Abstract
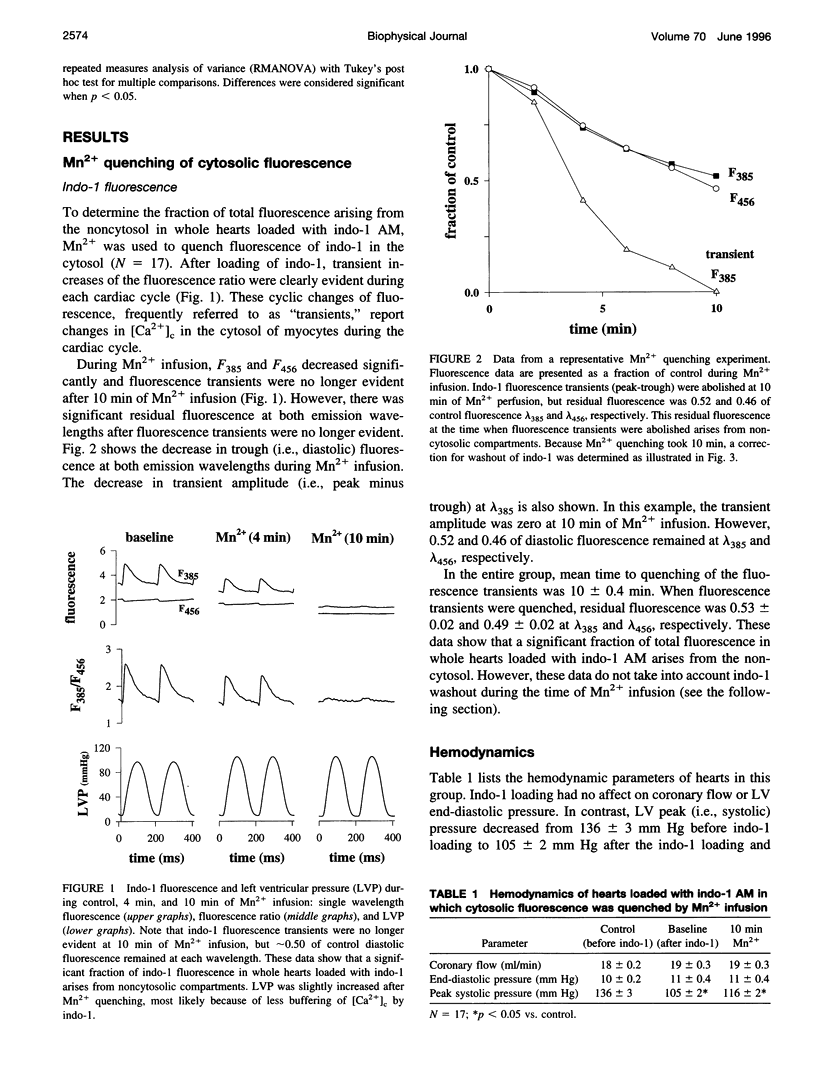
Assessment of free cytosolic [Ca2+] ([Ca2+]c) using the acetoxymethyl ester (AM) form of indo-1 may be compromised by loading of indo-1 into noncytosolic compartments, primarily mitochondria. To determine the fraction of noncytosolic fluorescence in whole hearts loaded with indo-1 AM, Mn2+ was used to quench cytosolic fluorescence. Residual (i.e., noncytosolic) fluorescence was subtracted from the total fluorescence before calculating [Ca2+]c. Noncytosolic fluorescence was used to estimate mitochondrial [Ca2+]. In hearts paced at 5 Hz (N = 17), noncytosolic fluorescence was 0.61 +/- 0.06 and 0.56 +/- 0.07 of total fluorescence at lambda 385 and lambda 456, respectively. After taking into account noncytosolic fluorescence, systolic and diastolic [Ca2+]c was 673 +/- 72 and 132 +/- 9 nM, respectively, noncytosolic [Ca2+] was 183 +/- 36 nM and increased to 272 +/- 12 when extracellular Ca2+ was increased from 2 to 6 mM. This increase in noncytosolic [Ca2+] was inhibited by ruthenium red, a blocker of Ca2+ uptake by mitochondria. We conclude that cytosolic and mitochondrial [Ca2+] can be determined in whole hearts loaded with indo-1 AM by using Mn2+ to quench cytosolic fluorescence.
