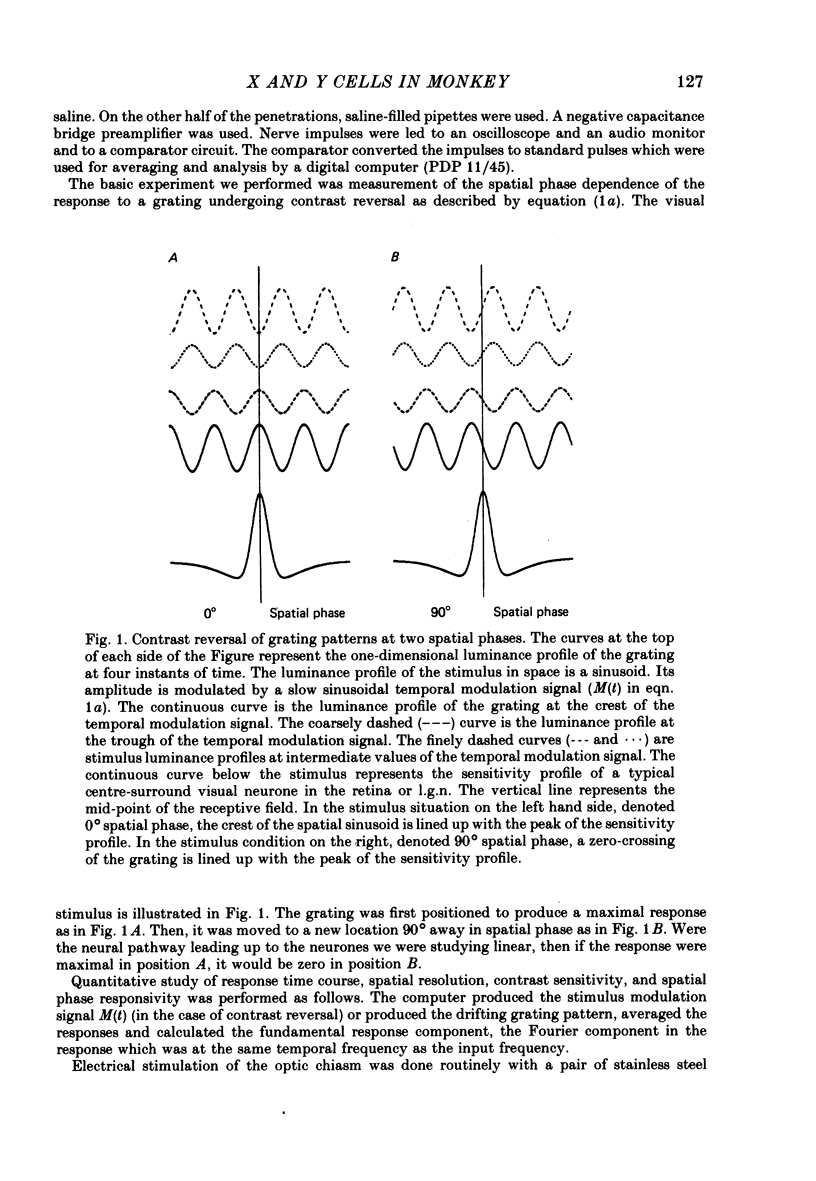
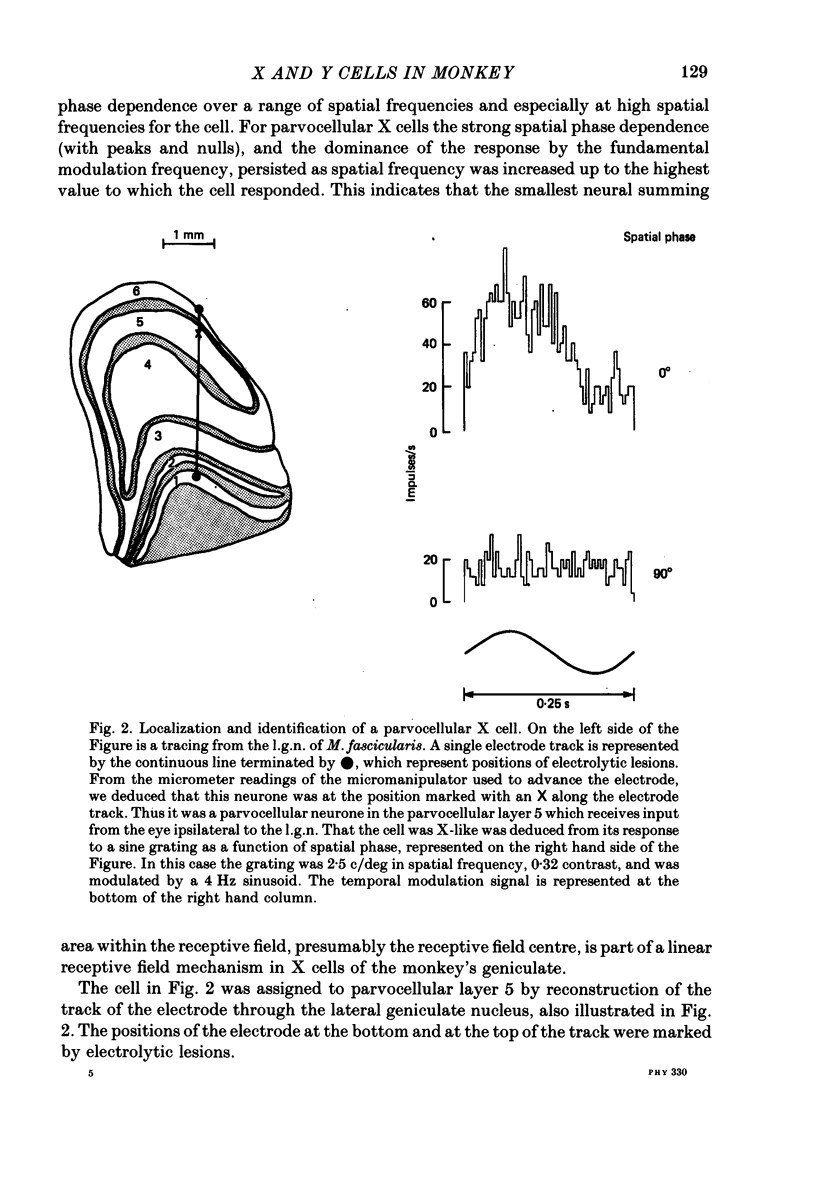
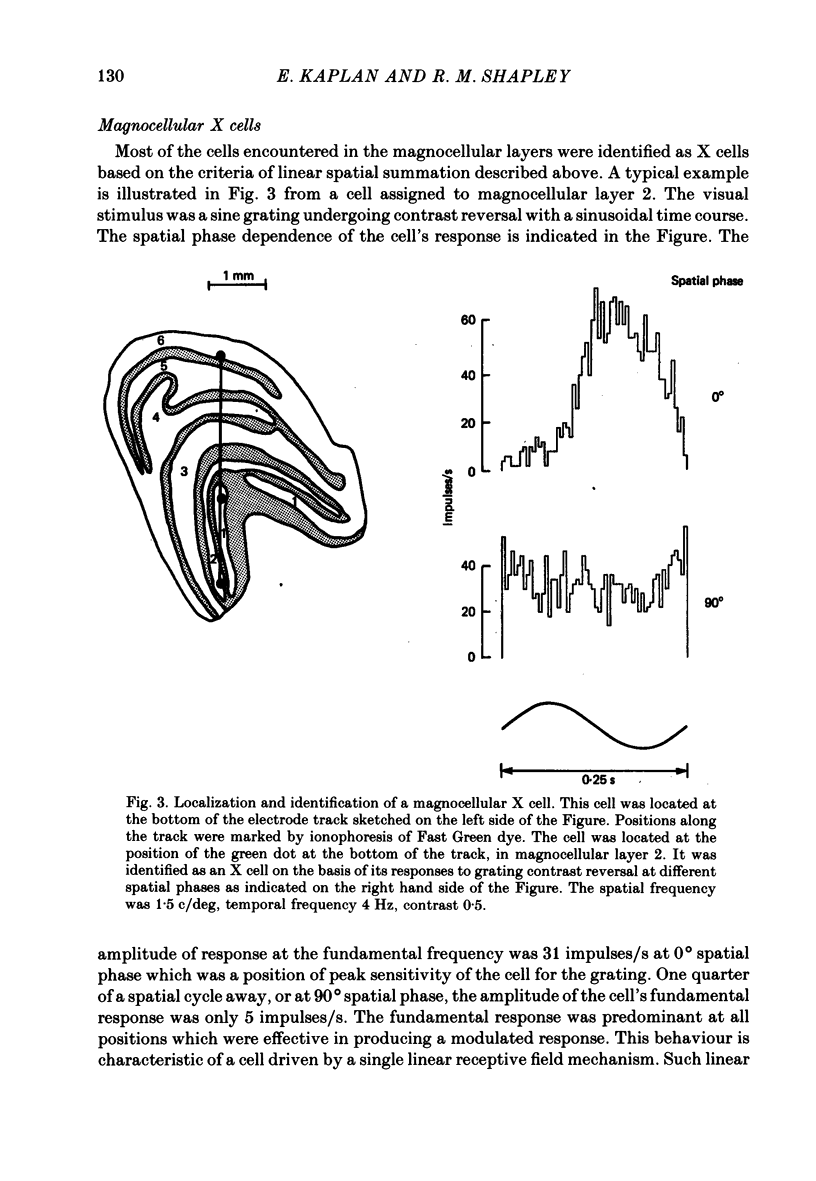
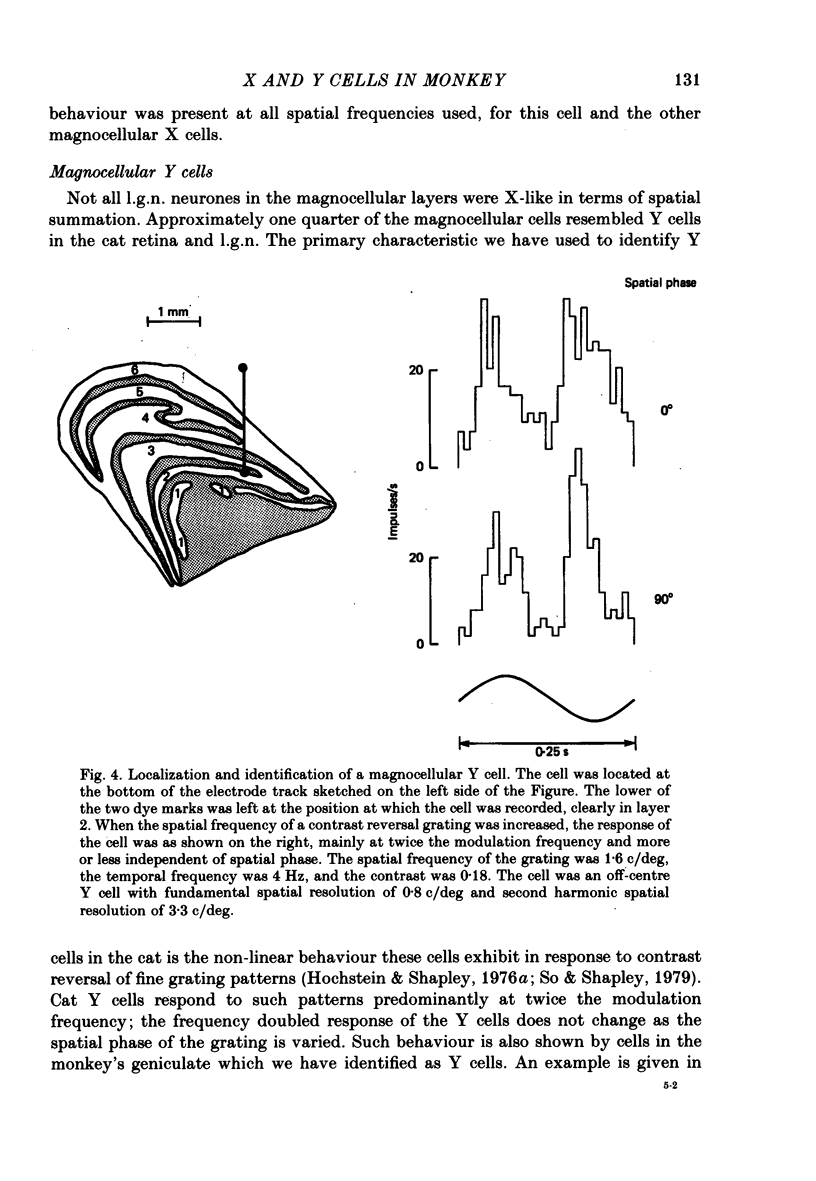
Abstract
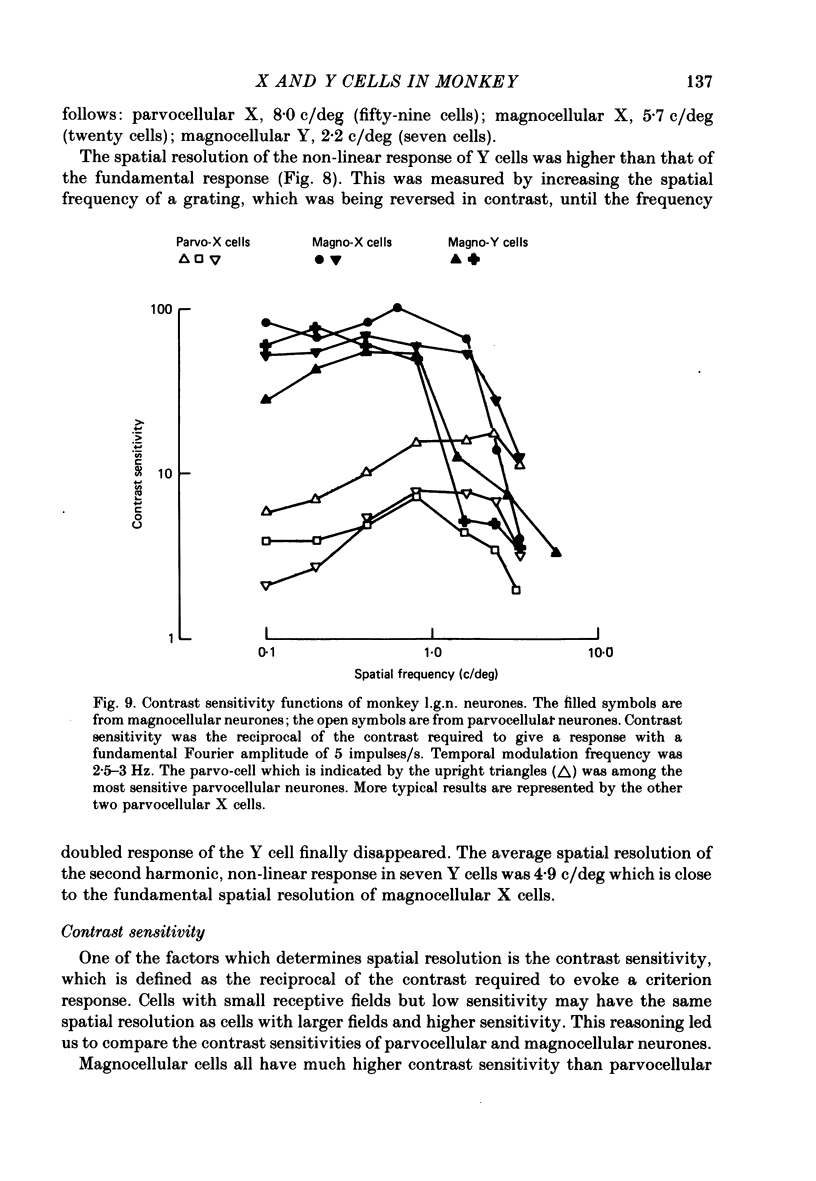
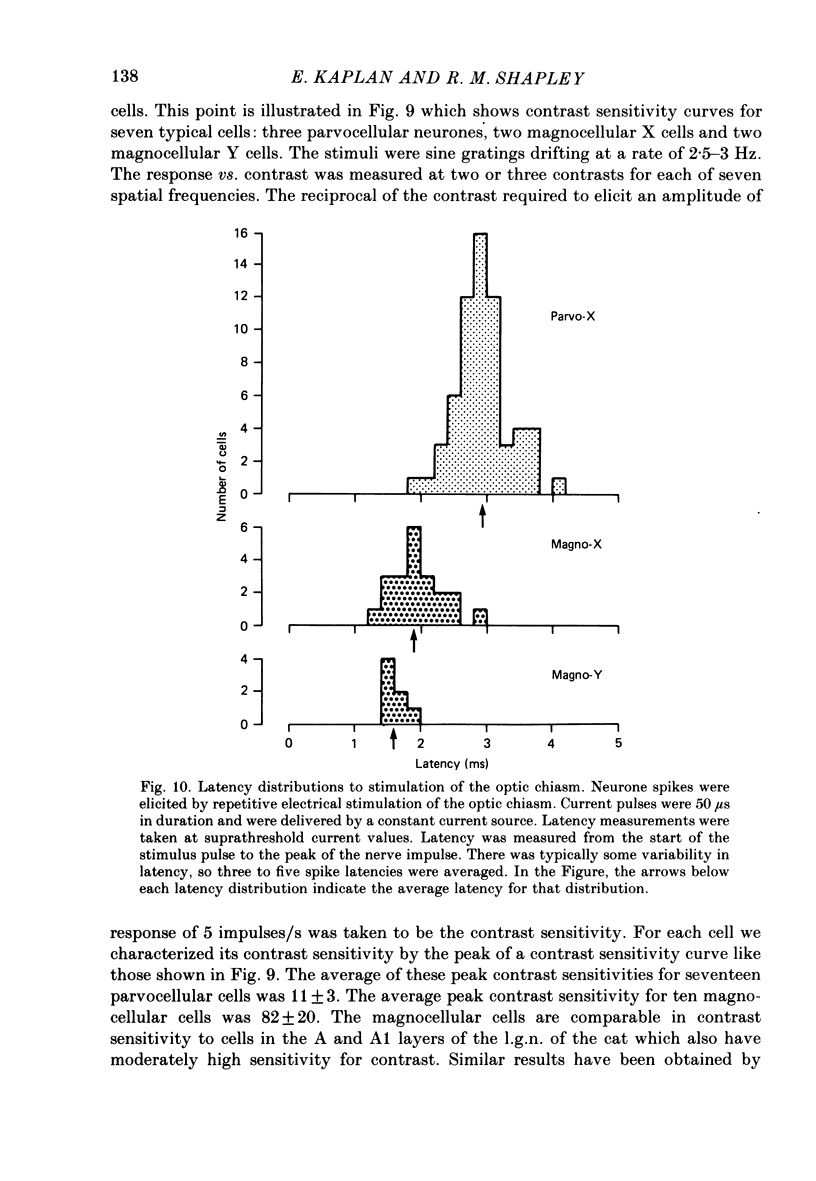
1. Cells of the lateral geniculate nucleus (l.g.n.) in macaque monkeys were sorted into two functional groups on the basis of spatial summation of visually evoked neural signals. 2. Cells were called X cells if their responses to contrast reversal of fine sine gratings were at the fundamental temporal modulation frequency with null positions one quarter of a cycle away from positions for peak response. Cells were