Abstract
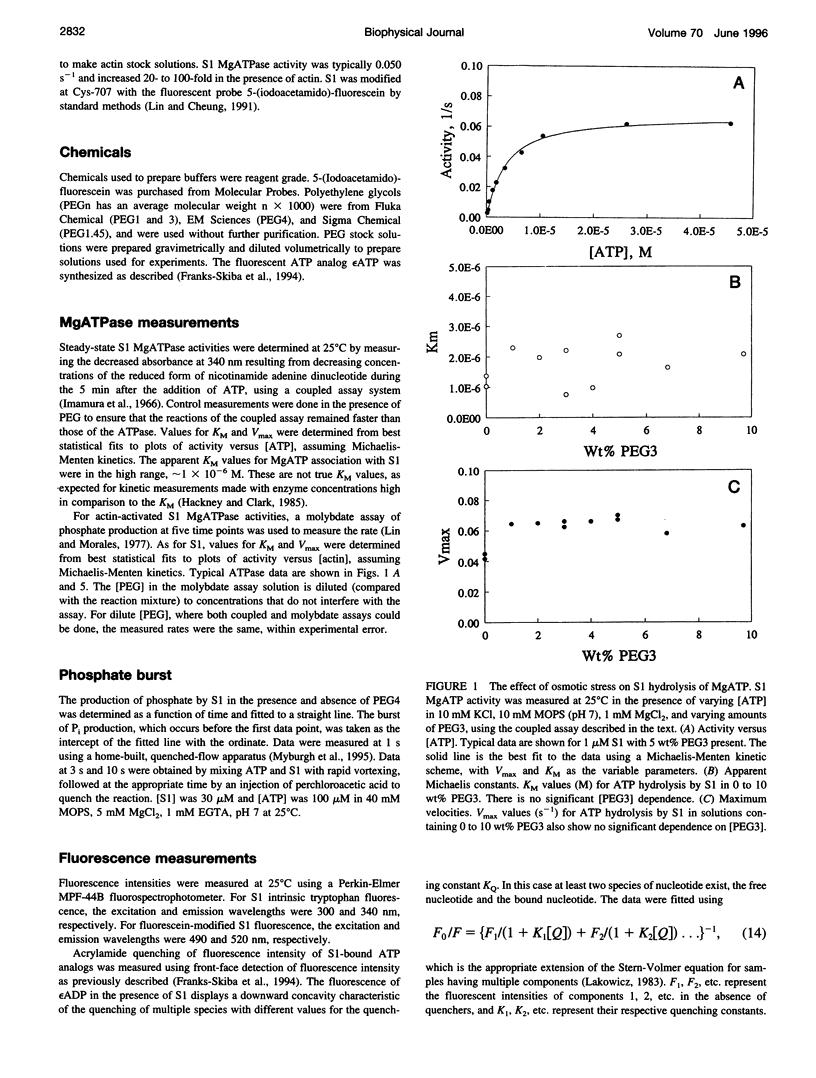
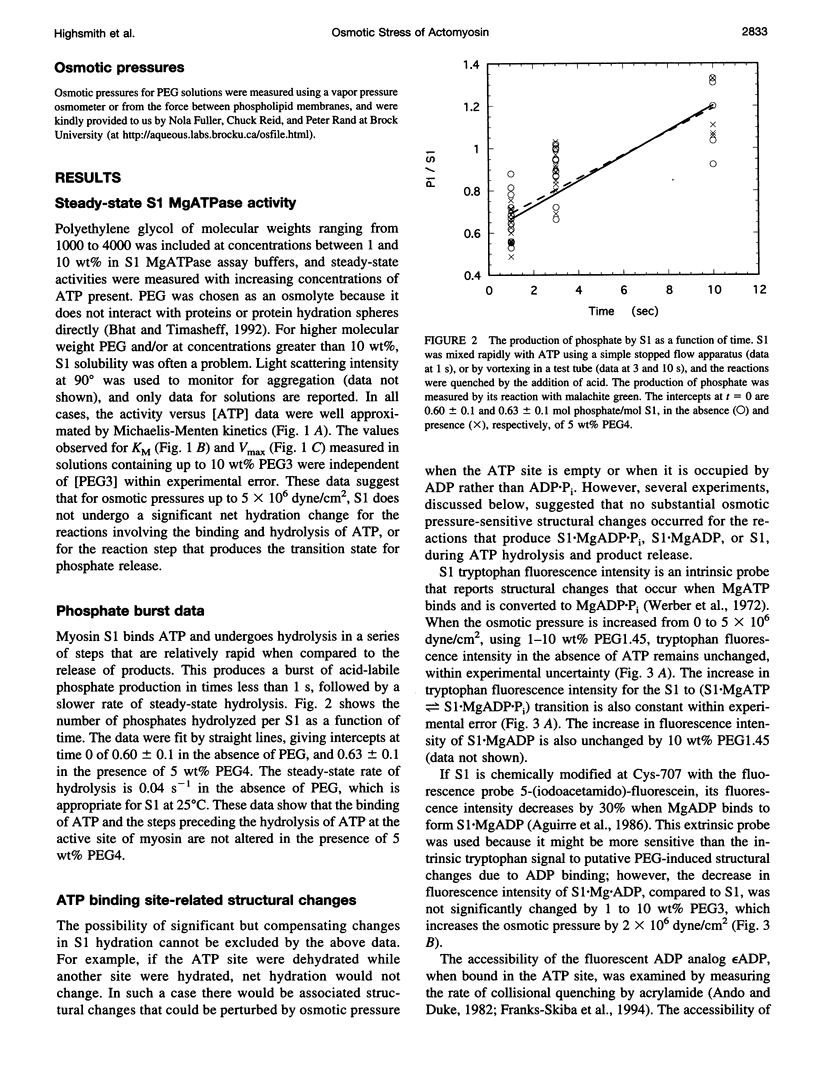
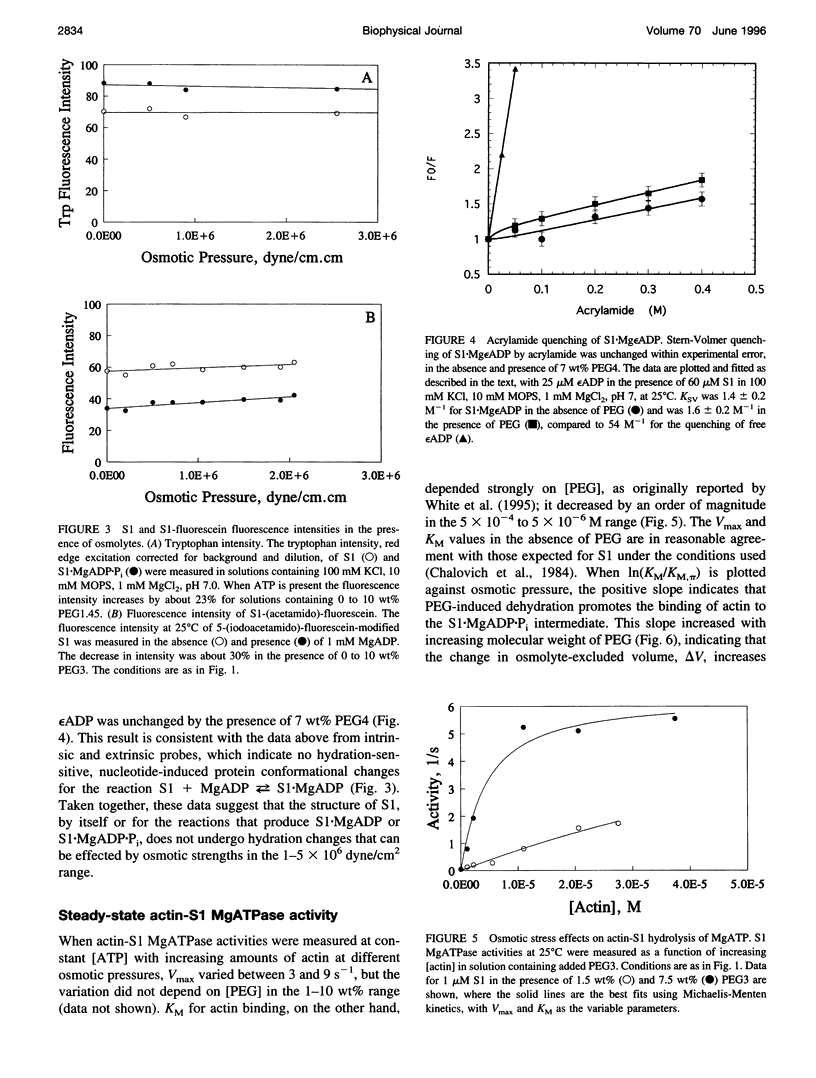
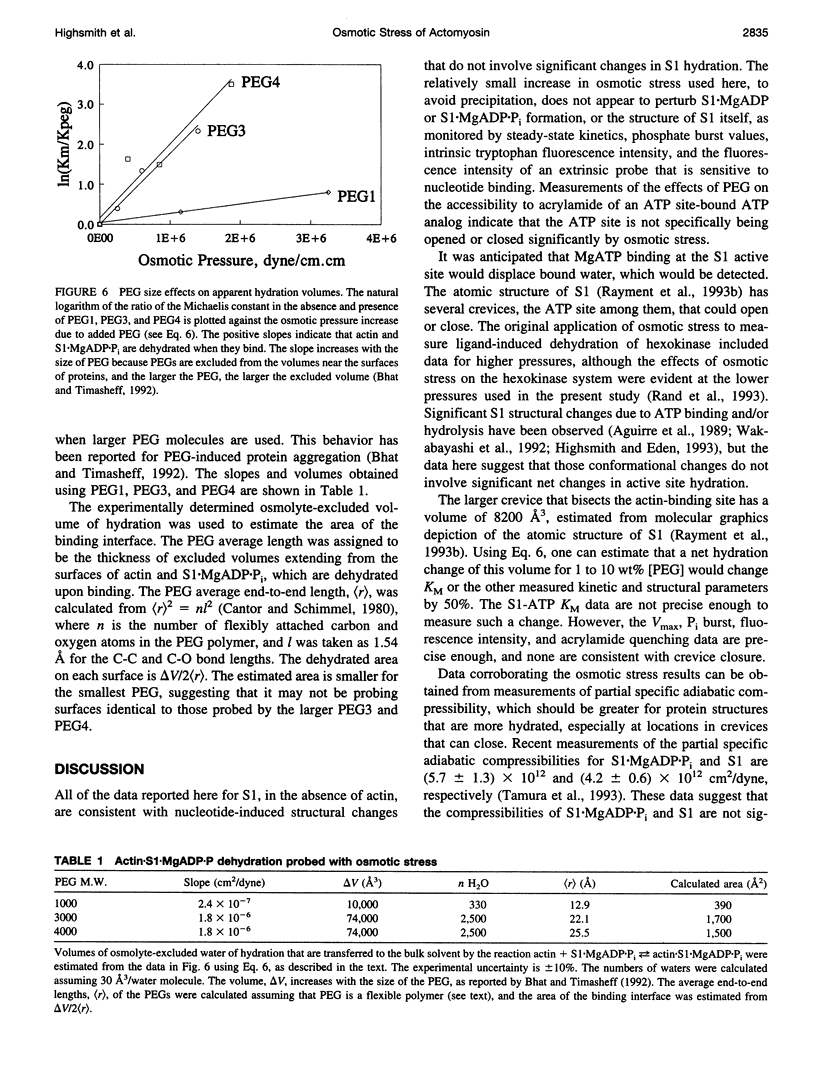
Osmotic stress in the 0.5-5 x 10(6) dyne/cm2 range was used to perturb the hydration of actin-myosin-ATP intermediates during steady-state hydrolysis. Polyethylene glycol (PEG) (1000 to 4000 Da), in the 1 to 10 wt% range, which does not cause protein precipitation, did not significantly affect the apparent KM or the Vmax for MgATP hydrolysis by myosin subfragment 1 (S1) alone, nor did it affect the value for the phosphate burst. Consistent with the kinetic data, osmotic stress did not affect nucleotide-induced changes in the fluorescence intensities of S1 tryptophans or of fluorescein attached to Cys-707. The accessibility of the fluorescent ATP analog, epsilon ADP, to acrylamide quenching was also unchanged. These data suggest that none of the steps in the ATP hydrolysis cycle involve substantial hydration changes, which might occur for the opening or closing of the ATP site or of other crevices in the S1 structure. In contrast, KM for the interaction of S1.MgADP.Pi with actin decreased tenfold in this range of osmotic pressure, suggesting that formation of actin.S1.MgADP.Pi involves net dehydration of the proteins. The dehydration volume increases as the size of the PEG is increased, as expected for a surface-excluded osmolyte. The measured dehydration volume for the formation of actin.S1.MgADP.Pi was used to estimate the surface area of the binding interface. This estimate was consistent with the area determined from the atomic structures of actin and myosin, indicating that osmotic stress is a reliable probe of actin.myosin.ATP interactions. The approach developed here should be useful for determining osmotic stress and excluded volume effects in situ, which are much larger than those of typical in vitro conditions.