Full text
PDF


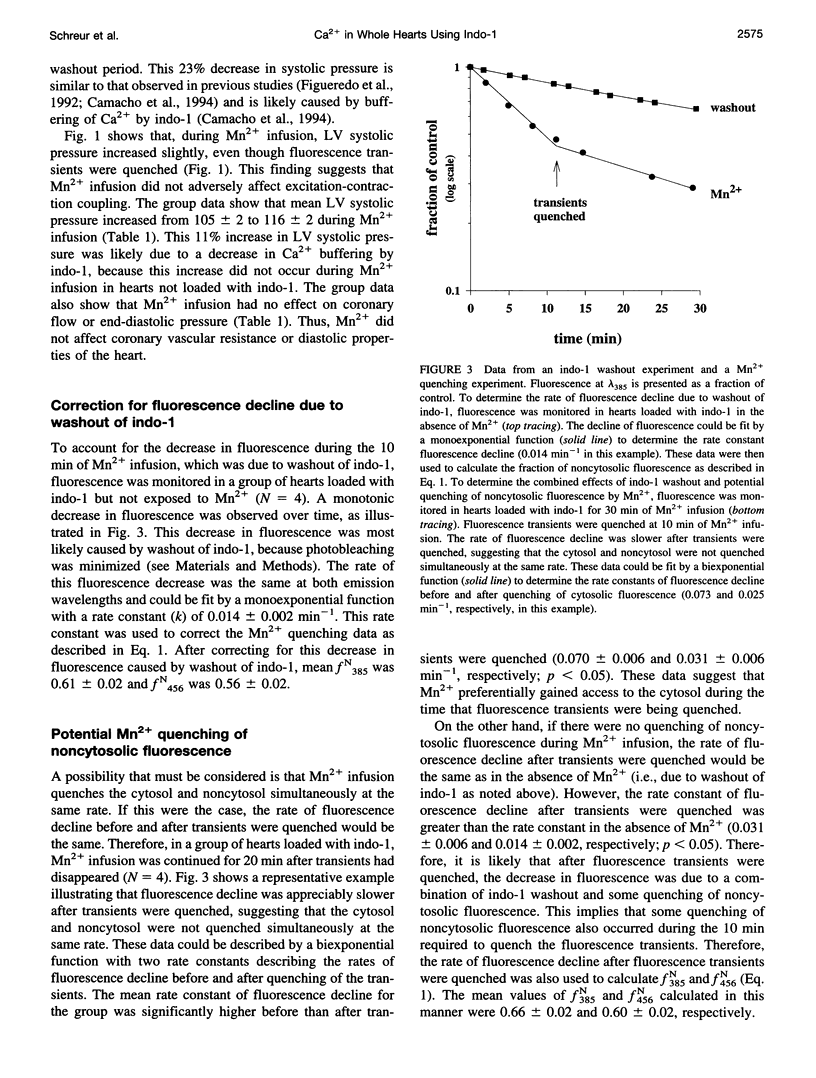




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen S. P., Stone D., McCormack J. G. The loading of fura-2 into mitochondria in the intact perfused rat heart and its use to estimate matrix Ca2+ under various conditions. J Mol Cell Cardiol. 1992 Jul;24(7):765–773. doi: 10.1016/0022-2828(92)93390-6. [DOI] [PubMed] [Google Scholar]
- Arkhammar P., Nilsson T., Berggren P. O. Glucose-stimulated efflux of indo-1 from pancreatic beta-cells is reduced by probenecid. FEBS Lett. 1990 Oct 29;273(1-2):182–184. doi: 10.1016/0014-5793(90)81079-4. [DOI] [PubMed] [Google Scholar]
- Ataka K., Chen D., Levitsky S., Jimenez E., Feinberg H. Effect of aging on intracellular Ca2+, pHi, and contractility during ischemia and reperfusion. Circulation. 1992 Nov;86(5 Suppl):II371–II376. [PubMed] [Google Scholar]
- Backx P. H., Ter Keurs H. E. Fluorescent properties of rat cardiac trabeculae microinjected with fura-2 salt. Am J Physiol. 1993 Apr;264(4 Pt 2):H1098–H1110. doi: 10.1152/ajpheart.1993.264.4.H1098. [DOI] [PubMed] [Google Scholar]
- Baker A. J., Brandes R., Schreur J. H., Camacho S. A., Weiner M. W. Protein and acidosis alter calcium-binding and fluorescence spectra of the calcium indicator indo-1. Biophys J. 1994 Oct;67(4):1646–1654. doi: 10.1016/S0006-3495(94)80637-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatter L. A., Wier W. G. Intracellular diffusion, binding, and compartmentalization of the fluorescent calcium indicators indo-1 and fura-2. Biophys J. 1990 Dec;58(6):1491–1499. doi: 10.1016/S0006-3495(90)82494-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
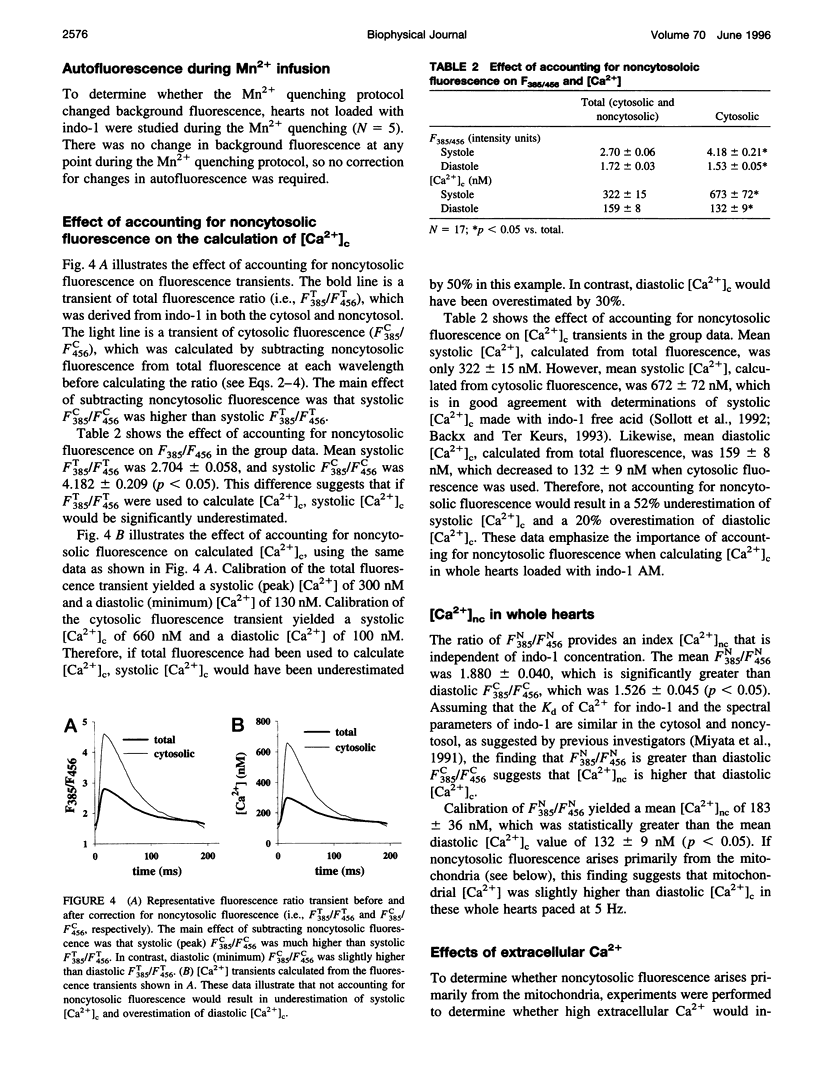
- Brandes R., Figueredo V. M., Camacho S. A., Baker A. J., Weiner M. W. Investigation of factors affecting fluorometric quantitation of cytosolic [Ca2+] in perfused hearts. Biophys J. 1993 Nov;65(5):1983–1993. doi: 10.1016/S0006-3495(93)81275-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandes R., Figueredo V. M., Camacho S. A., Baker A. J., Weiner M. W. Quantitation of cytosolic [Ca2+] in whole perfused rat hearts using Indo-1 fluorometry. Biophys J. 1993 Nov;65(5):1973–1982. doi: 10.1016/S0006-3495(93)81274-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandes R., Figueredo V. M., Camacho S. A., Massie B. M., Weiner M. W. Suppression of motion artifacts in fluorescence spectroscopy of perfused hearts. Am J Physiol. 1992 Sep;263(3 Pt 2):H972–H980. doi: 10.1152/ajpheart.1992.263.3.H972. [DOI] [PubMed] [Google Scholar]
