Abstract
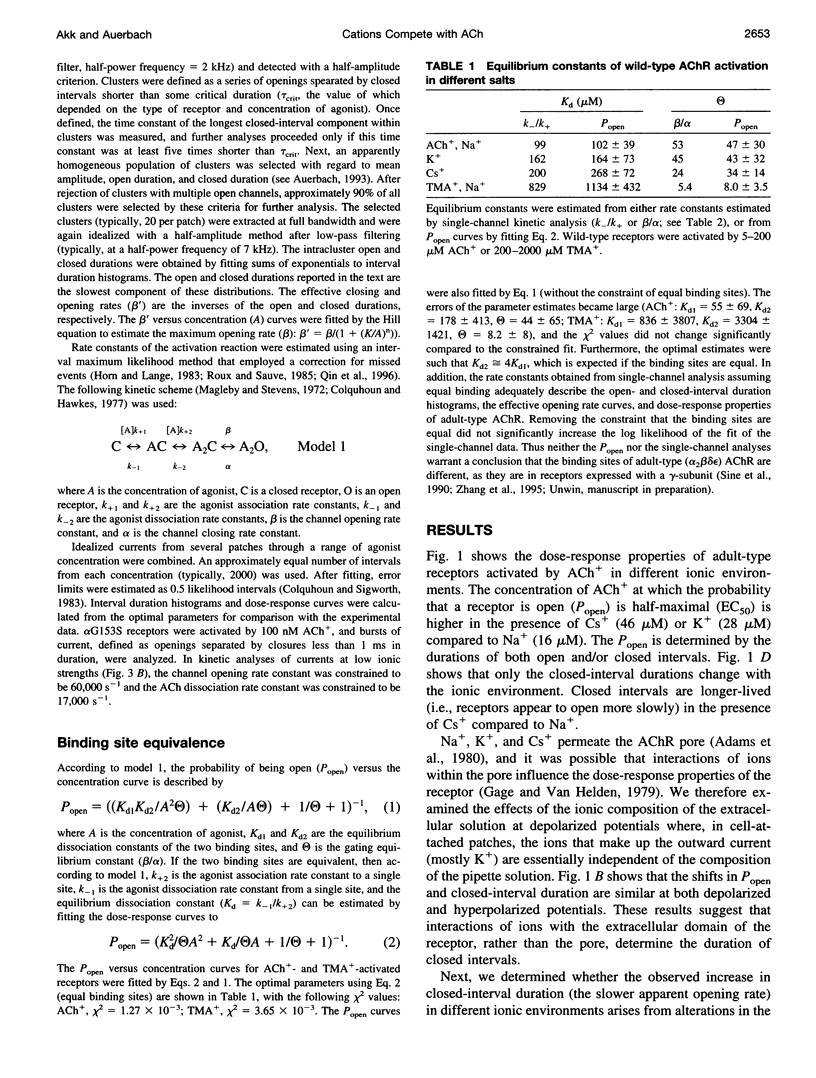
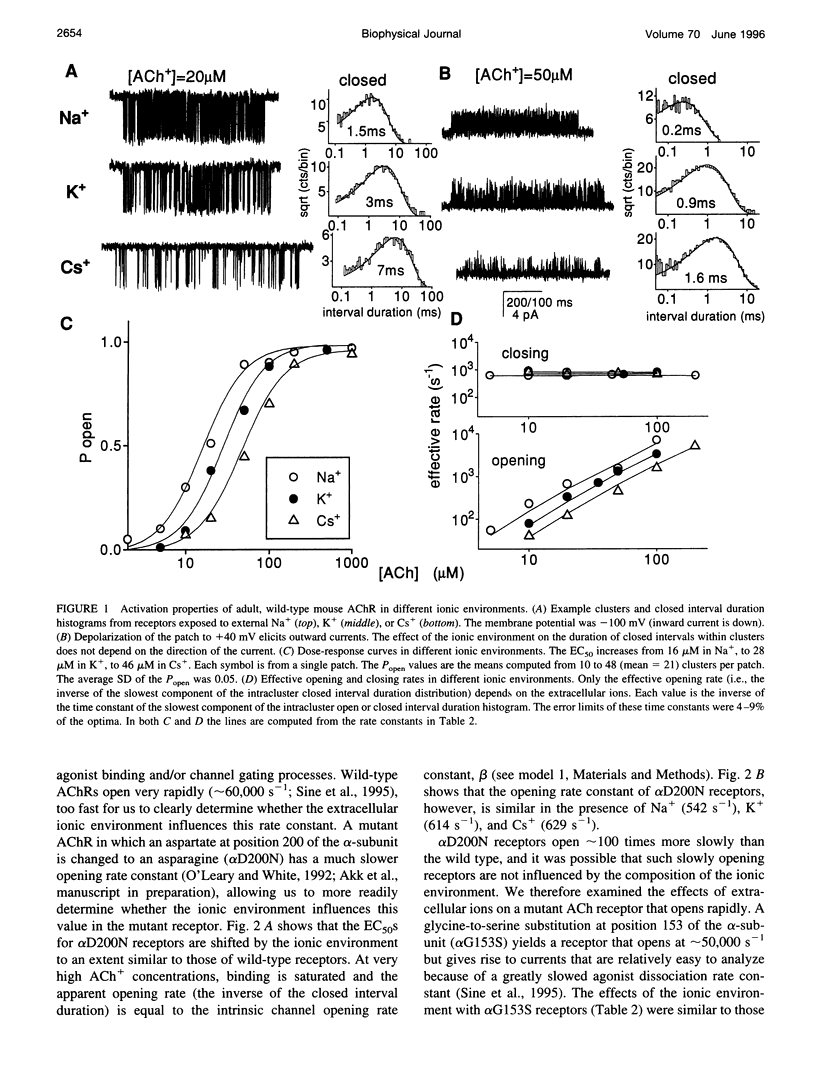
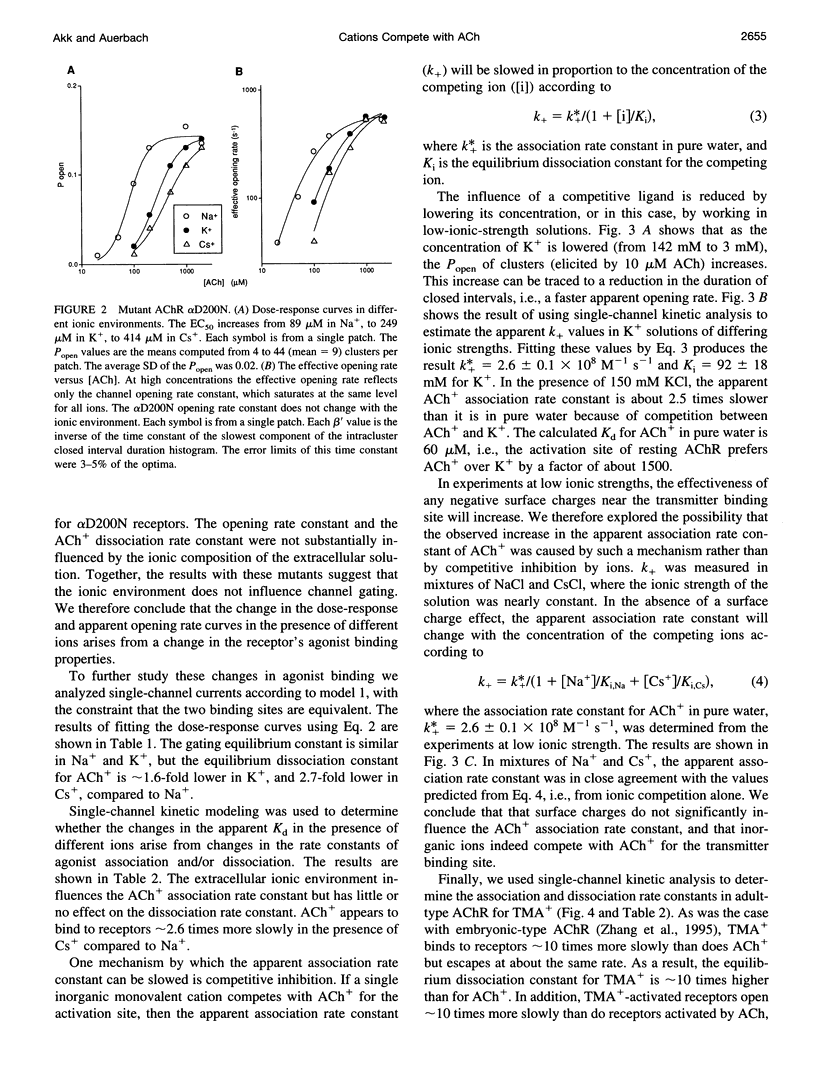
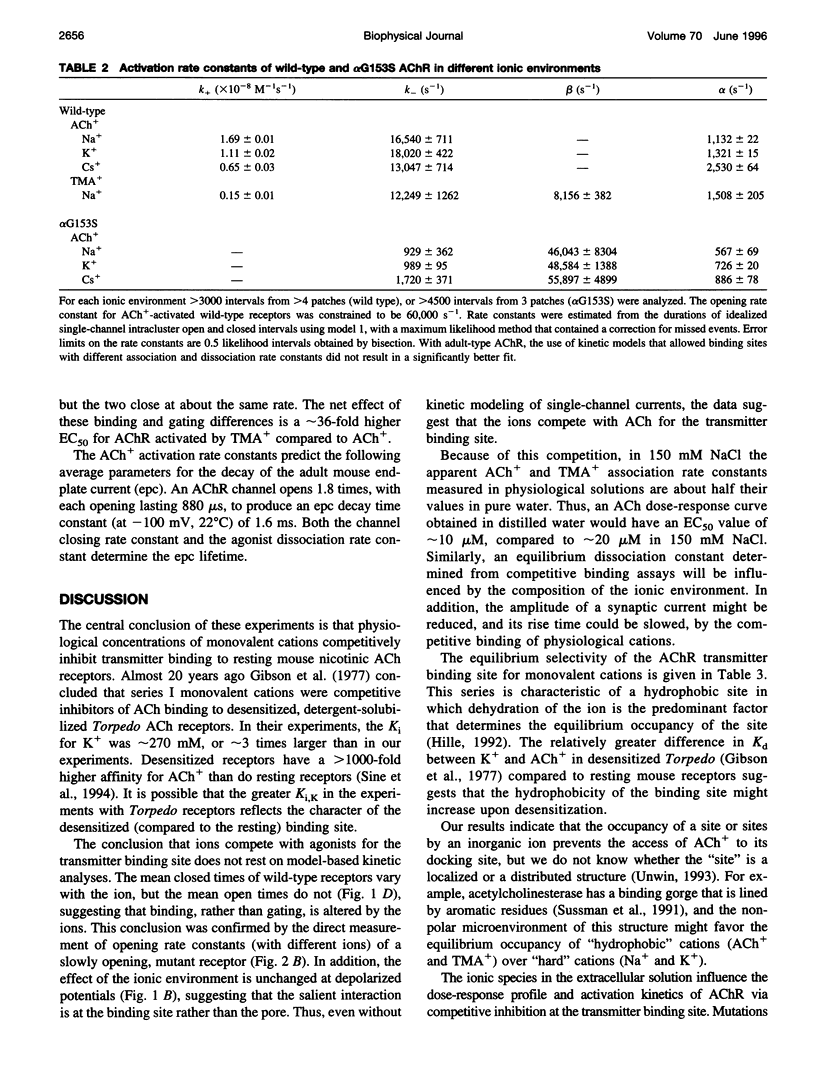
The properties of adult mouse recombinant nicotinic acetylcholine receptors activated by acetylcholine (ACh+) or tetramethylammonium (TMA+) were examined at the single-channel level. The midpoint of the dose-response curve depended on the type of monovalent cation present in the extracellular solution. The shifts in the midpoint were apparent with both inward and outward currents, suggesting that the salient interaction is with the extracellular domain of the receptor. Kinetic modeling was used to estimate the rate constants for agonist binding and channel gating in both wild-type and mutant receptors exposed to Na+, K+, or Cs+. The results indicate that in adult receptors, the two binding sites have the same equilibrium dissociation constant for agonists. The agonist association rate constant was influenced by the ionic composition of the extracellular solution whereas the rate constants for agonist dissociation, channel opening, and channel closing were not. In low-ionic-strength solutions the apparent association rate constant increased in a manner that suggests that inorganic cations are competitive inhibitors of ACh+ binding. There was no evidence of an electrostatic potential at the transmitter binding site. The equilibrium dissociation constants for inorganic ions (Na+, 151 mM; K+, 92 mM; Cs+, 38 mM) and agonists (TMA+, 0.5 mM) indicate that the transmitter binding site is hydrophobic. Under physiological conditions, about half of the binding sites in resting receptors are occupied by Na+.