called Y cells if their responses to such stimuli were at twice the modulation frequency and were approximately independent of spatial phase. 3. Ninety-nine percent of the cells in the four dorsal parvocellular layers of the l.g.n. were X cells; about seventy-five percent of the cells in the two ventral magnocellular layers were also X cells. The remainder were Y cells. 4. We confirmed previous findings that magnocellular cells had a shorter latency of response to electrical stimulation of the optic chiasm. 5. Magnocellular cells had much higher contrast sensitivities than did parvocellular cells. 6. Therefore, two distinct classes of X cells exist in the macaque l.g.n.: parvocellular X cells and magnocellular X cells. The great difference in their properties suggests that they have different functions in vision. The Y cells in the magnocellular layers form a third functional group with spatial properties distinctly different from the X cells. 7. We propose that the magnocellular layers of the macaque monkey's l.g.n. may be homologous to the A and A1 layers of the cat's l.g.n.
Full text
PDF


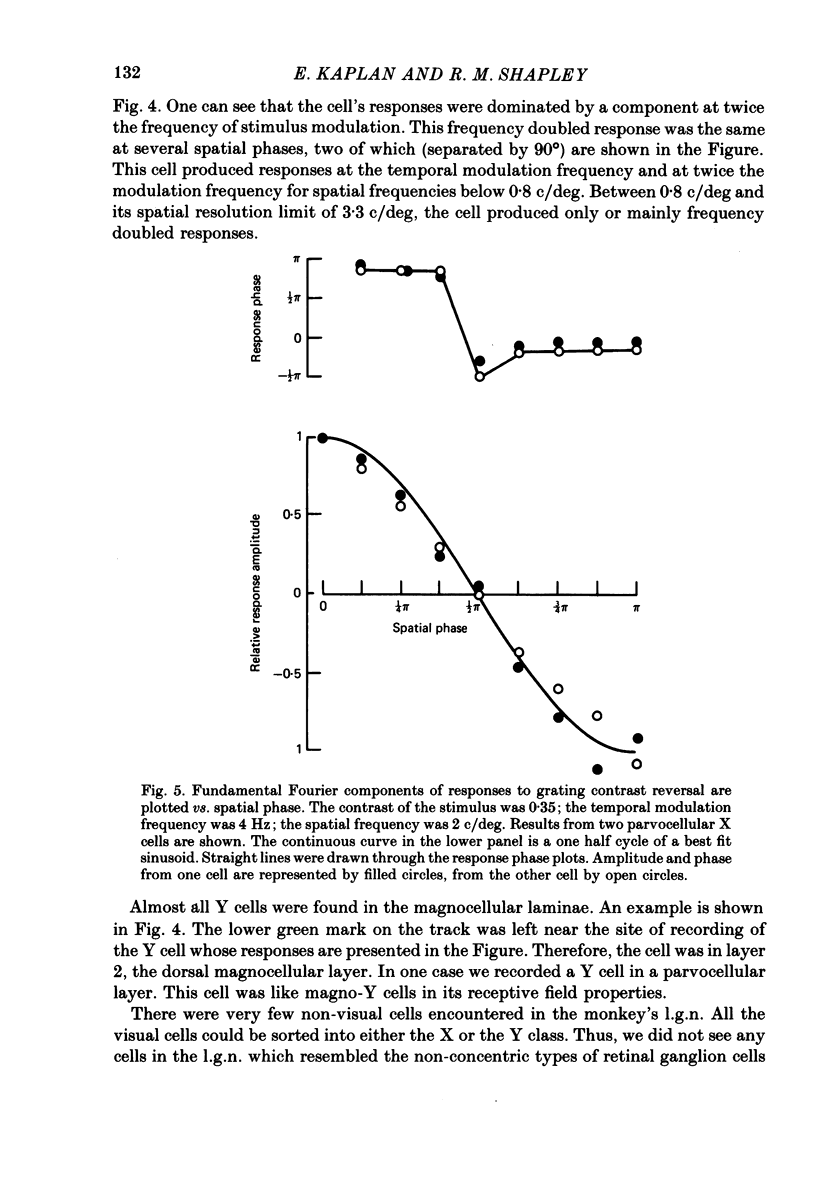
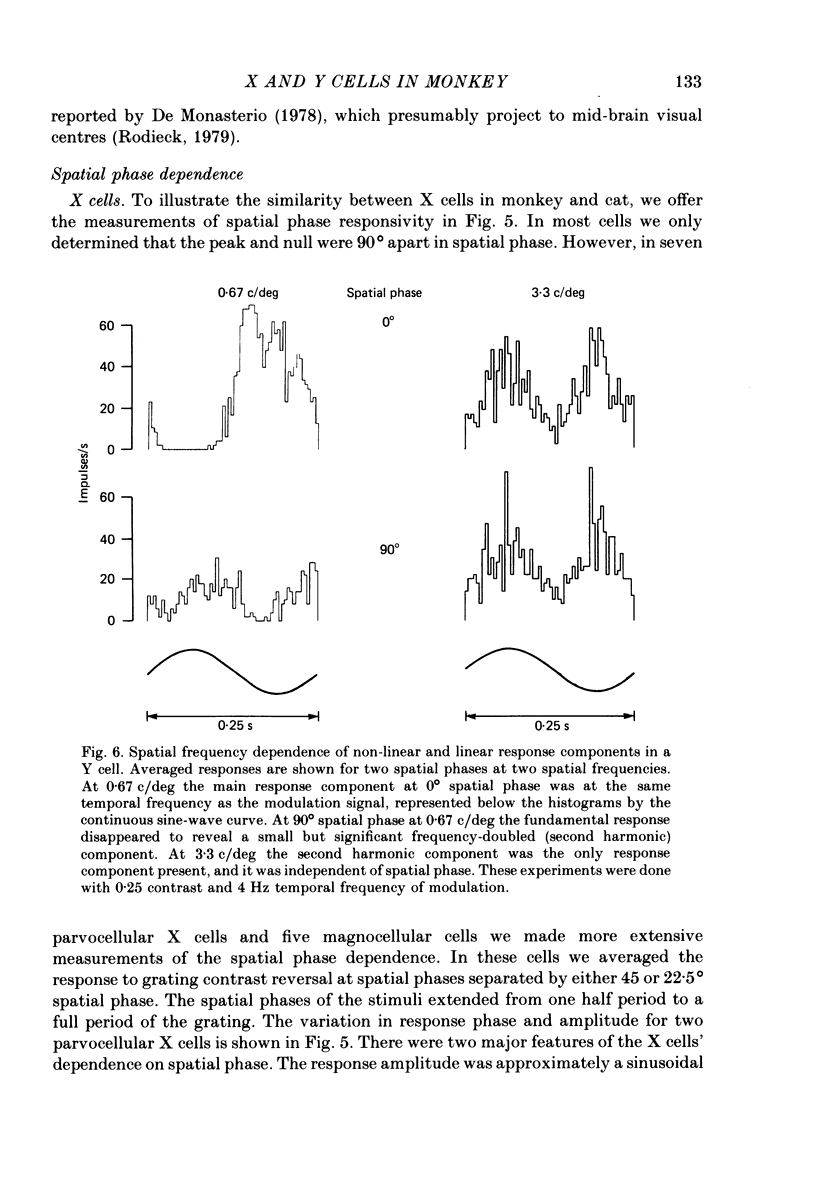

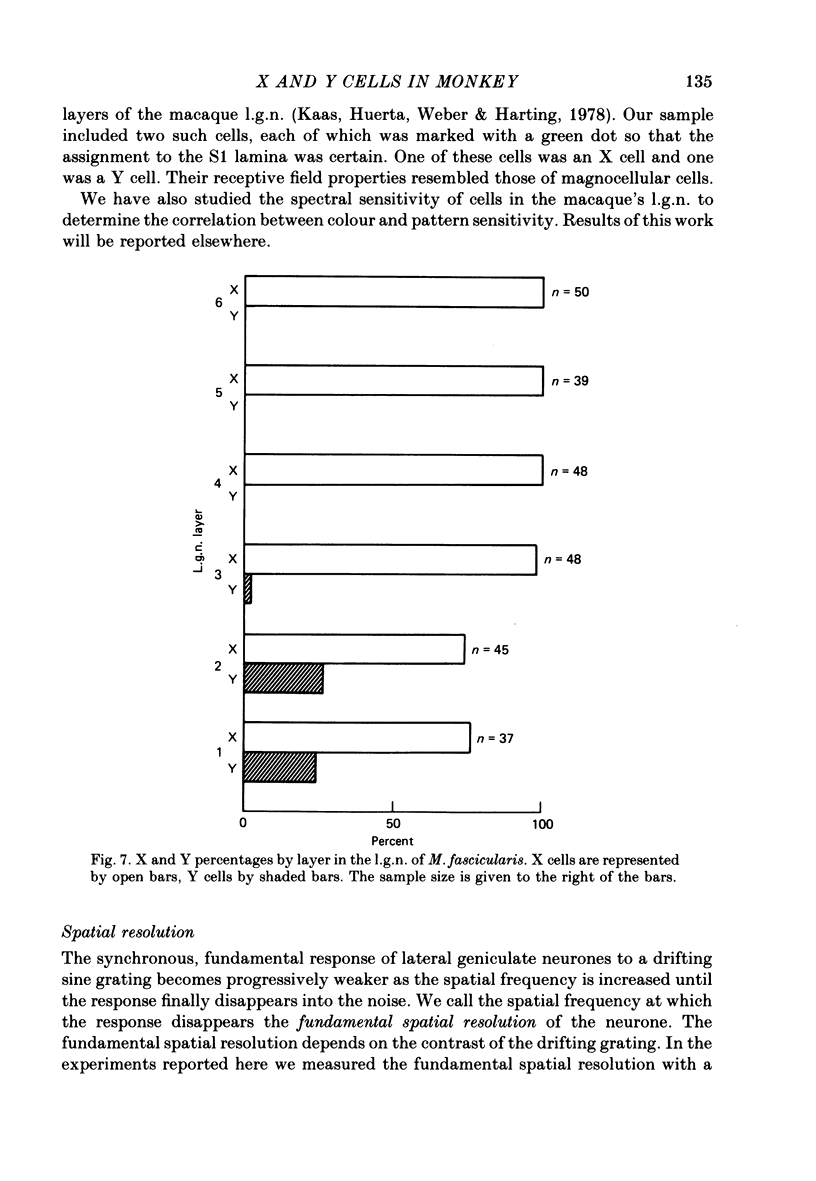
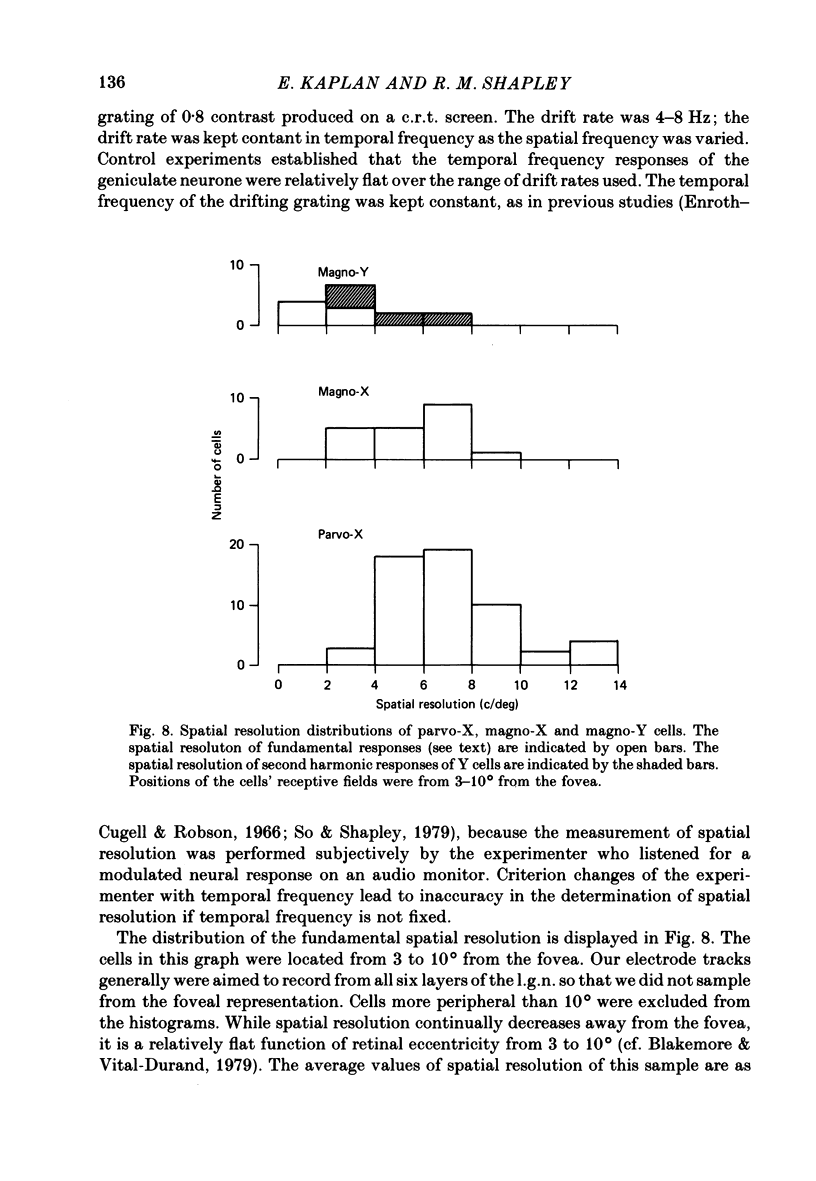





