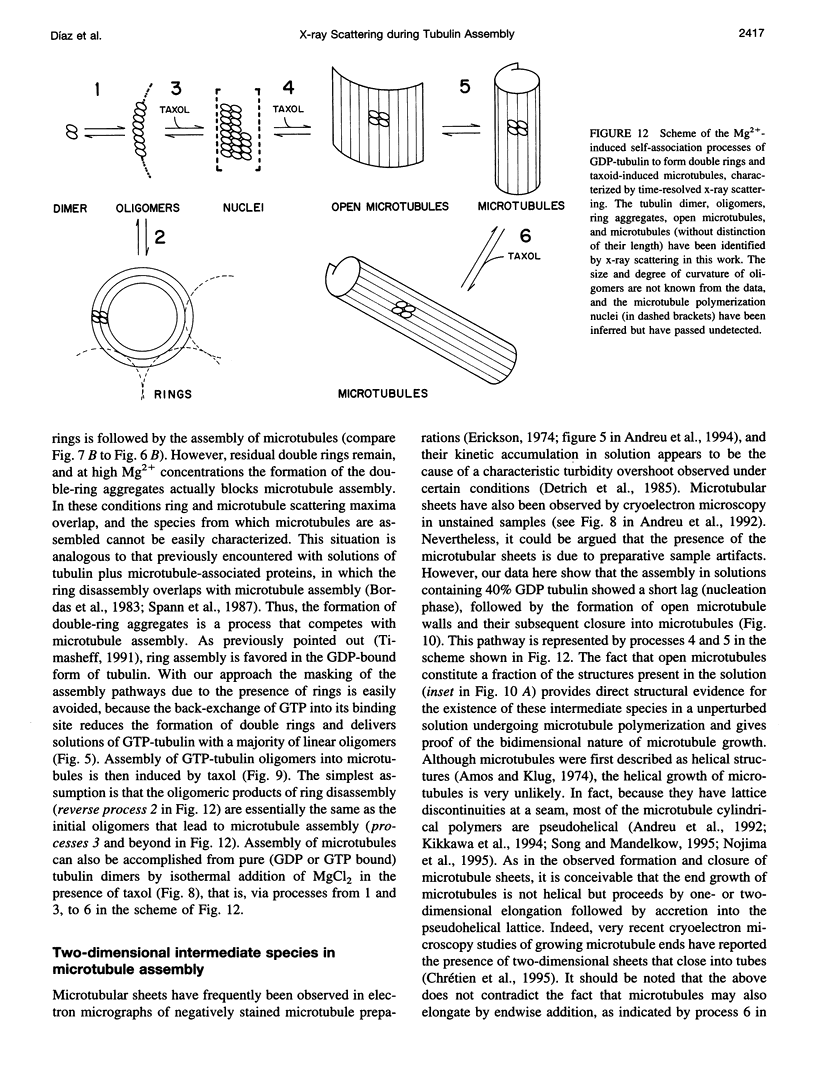
We have studied the self-association reactions of purified GDP-liganded tubulin into double rings and taxoid-induced microtubules, employing synchrotron time-resolved x-ray solution scattering. The experimental scattering profiles have been interpreted by reference to the known scattering profiles to 3 nm resolution and to the low-resolution structures of the tubulin dimer, tubulin double rings, and microtubules, and by comparison with oligomer models and model mixtures. The time courses of the scattering bands corresponding to the different structural features were monitored during the assembly reactions under varying biochemical conditions. GDP-tubulin essentially stays as a dimer at low Mg(2+) ion activity, in either the absence or presence of taxoid. Upon addition of the divalent cations, it associates into either double-ring aggregates or taxoid-induced microtubules by different pathways. Both processes have the formation of small linear (short protofilament-like) tubulin oligomers in common. Tubulin double-ring aggregate formation, which is shown by x-ray scattering to be favored in the GDP- versus the GTP-liganded protein, can actually block microtubule assembly. The tubulin self-association leading to double rings, as determined by sedimentation velocity, is endothermic. The formation of the double-ring aggregates from oligomers, which involves additional intermolecular contacts, is exothermic, as shown by x-ray and light scattering. Microtubule assembly can be initiated from GDP-tubulin dimers or oligomers. Under fast polymerization conditions, after a short lag time, open taxoid-induced microtubular sheets have been clearly detected (monitored by the central scattering and the maximum corresponding to the J(n) Bessel function), which slowly close into microtubules (monitored by the appearance of their characteristic J(0), J(3), and J (n) - (3) Bessel function maxima). This provides direct evidence for the bidimensional assembly of taxoid-induced microtubule polymers in solution and argues against helical growth. The rate of microtubule formation was increased by the same factors known to enhance taxoid-induced microtubule stability. The results suggest that taxoids induce the accretion of the existing Mg(2+)-induced GDP-tubulin oligomers, thus forming small bidimensional polymers that are necessary to nucleate the microtubular sheets, possibly by binding to or modifying the lateral interaction sites between tubulin dimers.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amos L., Klug A. Arrangement of subunits in flagellar microtubules. J Cell Sci. 1974 May;14(3):523–549. doi: 10.1242/jcs.14.3.523. [DOI] [PubMed] [Google Scholar]
- Andreu J. M., Bordas J., Diaz J. F., García de Ancos J., Gil R., Medrano F. J., Nogales E., Pantos E., Towns-Andrews E. Low resolution structure of microtubules in solution. Synchrotron X-ray scattering and electron microscopy of taxol-induced microtubules assembled from purified tubulin in comparison with glycerol and MAP-induced microtubules. J Mol Biol. 1992 Jul 5;226(1):169–184. doi: 10.1016/0022-2836(92)90132-4. [DOI] [PubMed] [Google Scholar]