- Brandes R., Figueredo V. M., Camacho S. A., Weiner M. W. Compensation for changes in tissue light absorption in fluorometry of hypoxic perfused rat hearts. Am J Physiol. 1994 Jun;266(6 Pt 2):H2554–H2567. doi: 10.1152/ajpheart.1994.266.6.H2554. [DOI] [PubMed] [Google Scholar]
- Camacho S. A., Brandes R., Figueredo V. M., Weiner M. W. Ca2+ transient decline and myocardial relaxation are slowed during low flow ischemia in rat hearts. J Clin Invest. 1994 Mar;93(3):951–957. doi: 10.1172/JCI117101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David H., Meyer R., Marx I., Guski H., Wenzelides K. Morphometric characterization of left ventricular myocardial cells of male rats during postnatal development. J Mol Cell Cardiol. 1979 Jul;11(7):631–638. doi: 10.1016/0022-2828(79)90377-8. [DOI] [PubMed] [Google Scholar]
- Davis M. H., Altschuld R. A., Jung D. W., Brierley G. P. Estimation of intramitochondrial pCa and pH by fura-2 and 2,7 biscarboxyethyl-5(6)-carboxyfluorescein (BCECF) fluorescence. Biochem Biophys Res Commun. 1987 Nov 30;149(1):40–45. doi: 10.1016/0006-291x(87)91602-0. [DOI] [PubMed] [Google Scholar]
- Figueredo V. M., Brandes R., Weiner M. W., Massie B. M., Camacho S. A. Cardiac contractile dysfunction during mild coronary flow reductions is due to an altered calcium-pressure relationship in rat hearts. J Clin Invest. 1992 Nov;90(5):1794–1802. doi: 10.1172/JCI116054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueredo V. M., Brandes R., Weiner M. W., Massie B. M., Camacho S. A. Endocardial versus epicardial differences of intracellular free calcium under normal and ischemic conditions in perfused rat hearts. Circ Res. 1993 May;72(5):1082–1090. doi: 10.1161/01.res.72.5.1082. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Gunter T. E., Restrepo D., Gunter K. K. Conversion of esterified fura-2 and indo-1 to Ca2+-sensitive forms by mitochondria. Am J Physiol. 1988 Sep;255(3 Pt 1):C304–C310. doi: 10.1152/ajpcell.1988.255.3.C304. [DOI] [PubMed] [Google Scholar]