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aguirre R., Gonsoulin F., Cheung H. C. Interaction of fluorescently labeled myosin subfragment 1 with nucleotides and actin. Biochemistry. 1986 Nov 4;25(22):6827–6835. doi: 10.1021/bi00370a015. [DOI] [PubMed] [Google Scholar]
- Aguirre R., Lin S. H., Gonsoulin F., Wang C. K., Cheung H. C. Characterization of the ethenoadenosine diphosphate binding site of myosin subfragment 1. Energetics of the equilibrium between two states of nucleotide.S1 and vanadate-induced global conformation changes detected by energy transfer. Biochemistry. 1989 Jan 24;28(2):799–807. doi: 10.1021/bi00428a058. [DOI] [PubMed] [Google Scholar]
- Ando T., Duke J. A. The process in which nucleotide is buried into the active site of heavy meromyosin. Biochem Biophys Res Commun. 1983 Aug 30;115(1):312–316. doi: 10.1016/0006-291x(83)91005-7. [DOI] [PubMed] [Google Scholar]
- Bhat R., Timasheff S. N. Steric exclusion is the principal source of the preferential hydration of proteins in the presence of polyethylene glycols. Protein Sci. 1992 Sep;1(9):1133–1143. doi: 10.1002/pro.5560010907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalovich J. M., Stein L. A., Greene L. E., Eisenberg E. Interaction of isozymes of myosin subfragment 1 with actin: effect of ionic strength and nucleotide. Biochemistry. 1984 Oct 9;23(21):4885–4889. doi: 10.1021/bi00316a011. [DOI] [PubMed] [Google Scholar]
