Abstract
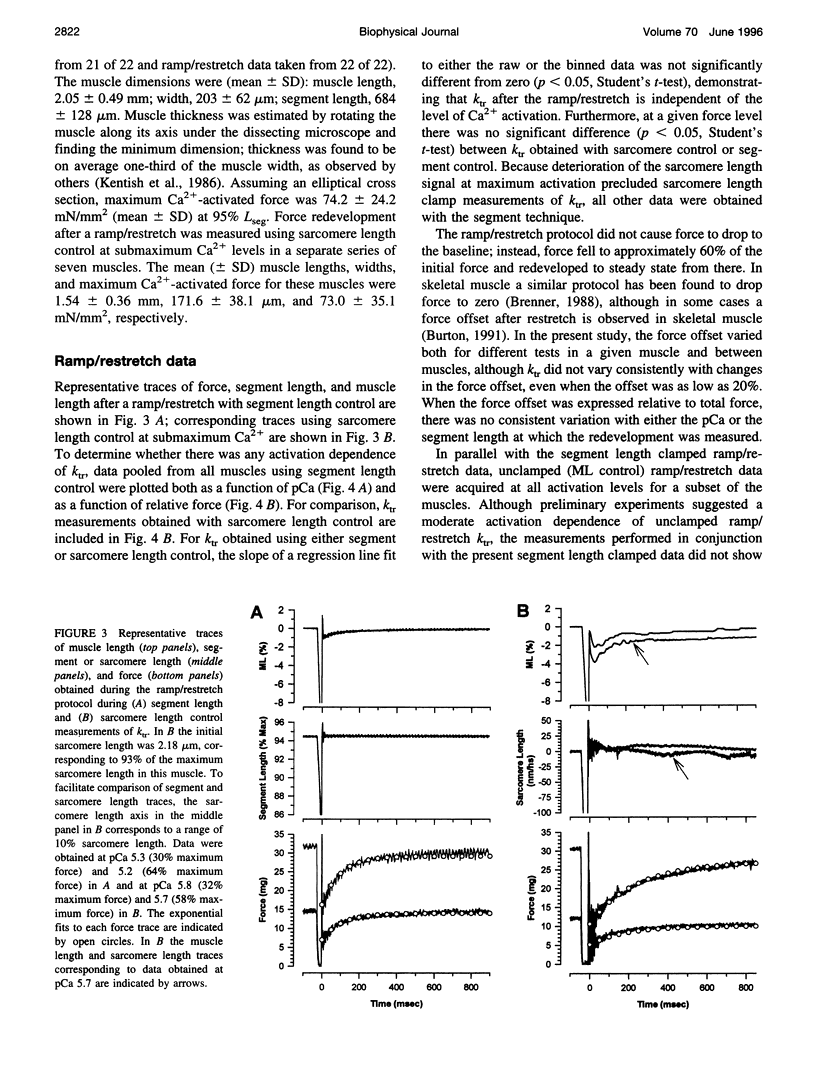
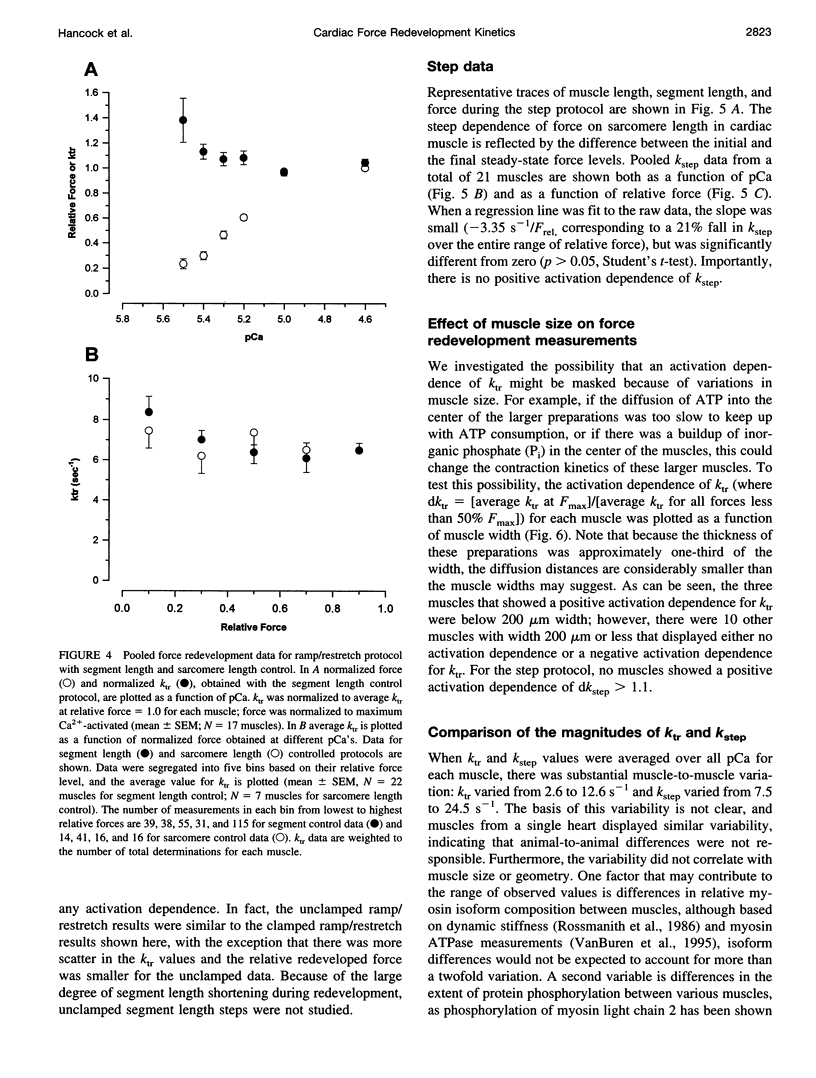
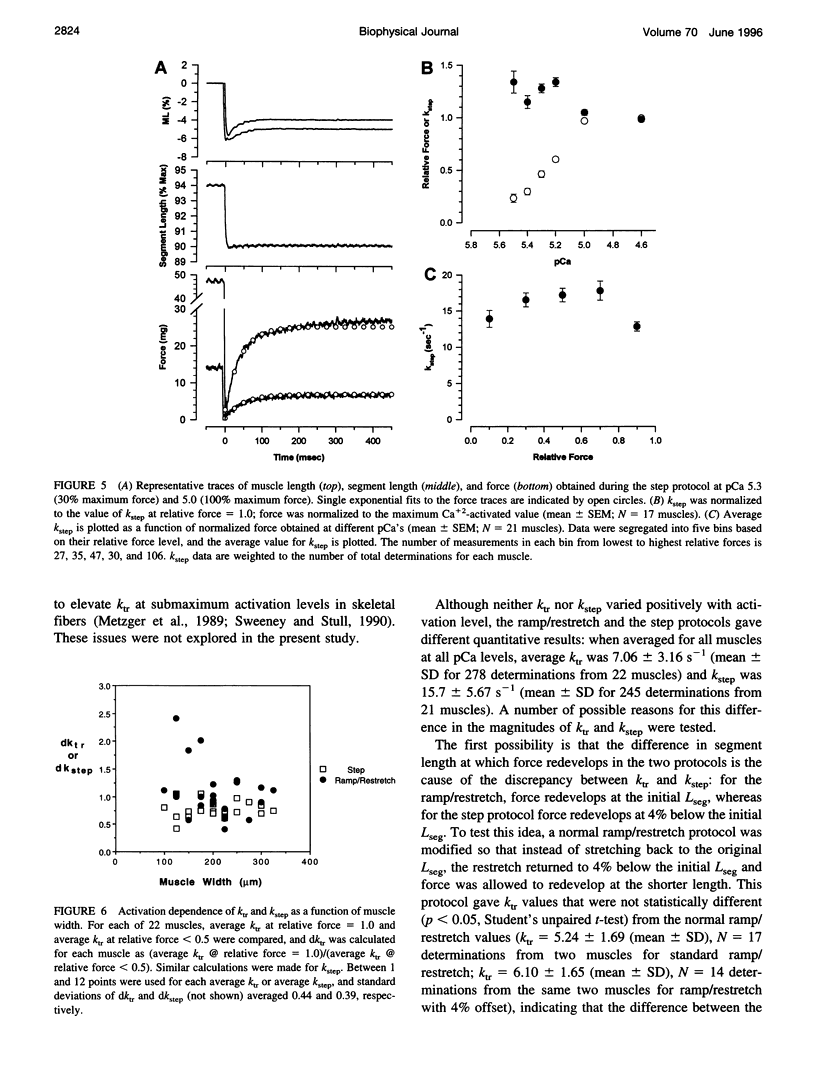
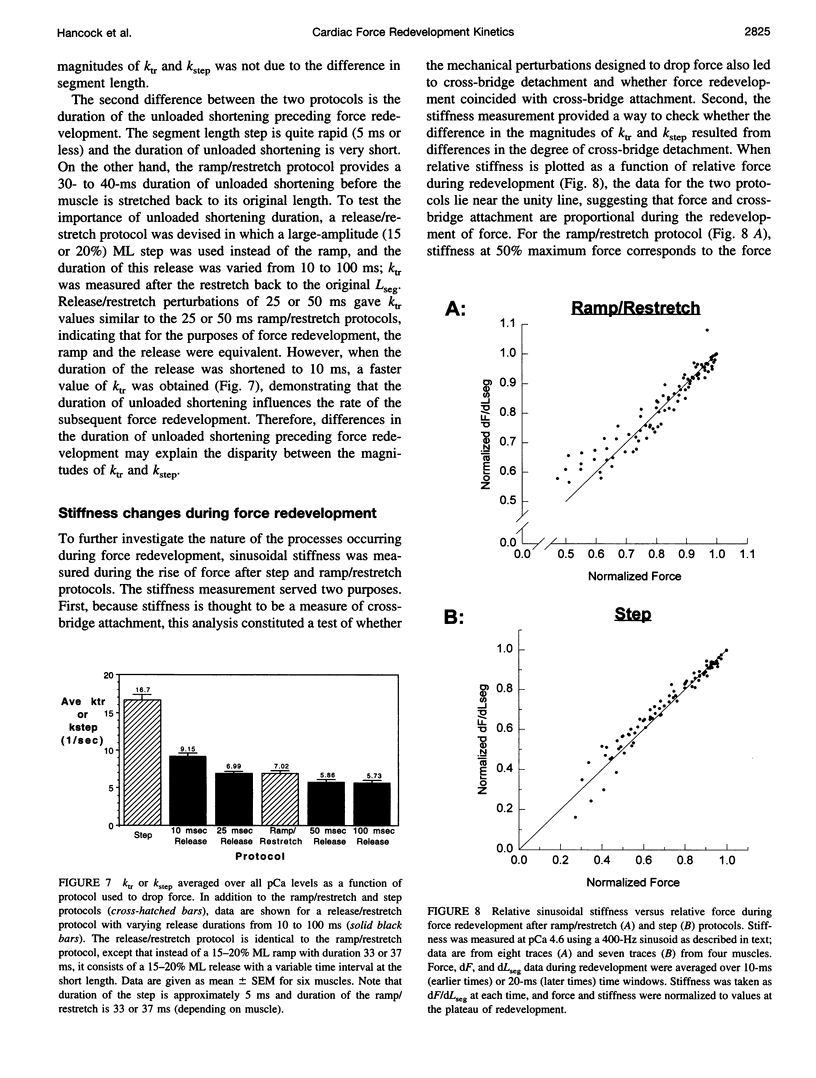
The influence of Ca2+ on isometric force kinetics was studied in skinned rat ventricular trabeculae by measuring the kinetics of force redevelopment after a transient decrease in force. Two protocols were employed to rapidly detach cycling myosin cross-bridges: a large-amplitude muscle length ramp followed by a restretch back to the original length or a 4% segment length step. During the recovery of force, the length of the central region of the muscle was controlled by using a segment marker technique and software feedback control. Tension redevelopment was fit by a rising exponential governed by the rate constant ktr for the ramp/restretch protocol and kstep for the step protocol. ktr and kstep averaged 7.06 s-1 and 15.7 s-1, respectively, at 15 degrees C; neither ktr nor kstep increased with the level of Ca2+ activation. Similar results were found at submaximum Ca2+ levels when sarcomere length control by laser diffraction was used. The lack of activation dependence of ktr contrasts with results from fast skeletal fibers, in which ktr varies 10-fold from low to high activation levels, and suggests that Ca2+ does not modulate the kinetics of cross-bridge attachment or detachment in mammalian cardiac muscle.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen D. G., Kentish J. C. Calcium concentration in the myoplasm of skinned ferret ventricular muscle following changes in muscle length. J Physiol. 1988 Dec;407:489–503. doi: 10.1113/jphysiol.1988.sp017427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araujo A., Walker J. W. Kinetics of tension development in skinned cardiac myocytes measured by photorelease of Ca2+. Am J Physiol. 1994 Nov;267(5 Pt 2):H1643–H1653. doi: 10.1152/ajpheart.1994.267.5.H1643. [DOI] [PubMed] [Google Scholar]