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. J., Dwyer T. M., Hille B. The permeability of endplate channels to monovalent and divalent metal cations. J Gen Physiol. 1980 May;75(5):493–510. doi: 10.1085/jgp.75.5.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auerbach A. A statistical analysis of acetylcholine receptor activation in Xenopus myocytes: stepwise versus concerted models of gating. J Physiol. 1993 Feb;461:339–378. doi: 10.1113/jphysiol.1993.sp019517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aylwin M. L., White M. M. Gating properties of mutant acetylcholine receptors. Mol Pharmacol. 1994 Dec;46(6):1149–1155. [PubMed] [Google Scholar]
- Changeux J. P., Galzi J. L., Devillers-Thiéry A., Bertrand D. The functional architecture of the acetylcholine nicotinic receptor explored by affinity labelling and site-directed mutagenesis. Q Rev Biophys. 1992 Nov;25(4):395–432. doi: 10.1017/s0033583500004352. [DOI] [PubMed] [Google Scholar]
- Chen J., Zhang Y., Akk G., Sine S., Auerbach A. Activation kinetics of recombinant mouse nicotinic acetylcholine receptors: mutations of alpha-subunit tyrosine 190 affect both binding and gating. Biophys J. 1995 Sep;69(3):849–859. doi: 10.1016/S0006-3495(95)79959-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D., Hawkes A. G. Relaxation and fluctuations of membrane currents that flow through drug-operated channels. Proc R Soc Lond B Biol Sci. 1977 Nov 14;199(1135):231–262. doi: 10.1098/rspb.1977.0137. [DOI] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Interaction at end-plate receptors between different choline derivatives. Proc R Soc Lond B Biol Sci. 1957 May 7;146(924):369–381. doi: 10.1098/rspb.1957.0018. [DOI] [PubMed] [Google Scholar]