- Coates J. H., Criddle A. H., Geeves M. A. Pressure-relaxation studies of pyrene-labelled actin and myosin subfragment 1 from rabbit skeletal muscle. Evidence for two states of acto-subfragment 1. Biochem J. 1985 Dec 1;232(2):351–356. doi: 10.1042/bj2320351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks-Skiba K., Hwang T., Cooke R. Quenching of fluorescent nucleotides bound to myosin: a probe of the active-site conformation. Biochemistry. 1994 Oct 25;33(42):12720–12728. doi: 10.1021/bi00208a025. [DOI] [PubMed] [Google Scholar]
- Garner M. M., Rau D. C. Water release associated with specific binding of gal repressor. EMBO J. 1995 Mar 15;14(6):1257–1263. doi: 10.1002/j.1460-2075.1995.tb07109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackney D. D., Clark P. K. Steady state kinetics at high enzyme concentration. The myosin MgATPase. J Biol Chem. 1985 May 10;260(9):5505–5510. [PubMed] [Google Scholar]
- Highsmith S., Eden D. Myosin-ATP chemomechanics. Biochemistry. 1993 Mar 16;32(10):2455–2458. doi: 10.1021/bi00061a001. [DOI] [PubMed] [Google Scholar]
- Highsmith S. The effects of temperature and salts on myosin subfragment-1 and F-actin association. Arch Biochem Biophys. 1977 Apr 30;180(2):404–408. doi: 10.1016/0003-9861(77)90054-6. [DOI] [PubMed] [Google Scholar]
- Imamura K., Tada M., Tonomura Y. The pre-steady state of the myosin--adenosine triphosphate system. IV. Liberation of ADP from the myosin--ATP system and effects of modifiers on the phosphorylation of myosin. J Biochem. 1966 Mar;59(3):280–289. [PubMed] [Google Scholar]
- Kabsch W., Mannherz H. G., Suck D., Pai E. F., Holmes K. C. Atomic structure of the actin:DNase I complex. Nature. 1990 Sep 6;347(6288):37–44. doi: 10.1038/347037a0. [DOI] [PubMed] [Google Scholar]
- Lin S. H., Cheung H. C. Two-state equilibria of myosin subfragment 1 and its complexes with ADP and actin. Biochemistry. 1991 Apr 30;30(17):4317–4322. doi: 10.1021/bi00231a030. [DOI] [PubMed] [Google Scholar]