- Backx P. H., Ter Keurs H. E. Fluorescent properties of rat cardiac trabeculae microinjected with fura-2 salt. Am J Physiol. 1993 Apr;264(4 Pt 2):H1098–H1110. doi: 10.1152/ajpheart.1993.264.4.H1098. [DOI] [PubMed] [Google Scholar]
- Brenner B. Effect of Ca2+ on cross-bridge turnover kinetics in skinned single rabbit psoas fibers: implications for regulation of muscle contraction. Proc Natl Acad Sci U S A. 1988 May;85(9):3265–3269. doi: 10.1073/pnas.85.9.3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner B., Eisenberg E. Rate of force generation in muscle: correlation with actomyosin ATPase activity in solution. Proc Natl Acad Sci U S A. 1986 May;83(10):3542–3546. doi: 10.1073/pnas.83.10.3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase P. B., Kushmerick M. J. Effect of physiological ADP concentrations on contraction of single skinned fibers from rabbit fast and slow muscles. Am J Physiol. 1995 Feb;268(2 Pt 1):C480–C489. doi: 10.1152/ajpcell.1995.268.2.C480. [DOI] [PubMed] [Google Scholar]
- Chase P. B., Kushmerick M. J. Effects of pH on contraction of rabbit fast and slow skeletal muscle fibers. Biophys J. 1988 Jun;53(6):935–946. doi: 10.1016/S0006-3495(88)83174-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase P. B., Martyn D. A., Hannon J. D. Isometric force redevelopment of skinned muscle fibers from rabbit activated with and without Ca2+. Biophys J. 1994 Nov;67(5):1994–2001. doi: 10.1016/S0006-3495(94)80682-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman Y. E., Simmons R. M. Control of sarcomere length in skinned muscle fibres of Rana temporaria during mechanical transients. J Physiol. 1984 May;350:497–518. doi: 10.1113/jphysiol.1984.sp015215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock W. O., Martyn D. A., Huntsman L. L. Ca2+ and segment length dependence of isometric force kinetics in intact ferret cardiac muscle. Circ Res. 1993 Oct;73(4):603–611. doi: 10.1161/01.res.73.4.603. [DOI] [PubMed] [Google Scholar]