- Gage P. W., Van Helden D. Effects of permeant monovalent cations on end-plate channels. J Physiol. 1979 Mar;288:509–528. [PMC free article] [PubMed] [Google Scholar]
- Gibson R. E., Juni S., O'Brien R. D. Monovalent ion effects on acetylcholine receptor from Torpedo californica. Arch Biochem Biophys. 1977 Feb;179(1):183–188. doi: 10.1016/0003-9861(77)90102-3. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hirano T., Kidokoro Y., Ohmori H. Acetylcholine dose-response relation and the effect of cesium ions in the rat adrenal chromaffin cell under voltage clamp. Pflugers Arch. 1987 Apr;408(4):401–407. doi: 10.1007/BF00581136. [DOI] [PubMed] [Google Scholar]
- Horn R., Lange K. Estimating kinetic constants from single channel data. Biophys J. 1983 Aug;43(2):207–223. doi: 10.1016/S0006-3495(83)84341-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magleby K. L., Stevens C. F. A quantitative description of end-plate currents. J Physiol. 1972 May;223(1):173–197. doi: 10.1113/jphysiol.1972.sp009840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall C. G., Ogden D., Colquhoun D. Activation of ion channels in the frog endplate by several analogues of acetylcholine. J Physiol. 1991 Feb;433:73–93. doi: 10.1113/jphysiol.1991.sp018415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Leary M. E., White M. M. Mutational analysis of ligand-induced activation of the Torpedo acetylcholine receptor. J Biol Chem. 1992 Apr 25;267(12):8360–8365. [PubMed] [Google Scholar]