- Andreu J. M., Díaz J. F., Gil R., de Pereda J. M., García de Lacoba M., Peyrot V., Briand C., Towns-Andrews E., Bordas J. Solution structure of Taxotere-induced microtubules to 3-nm resolution. The change in protofilament number is linked to the binding of the taxol side chain. J Biol Chem. 1994 Dec 16;269(50):31785–31792. [PubMed] [Google Scholar]
- Beese L., Stubbs G., Cohen C. Microtubule structure at 18 A resolution. J Mol Biol. 1987 Mar 20;194(2):257–264. doi: 10.1016/0022-2836(87)90373-1. [DOI] [PubMed] [Google Scholar]
- Bollag D. M., McQueney P. A., Zhu J., Hensens O., Koupal L., Liesch J., Goetz M., Lazarides E., Woods C. M. Epothilones, a new class of microtubule-stabilizing agents with a taxol-like mechanism of action. Cancer Res. 1995 Jun 1;55(11):2325–2333. [PubMed] [Google Scholar]
- Bordas J., Mandelkow E. M., Mandelkow E. Stages of tubulin assembly and disassembly studied by time-resolved synchrotron X-ray scattering. J Mol Biol. 1983 Feb 15;164(1):89–135. doi: 10.1016/0022-2836(83)90089-x. [DOI] [PubMed] [Google Scholar]
- Butner K. A., Kirschner M. W. Tau protein binds to microtubules through a flexible array of distributed weak sites. J Cell Biol. 1991 Nov;115(3):717–730. doi: 10.1083/jcb.115.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrétien D., Fuller S. D., Karsenti E. Structure of growing microtubule ends: two-dimensional sheets close into tubes at variable rates. J Cell Biol. 1995 Jun;129(5):1311–1328. doi: 10.1083/jcb.129.5.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derry W. B., Wilson L., Jordan M. A. Substoichiometric binding of taxol suppresses microtubule dynamics. Biochemistry. 1995 Feb 21;34(7):2203–2211. doi: 10.1021/bi00007a014. [DOI] [PubMed] [Google Scholar]
- Detrich H. W., 3rd, Jordan M. A., Wilson L., Williams R. C., Jr Mechanism of microtubule assembly. Changes in polymer structure and organization during assembly of sea urchin egg tubulin. J Biol Chem. 1985 Aug 5;260(16):9479–9490. [PubMed] [Google Scholar]
- Drechsel D. N., Kirschner M. W. The minimum GTP cap required to stabilize microtubules. Curr Biol. 1994 Dec 1;4(12):1053–1061. doi: 10.1016/s0960-9822(00)00243-8. [DOI] [PubMed] [Google Scholar]
- Dye R. B., Fink S. P., Williams R. C., Jr Taxol-induced flexibility of microtubules and its reversal by MAP-2 and Tau. J Biol Chem. 1993 Apr 5;268(10):6847–6850. [PubMed] [Google Scholar]
- Díaz J. F., Andreu J. M. Assembly of purified GDP-tubulin into microtubules induced by taxol and taxotere: reversibility, ligand stoichiometry, and competition. Biochemistry. 1993 Mar 23;32(11):2747–2755. doi: 10.1021/bi00062a003. [DOI] [PubMed] [Google Scholar]
- Díaz J. F., Menéndez M., Andreu J. M. Thermodynamics of ligand-induced assembly of tubulin. Biochemistry. 1993 Sep 28;32(38):10067–10077. doi: 10.1021/bi00089a023. [DOI] [PubMed] [Google Scholar]