- Hoh J. F., Rossmanith G. H., Kwan L. J., Hamilton A. M. Adrenaline increases the rate of cycling of crossbridges in rat cardiac muscle as measured by pseudo-random binary noise-modulated perturbation analysis. Circ Res. 1988 Mar;62(3):452–461. doi: 10.1161/01.res.62.3.452. [DOI] [PubMed] [Google Scholar]
- Huntsman L. L., Rondinone J. F., Martyn D. A. Force-length relations in cardiac muscle segments. Am J Physiol. 1983 May;244(5):H701–H707. doi: 10.1152/ajpheart.1983.244.5.H701. [DOI] [PubMed] [Google Scholar]
- Kentish J. C., Stienen G. J. Differential effects of length on maximum force production and myofibrillar ATPase activity in rat skinned cardiac muscle. J Physiol. 1994 Feb 15;475(1):175–184. doi: 10.1113/jphysiol.1994.sp020059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kentish J. C. The effects of inorganic phosphate and creatine phosphate on force production in skinned muscles from rat ventricle. J Physiol. 1986 Jan;370:585–604. doi: 10.1113/jphysiol.1986.sp015952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kentish J. C., ter Keurs H. E., Ricciardi L., Bucx J. J., Noble M. I. Comparison between the sarcomere length-force relations of intact and skinned trabeculae from rat right ventricle. Influence of calcium concentrations on these relations. Circ Res. 1986 Jun;58(6):755–768. doi: 10.1161/01.res.58.6.755. [DOI] [PubMed] [Google Scholar]
- Kress M., Huxley H. E., Faruqi A. R., Hendrix J. Structural changes during activation of frog muscle studied by time-resolved X-ray diffraction. J Mol Biol. 1986 Apr 5;188(3):325–342. doi: 10.1016/0022-2836(86)90158-0. [DOI] [PubMed] [Google Scholar]
