Abstract
The near-infrared charge transfer band at 760 nm (band III) has been investigated in deoxy and photodissociated dimeric Scapharca hemoglobin. At 300 K, the 10-ns spectrum of the carbonmonoxy derivative photoproduct is shifted by about 6 nm toward longer wavelengths with respect to the deoxy spectrum, both in buffer and in glycerol/buffer solutions. Moreover, the band III peak occurs at about the same wavelength at 300 K and at 10 K for the 10-ns photodissociated derivative, whereas in the deoxy derivative large changes in peak position and linewidth are observed as a function of temperature. These findings suggest that in dimeric Scapharca hemoglobin the photoproduct has not relaxed after 10 ns. The complete time dependence of the relaxation process has been studied both in buffer and in glycerol/buffer solutions at room temperature. The relaxation from the photoproduct to the deoxy species occurs on a microsecond time scale, in line with recent optical absorption and resonance Raman measurements.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ansari A., Jones C. M., Henry E. R., Hofrichter J., Eaton W. A. Conformational relaxation and ligand binding in myoglobin. Biochemistry. 1994 May 3;33(17):5128–5145. doi: 10.1021/bi00183a017. [DOI] [PubMed] [Google Scholar]
- Ansari A., Jones C. M., Henry E. R., Hofrichter J., Eaton W. A. The role of solvent viscosity in the dynamics of protein conformational changes. Science. 1992 Jun 26;256(5065):1796–1798. doi: 10.1126/science.1615323. [DOI] [PubMed] [Google Scholar]
- Boffi A., Verzili D., Chiancone E., Leone M., Cupane A., Militello V., Vitrano E., Cordone L., Yu W., Di Iorio E. E. Stereodynamic properties of the cooperative homodimeric Scapharca inaequivalvis hemoglobin studied through optical absorption spectroscopy and ligand rebinding kinetics. Biophys J. 1994 Oct;67(4):1713–1723. doi: 10.1016/S0006-3495(94)80645-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavez M. D., Courtney S. H., Chance M. R., Kiula D., Nocek J., Hoffman B. M., Friedman J. M., Ondrias M. R. Structural and functional significance of inhomogeneous line broadening of band III in hemoglobin and Fe-Mn hybrid hemoglobins. Biochemistry. 1990 May 22;29(20):4844–4852. doi: 10.1021/bi00472a014. [DOI] [PubMed] [Google Scholar]
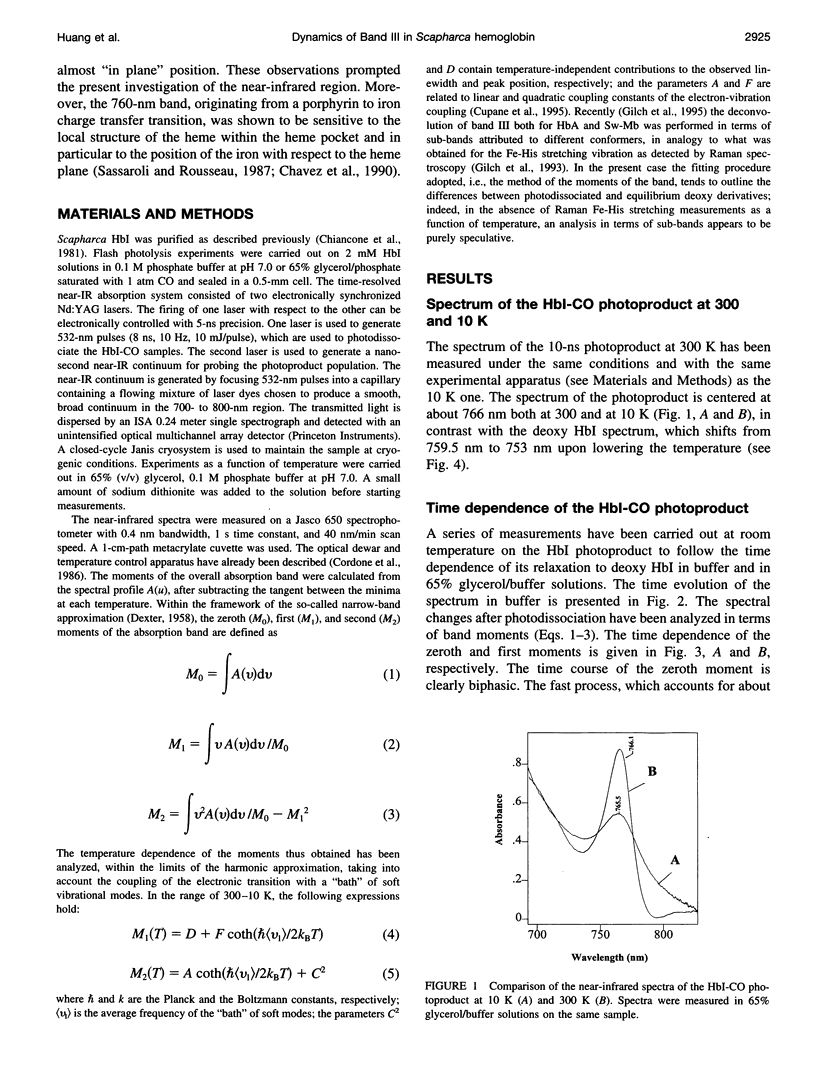
- Chiancone E., Elber R., Royer W. E., Jr, Regan R., Gibson Q. H. Ligand binding and conformation change in the dimeric hemoglobin of the clam Scapharca inaequivalvis. J Biol Chem. 1993 Mar 15;268(8):5711–5718. [PubMed] [Google Scholar]