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blakemore C., Vital-Durand F. Development of the neural basis of visual acuity in monkeys: speculation on the origin of deprivation amblyopia. Trans Ophthalmol Soc U K. 1979;99(3):363–368. [PubMed] [Google Scholar]
- Cleland B. G., Dubin M. W., Levick W. R. Sustained and transient neurones in the cat's retina and lateral geniculate nucleus. J Physiol. 1971 Sep;217(2):473–496. doi: 10.1113/jphysiol.1971.sp009581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Valois R. L., Morgan H., Snodderly D. M. Psychophysical studies of monkey vision. 3. Spatial luminance contrast sensitivity tests of macaque and human observers. Vision Res. 1974 Jan;14(1):75–81. doi: 10.1016/0042-6989(74)90118-7. [DOI] [PubMed] [Google Scholar]
- Dreher B., Fukada Y., Rodieck R. W. Identification, classification and anatomical segregation of cells with X-like and Y-like properties in the lateral geniculate nucleus of old-world primates. J Physiol. 1976 Jun;258(2):433–452. doi: 10.1113/jphysiol.1976.sp011429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enroth-Cugell C., Robson J. G. The contrast sensitivity of retinal ganglion cells of the cat. J Physiol. 1966 Dec;187(3):517–552. doi: 10.1113/jphysiol.1966.sp008107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochstein S., Shapley R. M. Linear and nonlinear spatial subunits in Y cat retinal ganglion cells. J Physiol. 