- Gupta M. P., Dixon I. M., Zhao D., Dhalla N. S. Influence of ruthenium red on rat heart subcellular calcium transport. Can J Cardiol. 1989 Jan-Feb;5(1):55–63. [PubMed] [Google Scholar]
- Haworth R. A., Goknur A. B., Berkoff H. A. Measurement of Ca channel activity of isolated adult rat heart cells using 54Mn. Arch Biochem Biophys. 1989 Feb 1;268(2):594–604. doi: 10.1016/0003-9861(89)90327-5. [DOI] [PubMed] [Google Scholar]
- Hove-Madsen L., Bers D. M. Indo-1 binding to protein in permeabilized ventricular myocytes alters its spectral and Ca binding properties. Biophys J. 1992 Jul;63(1):89–97. doi: 10.1016/S0006-3495(92)81597-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter D. R., Haworth R. A., Berkoff H. A. Cellular manganese uptake by the isolated perfused rat heart: a probe for the sarcolemma calcium channel. J Mol Cell Cardiol. 1981 Sep;13(9):823–832. doi: 10.1016/0022-2828(81)90239-x. [DOI] [PubMed] [Google Scholar]
- Kihara Y., Grossman W., Morgan J. P. Direct measurement of changes in intracellular calcium transients during hypoxia, ischemia, and reperfusion of the intact mammalian heart. Circ Res. 1989 Oct;65(4):1029–1044. doi: 10.1161/01.res.65.4.1029. [DOI] [PubMed] [Google Scholar]
- Kitakaze M., Marban E. Cellular mechanism of the modulation of contractile function by coronary perfusion pressure in ferret hearts. J Physiol. 1989 Jul;414:455–472. doi: 10.1113/jphysiol.1989.sp017698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H. C., Mohabir R., Smith N., Franz M. R., Clusin W. T. Effect of ischemia on calcium-dependent fluorescence transients in rabbit hearts containing indo 1. Correlation with monophasic action potentials and contraction. Circulation. 1988 Oct;78(4):1047–1059. doi: 10.1161/01.cir.78.4.1047. [DOI] [PubMed] [Google Scholar]
- Lorell B. H., Apstein C. S., Cunningham M. J., Schoen F. J., Weinberg E. O., Peeters G. A., Barry W. H. Contribution of endothelial cells to calcium-dependent fluorescence transients in rabbit hearts loaded with indo 1. Circ Res. 1990 Aug;67(2):415–425. doi: 10.1161/01.res.67.2.415. [DOI] [PubMed] [Google Scholar]
- Miyata H., Lakatta E. G., Stern M. D., Silverman H. S. Relation of mitochondrial and cytosolic free calcium to cardiac myocyte recovery after exposure to anoxia. Circ Res. 1992 Sep;71(3):605–613. doi: 10.1161/01.res.71.3.605. [DOI] [PubMed] [Google Scholar]