- Chiancone E., Vecchini P., Verzili D., Ascoli F., Antonini E. Dimeric and tetrameric hemoglobins from the mollusc Scapharca inaequivalvis. Structural and functional properties. J Mol Biol. 1981 Nov 5;152(3):577–592. doi: 10.1016/0022-2836(81)90270-9. [DOI] [PubMed] [Google Scholar]
- Cordone L., Cupane A., Leone M., Vitrano E. Optical absorption spectra of deoxy- and oxyhemoglobin in the temperature range 300-20 K. Relation with protein dynamics. Biophys Chem. 1986 Aug;24(3):259–275. doi: 10.1016/0301-4622(86)85031-1. [DOI] [PubMed] [Google Scholar]
- Cordone L., Cupane A., Leone M., Vitrano E. Thermal behavior of the 760-nm absorption band in photodissociated sperm whale carbonmonoxymyoglobin at cryogenic temperature: dependence on external medium. Biopolymers. 1990 Feb 15;29(3):639–643. doi: 10.1002/bip.360290316. [DOI] [PubMed] [Google Scholar]
- Cupane A., Leone M., Vitrano E., Cordone L. Low temperature optical absorption spectroscopy: an approach to the study of stereodynamic properties of hemeproteins. Eur Biophys J. 1995;23(6):385–398. doi: 10.1007/BF00196825. [DOI] [PubMed] [Google Scholar]
- Cupane A., Leone M., Vitrano E., Cordone L. Structural and dynamic properties of the heme pocket in myoglobin probed by optical spectroscopy. Biopolymers. 1988 Dec;27(12):1977–1997. doi: 10.1002/bip.360271209. [DOI] [PubMed] [Google Scholar]
- Dunn R. C., Simon J. D. Picosecond study of the near infrared absorption band of hemoglobin after photolysis of carbonmonoxyhemoglobin. Biophys J. 1991 Oct;60(4):884–889. doi: 10.1016/S0006-3495(91)82122-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilch H., Schweitzer-Stenner R., Dreybrodt W. Structural heterogeneity of the Fe(2+)-N epsilon (HisF8) bond in various hemoglobin and myoglobin derivatives probed by the Raman-active iron histidine stretching mode. Biophys J. 1993 Oct;65(4):1470–1485. doi: 10.1016/S0006-3495(93)81216-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambright D. G., Balasubramanian S., Boxer S. G. Dynamics of protein relaxation in site-specific mutants of human myoglobin. Biochemistry. 1993 Sep 28;32(38):10116–10124. doi: 10.1021/bi00089a030. [DOI] [PubMed] [Google Scholar]
- Leone M., Cupane A., Militello V., Cordone L. Thermal broadening of the Soret band in heme complexes and in heme-proteins: role of iron dynamics. Eur Biophys J. 1994;23(5):349–352. doi: 10.1007/BF00188658. [DOI] [PubMed] [Google Scholar]