- Lehman W., Craig R., Vibert P. Ca(2+)-induced tropomyosin movement in Limulus thin filaments revealed by three-dimensional reconstruction. Nature. 1994 Mar 3;368(6466):65–67. doi: 10.1038/368065a0. [DOI] [PubMed] [Google Scholar]
- Lompré A. M., Nadal-Ginard B., Mahdavi V. Expression of the cardiac ventricular alpha- and beta-myosin heavy chain genes is developmentally and hormonally regulated. J Biol Chem. 1984 May 25;259(10):6437–6446. [PubMed] [Google Scholar]
- Martyn D. A., Gordon A. M. Force and stiffness in glycerinated rabbit psoas fibers. Effects of calcium and elevated phosphate. J Gen Physiol. 1992 May;99(5):795–816. doi: 10.1085/jgp.99.5.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martyn D. A., Gordon A. M. Length and myofilament spacing-dependent changes in calcium sensitivity of skeletal fibres: effects of pH and ionic strength. J Muscle Res Cell Motil. 1988 Oct;9(5):428–445. doi: 10.1007/BF01774069. [DOI] [PubMed] [Google Scholar]
- Metzger J. M., Greaser M. L., Moss R. L. Variations in cross-bridge attachment rate and tension with phosphorylation of myosin in mammalian skinned skeletal muscle fibers. Implications for twitch potentiation in intact muscle. J Gen Physiol. 1989 May;93(5):855–883. doi: 10.1085/jgp.93.5.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger J. M., Moss R. L. Calcium-sensitive cross-bridge transitions in mammalian fast and slow skeletal muscle fibers. Science. 1990 Mar 2;247(4946):1088–1090. doi: 10.1126/science.2309121. [DOI] [PubMed] [Google Scholar]
- Millar N. C., Homsher E. The effect of phosphate and calcium on force generation in glycerinated rabbit skeletal muscle fibers. A steady-state and transient kinetic study. J Biol Chem. 1990 Nov 25;265(33):20234–20240. [PubMed] [Google Scholar]