- Qin F., Auerbach A., Sachs F. Estimating single-channel kinetic parameters from idealized patch-clamp data containing missed events. Biophys J. 1996 Jan;70(1):264–280. doi: 10.1016/S0006-3495(96)79568-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux B., Sauvé R. A general solution to the time interval omission problem applied to single channel analysis. Biophys J. 1985 Jul;48(1):149–158. doi: 10.1016/S0006-3495(85)83768-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakmann B., Patlak J., Neher E. Single acetylcholine-activated channels show burst-kinetics in presence of desensitizing concentrations of agonist. Nature. 1980 Jul 3;286(5768):71–73. doi: 10.1038/286071a0. [DOI] [PubMed] [Google Scholar]
- Sine S. M., Claudio T., Sigworth F. J. Activation of Torpedo acetylcholine receptors expressed in mouse fibroblasts. Single channel current kinetics reveal distinct agonist binding affinities. J Gen Physiol. 1990 Aug;96(2):395–437. doi: 10.1085/jgp.96.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sine S. M. Molecular dissection of subunit interfaces in the acetylcholine receptor: identification of residues that determine curare selectivity. Proc Natl Acad Sci U S A. 1993 Oct 15;90(20):9436–9440. doi: 10.1073/pnas.90.20.9436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sine S. M., Ohno K., Bouzat C., Auerbach A., Milone M., Pruitt J. N., Engel A. G. Mutation of the acetylcholine receptor alpha subunit causes a slow-channel myasthenic syndrome by enhancing agonist binding affinity. Neuron. 1995 Jul;15(1):229–239. doi: 10.1016/0896-6273(95)90080-2. [DOI] [PubMed] [Google Scholar]