- Martin J. L., Migus A., Poyart C., Lecarpentier Y., Astier R., Antonetti A. Femtosecond photolysis of CO-ligated protoheme and hemoproteins: appearance of deoxy species with a 350-fsec time constant. Proc Natl Acad Sci U S A. 1983 Jan;80(1):173–177. doi: 10.1073/pnas.80.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narisawa S., Smans K. A., Avis J., Hoylaerts M. F., Millán J. L. Transgenic mice expressing the tumor marker germ cell alkaline phosphatase: an in vivo tumor model for human cancer antigens. Proc Natl Acad Sci U S A. 1993 Jun 1;90(11):5081–5085. doi: 10.1073/pnas.90.11.5081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrich J. W., Martin J. L., Houde D., Poyart C., Orszag A. Time-resolved Raman spectroscopy with subpicosecond resolution: vibrational cooling and delocalization of strain energy in photodissociated (carbonmonoxy)hemoglobin. Biochemistry. 1987 Dec 1;26(24):7914–7923. doi: 10.1021/bi00398a056. [DOI] [PubMed] [Google Scholar]
- Rousseau D. L., Song S., Friedman J. M., Boffi A., Chiancone E. Heme-heme interactions in a homodimeric cooperative hemoglobin. Evidence from transient Raman scattering. J Biol Chem. 1993 Mar 15;268(8):5719–5723. [PubMed] [Google Scholar]
- Royer W. E., Jr, Hendrickson W. A., Chiancone E. Structural transitions upon ligand binding in a cooperative dimeric hemoglobin. Science. 1990 Aug 3;249(4968):518–521. doi: 10.1126/science.2382132. [DOI] [PubMed] [Google Scholar]
- Royer W. E., Jr, Hendrickson W. A., Chiancone E. The 2.4-A crystal structure of Scapharca dimeric hemoglobin. Cooperativity based on directly communicating hemes at a novel subunit interface. J Biol Chem. 1989 Dec 15;264(35):21052–21061. [PubMed] [Google Scholar]
- Royer W. E., Jr High-resolution crystallographic analysis of a co-operative dimeric hemoglobin. J Mol Biol. 1994 Jan 14;235(2):657–681. doi: 10.1006/jmbi.1994.1019. [DOI] [PubMed] [Google Scholar]
- Sassaroli M., Rousseau D. L. Time dependence of near-infrared spectra of photodissociated hemoglobin and myoglobin. Biochemistry. 1987 Jun 2;26(11):3092–3098. doi: 10.1021/bi00385a022. [DOI] [PubMed] [Google Scholar]
- Srajer V., Champion P. M. Investigations of optical line shapes and kinetic hole burning in myoglobin. Biochemistry. 1991 Jul 30;30(30):7390–7402. doi: 10.1021/bi00244a005. [DOI] [PubMed] [Google Scholar]
- Srajer V, V, Schomacker KT, Champion PM. Spectral broadening in biomolecules. Phys Rev Lett. 1986 Sep 8;57(10):1267–1270. doi: 10.1103/PhysRevLett.57.1267. [DOI] [PubMed] [Google Scholar]
- Xie X. L., Simon J. D. Protein conformational relaxation following photodissociation of CO from carbonmonoxymyoglobin: picosecond circular dichroism and absorption studies. Biochemistry. 1991 Apr 16;30(15):3682–3692. doi: 10.1021/bi00229a013. [DOI] [PubMed] [Google Scholar]