- Miyata H., Silverman H. S., Sollott S. J., Lakatta E. G., Stern M. D., Hansford R. G. Measurement of mitochondrial free Ca2+ concentration in living single rat cardiac myocytes. Am J Physiol. 1991 Oct;261(4 Pt 2):H1123–H1134. doi: 10.1152/ajpheart.1991.261.4.H1123. [DOI] [PubMed] [Google Scholar]
- Moore C. L. Specific inhibition of mitochondrial Ca++ transport by ruthenium red. Biochem Biophys Res Commun. 1971 Jan 22;42(2):298–305. doi: 10.1016/0006-291x(71)90102-1. [DOI] [PubMed] [Google Scholar]
- Page E. Quantitative ultrastructural analysis in cardiac membrane physiology. Am J Physiol. 1978 Nov;235(5):C147–C158. doi: 10.1152/ajpcell.1978.235.5.C147. [DOI] [PubMed] [Google Scholar]
- Payet M. D., Schanne O. F., Ruiz-Ceretti E. Competition for slow channel of Ca2+, Mn2+, Verapamil, and D-600 in rat ventricular muscle? J Mol Cell Cardiol. 1980 Jun;12(6):635–638. doi: 10.1016/0022-2828(80)90020-6. [DOI] [PubMed] [Google Scholar]
- Perreault C. L., Shannon R. P., Komamura K., Vatner S. F., Morgan J. P. Abnormalities in intracellular calcium regulation and contractile function in myocardium from dogs with pacing-induced heart failure. J Clin Invest. 1992 Mar;89(3):932–938. doi: 10.1172/JCI115674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sollott S. J., Ziman B. D., Lakatta E. G. Novel technique to load indo-1 free acid into single adult cardiac myocytes to assess cytosolic Ca2+. Am J Physiol. 1992 Jun;262(6 Pt 2):H1941–H1949. doi: 10.1152/ajpheart.1992.262.6.H1941. [DOI] [PubMed] [Google Scholar]
- Spurgeon H. A., Stern M. D., Baartz G., Raffaeli S., Hansford R. G., Talo A., Lakatta E. G., Capogrossi M. C. Simultaneous measurement of Ca2+, contraction, and potential in cardiac myocytes. Am J Physiol. 1990 Feb;258(2 Pt 2):H574–H586. doi: 10.1152/ajpheart.1990.258.2.H574. [DOI] [PubMed] [Google Scholar]
- Steenbergen C., Murphy E., Levy L., London R. E. Elevation in cytosolic free calcium concentration early in myocardial ischemia in perfused rat heart. Circ Res. 1987 May;60(5):700–707. doi: 10.1161/01.res.60.5.700. [DOI] [PubMed] [Google Scholar]
- Wikman-Coffelt J., Wu S. T., Parmley W. W. Intracellular endocardial calcium and myocardial function in rat hearts. Cell Calcium. 1991 Jan;12(1):39–50. doi: 10.1016/0143-4160(91)90083-q. [DOI] [PubMed] [Google Scholar]