- Díaz J. F., Pantos E., Bordas J., Andreu J. M. Solution structure of GDP-tubulin double rings to 3 nm resolution and comparison with microtubules. J Mol Biol. 1994 Apr 29;238(2):214–225. doi: 10.1006/jmbi.1994.1282. [DOI] [PubMed] [Google Scholar]
- Erickson H. P. Microtubule surface lattice and subunit structure and observations on reassembly. J Cell Biol. 1974 Jan;60(1):153–167. doi: 10.1083/jcb.60.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson H. P., O'Brien E. T. Microtubule dynamic instability and GTP hydrolysis. Annu Rev Biophys Biomol Struct. 1992;21:145–166. doi: 10.1146/annurev.bb.21.060192.001045. [DOI] [PubMed] [Google Scholar]
- Erickson H. P., Pantaloni D. The role of subunit entropy in cooperative assembly. Nucleation of microtubules and other two-dimensional polymers. Biophys J. 1981 May;34(2):293–309. doi: 10.1016/S0006-3495(81)84850-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frigon R. P., Timasheff S. N. Magnesium-induced self-association of calf brain tubulin. I. Stoichiometry. Biochemistry. 1975 Oct 21;14(21):4559–4566. doi: 10.1021/bi00692a001. [DOI] [PubMed] [Google Scholar]
- Frigon R. P., Timasheff S. N. Magnesium-induced self-association of calf brain tubulin. II. Thermodynamics. Biochemistry. 1975 Oct 21;14(21):4567–4573. doi: 10.1021/bi00692a002. [DOI] [PubMed] [Google Scholar]
- García de la Torre J., Andreu J. M. Hydrodynamic analysis of tubulin dimer and double rings. J Mol Biol. 1994 Apr 29;238(2):223–225. doi: 10.1006/jmbi.1994.1283. [DOI] [PubMed] [Google Scholar]
- Gilbert S. P., Webb M. R., Brune M., Johnson K. A. Pathway of processive ATP hydrolysis by kinesin. Nature. 1995 Feb 23;373(6516):671–676. doi: 10.1038/373671a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirose K., Lockhart A., Cross R. A., Amos L. A. Nucleotide-dependent angular change in kinesin motor domain bound to tubulin. Nature. 1995 Jul 20;376(6537):277–279. doi: 10.1038/376277a0. [DOI] [PubMed] [Google Scholar]
- Hoenger A., Sablin E. P., Vale R. D., Fletterick R. J., Milligan R. A. Three-dimensional structure of a tubulin-motor-protein complex. Nature. 1995 Jul 20;376(6537):271–274. doi: 10.1038/376271a0. [DOI] [PubMed] [Google Scholar]
- Horwitz S. B. How to make taxol from scratch. Nature. 1994 Feb 17;367(6464):593–594. doi: 10.1038/367593a0. [DOI] [PubMed] [Google Scholar]
- Howard W. D., Timasheff S. N. GDP state of tubulin: stabilization of double rings. Biochemistry. 1986 Dec 16;25(25):8292–8300. doi: 10.1021/bi00373a025. [DOI] [PubMed] [Google Scholar]