- Lin T. I., Morales M. F. Application of a one-step procedure for measuring inorganic phosphate in the presence of proteins: the actomyosin ATPase system. Anal Biochem. 1977 Jan;77(1):10–17. doi: 10.1016/0003-2697(77)90284-6. [DOI] [PubMed] [Google Scholar]
- Margossian S. S., Lowey S. Preparation of myosin and its subfragments from rabbit skeletal muscle. Methods Enzymol. 1982;85(Pt B):55–71. doi: 10.1016/0076-6879(82)85009-x. [DOI] [PubMed] [Google Scholar]
- Nauss K. M., Kitagawa S., Gergely J. Pyrophosphate binding to and adenosine triphosphatase activity of myosin and its proteolytic fragments. Implications for the substructure of myosin. J Biol Chem. 1969 Feb 25;244(4):755–765. [PubMed] [Google Scholar]
- Oplatka A. The role of water in the mechanism of muscular contraction. FEBS Lett. 1994 Nov 21;355(1):1–3. doi: 10.1016/0014-5793(94)01158-3. [DOI] [PubMed] [Google Scholar]
- Otting G., Liepinsh E., Wüthrich K. Protein hydration in aqueous solution. Science. 1991 Nov 15;254(5034):974–980. doi: 10.1126/science.1948083. [DOI] [PubMed] [Google Scholar]
- Parsegian V. A., Rand R. P., Fuller N. L., Rau D. C. Osmotic stress for the direct measurement of intermolecular forces. Methods Enzymol. 1986;127:400–416. doi: 10.1016/0076-6879(86)27032-9. [DOI] [PubMed] [Google Scholar]
- Rand R. P., Fuller N. L., Butko P., Francis G., Nicholls P. Measured change in protein solvation with substrate binding and turnover. Biochemistry. 1993 Jun 15;32(23):5925–5929. doi: 10.1021/bi00074a001. [DOI] [PubMed] [Google Scholar]
- Rayment I., Holden H. M., Whittaker M., Yohn C. B., Lorenz M., Holmes K. C., Milligan R. A. Structure of the actin-myosin complex and its implications for muscle contraction. Science. 1993 Jul 2;261(5117):58–65. doi: 10.1126/science.8316858. [DOI] [PubMed] [Google Scholar]