- Sine S. M., Quiram P., Papanikolaou F., Kreienkamp H. J., Taylor P. Conserved tyrosines in the alpha subunit of the nicotinic acetylcholine receptor stabilize quaternary ammonium groups of agonists and curariform antagonists. J Biol Chem. 1994 Mar 25;269(12):8808–8816. [PubMed] [Google Scholar]
- Smith S. M., McBurney R. N. Caesium ions: a glycine-activated channel agonist in rat spinal cord neurones grown in cell culture. Br J Pharmacol. 1989 Apr;96(4):940–948. doi: 10.1111/j.1476-5381.1989.tb11905.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussman J. L., Harel M., Frolow F., Oefner C., Goldman A., Toker L., Silman I. Atomic structure of acetylcholinesterase from Torpedo californica: a prototypic acetylcholine-binding protein. Science. 1991 Aug 23;253(5022):872–879. doi: 10.1126/science.1678899. [DOI] [PubMed] [Google Scholar]
- Tomaselli G. F., McLaughlin J. T., Jurman M. E., Hawrot E., Yellen G. Mutations affecting agonist sensitivity of the nicotinic acetylcholine receptor. Biophys J. 1991 Sep;60(3):721–727. doi: 10.1016/S0006-3495(91)82102-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unwin N. Nicotinic acetylcholine receptor at 9 A resolution. J Mol Biol. 1993 Feb 20;229(4):1101–1124. doi: 10.1006/jmbi.1993.1107. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Chen J., Auerbach A. Activation of recombinant mouse acetylcholine receptors by acetylcholine, carbamylcholine and tetramethylammonium. J Physiol. 1995 Jul 1;486(Pt 1):189–206. doi: 10.1113/jphysiol.1995.sp020802. [DOI] [PMC free article] [PubMed] [Google Scholar]