- Hyman A. A., Chrétien D., Arnal I., Wade R. H. Structural changes accompanying GTP hydrolysis in microtubules: information from a slowly hydrolyzable analogue guanylyl-(alpha,beta)-methylene-diphosphonate. J Cell Biol. 1995 Jan;128(1-2):117–125. doi: 10.1083/jcb.128.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikkawa M., Ishikawa T., Nakata T., Wakabayashi T., Hirokawa N. Direct visualization of the microtubule lattice seam both in vitro and in vivo. J Cell Biol. 1994 Dec;127(6 Pt 2):1965–1971. doi: 10.1083/jcb.127.6.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikkawa M., Ishikawa T., Wakabayashi T., Hirokawa N. Three-dimensional structure of the kinesin head-microtubule complex. Nature. 1995 Jul 20;376(6537):274–277. doi: 10.1038/376274a0. [DOI] [PubMed] [Google Scholar]
- Kingston D. G. Taxol: the chemistry and structure-activity relationships of a novel anticancer agent. Trends Biotechnol. 1994 Jun;12(6):222–227. doi: 10.1016/0167-7799(94)90120-1. [DOI] [PubMed] [Google Scholar]
- Kurz J. C., Williams R. C., Jr Microtubule-associated proteins and the flexibility of microtubules. Biochemistry. 1995 Oct 17;34(41):13374–13380. doi: 10.1021/bi00041a014. [DOI] [PubMed] [Google Scholar]
- Lee J. C., Timasheff S. N. The reconstitution of microtubules from purified calf brain tubulin. Biochemistry. 1975 Nov 18;14(23):5183–5187. doi: 10.1021/bi00694a025. [DOI] [PubMed] [Google Scholar]
- Mandelkow E. M., Lange G., Jagla A., Spann U., Mandelkow E. Dynamics of the microtubule oscillator: role of nucleotides and tubulin-MAP interactions. EMBO J. 1988 Feb;7(2):357–365. doi: 10.1002/j.1460-2075.1988.tb02821.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandelkow E., Mandelkow E. M. Microtubules and microtubule-associated proteins. Curr Opin Cell Biol. 1995 Feb;7(1):72–81. doi: 10.1016/0955-0674(95)80047-6. [DOI] [PubMed] [Google Scholar]
- Manuel Andreu J., Garcia de Ancos J., Starling D., Hodgkinson J. L., Bordas J. A synchrotron X-ray scattering characterization of purified tubulin and of its expansion induced by mild detergent binding. Biochemistry. 1989 May 2;28(9):4036–4040. doi: 10.1021/bi00435a060. [DOI] [PubMed] [Google Scholar]
- Martin S. R., Schilstra M. J., Bayley P. M. Dynamic instability of microtubules: Monte Carlo simulation and application to different types of microtubule lattice. Biophys J. 1993 Aug;65(2):578–596. doi: 10.1016/S0006-3495(93)81091-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melki R., Carlier M. F., Pantaloni D. Oscillations in microtubule polymerization: the rate of GTP regeneration on tubulin controls the period. EMBO J. 1988 Sep;7(9):2653–2659. doi: 10.1002/j.1460-2075.1988.tb03118.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melki R., Carlier M. F., Pantaloni D., Timasheff S. N. Cold depolymerization of microtubules to double rings: geometric stabilization of assemblies. Biochemistry. 1989 Nov 14;28(23):9143–9152. doi: 10.1021/bi00449a028. [DOI] [PubMed] [Google Scholar]
- Mickey B., Howard J. Rigidity of microtubules is increased by stabilizing agents. J Cell Biol. 1995 Aug;130(4):909–917. doi: 10.1083/jcb.130.4.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogales E., Wolf S. G., Khan I. A., Ludueña R. F., Downing K. H. Structure of tubulin at 6.5 A and location of the taxol-binding site. Nature. 1995 Jun 1;375(6530):424–427. doi: 10.1038/375424a0. [DOI] [PubMed] [Google Scholar]