- Rayment I., Rypniewski W. R., Schmidt-Bäse K., Smith R., Tomchick D. R., Benning M. M., Winkelmann D. A., Wesenberg G., Holden H. M. Three-dimensional structure of myosin subfragment-1: a molecular motor. Science. 1993 Jul 2;261(5117):50–58. doi: 10.1126/science.8316857. [DOI] [PubMed] [Google Scholar]
- Robinson C. R., Sligar S. G. Hydrostatic pressure reverses osmotic pressure effects on the specificity of EcoRI-DNA interactions. Biochemistry. 1994 Apr 5;33(13):3787–3793. doi: 10.1021/bi00179a001. [DOI] [PubMed] [Google Scholar]
- Spudich J. A., Watt S. The regulation of rabbit skeletal muscle contraction. I. Biochemical studies of the interaction of the tropomyosin-troponin complex with actin and the proteolytic fragments of myosin. J Biol Chem. 1971 Aug 10;246(15):4866–4871. [PubMed] [Google Scholar]
- Tamura Y., Suzuki N., Mihashi K. Adiabatic compressibility of myosin subfragment-1 and heavy meromyosin with or without nucleotide. Biophys J. 1993 Nov;65(5):1899–1905. doi: 10.1016/S0006-3495(93)81260-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi K., Tokunaga M., Kohno I., Sugimoto Y., Hamanaka T., Takezawa Y., Wakabayashi T., Amemiya Y. Small-angle synchrotron x-ray scattering reveals distinct shape changes of the myosin head during hydrolysis of ATP. Science. 1992 Oct 16;258(5081):443–447. doi: 10.1126/science.1411537. [DOI] [PubMed] [Google Scholar]
- Weeds A. G., Taylor R. S. Separation of subfragment-1 isoenzymes from rabbit skeletal muscle myosin. Nature. 1975 Sep 4;257(5521):54–56. doi: 10.1038/257054a0. [DOI] [PubMed] [Google Scholar]
- Werber M. M., Szent-Györgyi A. G., Fasman G. D. Fluorescence studies on heavy meromyosin-substrate interaction. Biochemistry. 1972 Jul 18;11(15):2872–2883. doi: 10.1021/bi00765a021. [DOI] [PubMed] [Google Scholar]