- Parry D. A., Squire J. M. Structural role of tropomyosin in muscle regulation: analysis of the x-ray diffraction patterns from relaxed and contracting muscles. J Mol Biol. 1973 Mar 25;75(1):33–55. doi: 10.1016/0022-2836(73)90527-5. [DOI] [PubMed] [Google Scholar]
- Rossmanith G. H., Hoh J. F., Kirman A., Kwan L. J. Influence of V1 and V3 isomyosins on the mechanical behaviour of rat papillary muscle as studied by pseudo-random binary noise modulated length perturbations. J Muscle Res Cell Motil. 1986 Aug;7(4):307–319. doi: 10.1007/BF01753651. [DOI] [PubMed] [Google Scholar]
- Shibata T., Hunter W. C., Yang A., Sagawa K. Dynamic stiffness measured in central segment of excised rabbit papillary muscles during barium contracture. Circ Res. 1987 May;60(5):756–769. doi: 10.1161/01.res.60.5.756. [DOI] [PubMed] [Google Scholar]
- Sweeney H. L., Stull J. T. Alteration of cross-bridge kinetics by myosin light chain phosphorylation in rabbit skeletal muscle: implications for regulation of actin-myosin interaction. Proc Natl Acad Sci U S A. 1990 Jan;87(1):414–418. doi: 10.1073/pnas.87.1.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanBuren P., Harris D. E., Alpert N. R., Warshaw D. M. Cardiac V1 and V3 myosins differ in their hydrolytic and mechanical activities in vitro. Circ Res. 1995 Aug;77(2):439–444. doi: 10.1161/01.res.77.2.439. [DOI] [PubMed] [Google Scholar]
- Wilkinson J. M. Troponin C from rabbit slow skeletal and cardiac muscle is the product of a single gene. Eur J Biochem. 1980 Jan;103(1):179–188. doi: 10.1111/j.1432-1033.1980.tb04302.x. [DOI] [PubMed] [Google Scholar]
- Wolff M. R., McDonald K. S., Moss R. L. Rate of tension development in cardiac muscle varies with level of activator calcium. Circ Res. 1995 Jan;76(1):154–160. doi: 10.1161/01.res.76.1.154. [DOI] [PubMed] [Google Scholar]