- Nojima D., Linck R. W., Egelman E. H. At least one of the protofilaments in flagellar microtubules is not composed of tubulin. Curr Biol. 1995 Feb 1;5(2):158–167. doi: 10.1016/s0960-9822(95)00037-6. [DOI] [PubMed] [Google Scholar]
- Novella I. S., Andreu J. M., Andreu D. Chemically synthesized 182-235 segment of tau protein and analogue peptides are efficient in vitro microtubule assembly inducers of low apparent sequence specificity. FEBS Lett. 1992 Oct 26;311(3):235–240. doi: 10.1016/0014-5793(92)81110-8. [DOI] [PubMed] [Google Scholar]
- Parness J., Horwitz S. B. Taxol binds to polymerized tubulin in vitro. J Cell Biol. 1981 Nov;91(2 Pt 1):479–487. doi: 10.1083/jcb.91.2.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao S., Krauss N. E., Heerding J. M., Swindell C. S., Ringel I., Orr G. A., Horwitz S. B. 3'-(p-azidobenzamido)taxol photolabels the N-terminal 31 amino acids of beta-tubulin. J Biol Chem. 1994 Feb 4;269(5):3132–3134. [PubMed] [Google Scholar]
- Rowinsky E. K., Donehower R. C. Paclitaxel (taxol) N Engl J Med. 1995 Apr 13;332(15):1004–1014. doi: 10.1056/NEJM199504133321507. [DOI] [PubMed] [Google Scholar]
- Shearwin K. E., Perez-Ramirez B., Timasheff S. N. Linkages between the dissociation of alpha beta tubulin into subunits and ligand binding: the ground state of tubulin is the GDP conformation. Biochemistry. 1994 Feb 1;33(4):885–893. doi: 10.1021/bi00170a006. [DOI] [PubMed] [Google Scholar]
- Shivanna B. D., Mejillano M. R., Williams T. D., Himes R. H. Exchangeable GTP binding site of beta-tubulin. Identification of cysteine 12 as the major site of cross-linking by direct photoaffinity labeling. J Biol Chem. 1993 Jan 5;268(1):127–132. [PubMed] [Google Scholar]
- Song Y. H., Mandelkow E. The anatomy of flagellar microtubules: polarity, seam, junctions, and lattice. J Cell Biol. 1995 Jan;128(1-2):81–94. doi: 10.1083/jcb.128.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spann U., Renner W., Mandelkow E. M., Bordas J., Mandelkow E. Tubulin oligomers and microtubule assembly studied by time-resolved X-ray scattering: separation of prenucleation and nucleation events. Biochemistry. 1987 Feb 24;26(4):1123–1132. doi: 10.1021/bi00378a021. [DOI] [PubMed] [Google Scholar]
- Vale R. D., Coppin C. M., Malik F., Kull F. J., Milligan R. A. Tubulin GTP hydrolysis influences the structure, mechanical properties, and kinesin-driven transport of microtubules. J Biol Chem. 1994 Sep 23;269(38):23769–23775. [PubMed] [Google Scholar]
- Venier P., Maggs A. C., Carlier M. F., Pantaloni D. Analysis of microtubule rigidity using hydrodynamic flow and thermal fluctuations. J Biol Chem. 1994 May 6;269(18):13353–13360. [PubMed] [Google Scholar]
- Williams R. C., Jr, Rone L. A. End-to-end joining of taxol-stabilized GDP-containing microtubules. J Biol Chem. 1989 Jan 25;264(3):1663–1670. [PubMed] [Google Scholar]