1976 Nov;262(2):265–284. doi: 10.1113/jphysiol.1976.sp011595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann K. P., Stone J., Sherman S. M. Relay of receptive-field properties in dorsal lateral geniculate nucleus of the cat. J Neurophysiol. 1972 Jul;35(4):518–531. doi: 10.1152/jn.1972.35.4.518. [DOI] [PubMed] [Google Scholar]
- Kaas J. H., Huerta M. F., Weber J. T., Harting J. K. Patterns of retinal terminations and laminar organization of the lateral geniculate nucleus of primates. J Comp Neurol. 1978 Dec 1;182(3):517–553. doi: 10.1002/cne.901820308. [DOI] [PubMed] [Google Scholar]
- Lee B. B., Creutzfeldt O. D., Elepfandt A. The responses of magno- and parvocellular cells of the monkey's lateral geniculate body to moving stimuli. Exp Brain Res. 1979 May 2;35(3):547–557. doi: 10.1007/BF00236771. [DOI] [PubMed] [Google Scholar]
- Lennie P. Parallel visual pathways: a review. Vision Res. 1980;20(7):561–594. doi: 10.1016/0042-6989(80)90115-7. [DOI] [PubMed] [Google Scholar]
- Lennie P. Perceptual signs of parallel pathways. Philos Trans R Soc Lond B Biol Sci. 1980 Jul 8;290(1038):23–37. doi: 10.1098/rstb.1980.0080. [DOI] [PubMed] [Google Scholar]
- Levick W. R. Form and function of cat retinal ganglion cells. Nature. 1975 Apr 24;254(5502):659–662. doi: 10.1038/254659a0. [DOI] [PubMed] [Google Scholar]
- Marrocco R. T. Sustained and transient cells in monkey lateral geniculate nucleus: conduction velocites and response properties. J Neurophysiol. 1976 Mar;39(2):340–353. doi: 10.1152/jn.1976.39.2.340. [DOI] [PubMed] [Google Scholar]
- Movshon J. A., Thompson I. D., Tolhurst D. J. Spatial summation in the receptive fields of simple cells in the cat's striate cortex. J Physiol. 1978 Oct;283:53–77. doi: 10.1113/jphysiol.1978.sp012488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P. Genesis of the dorsal lateral geniculate nucleus in the rhesus monkey: site and time of origin, kinetics of proliferation, routes of migration and pattern of distribution of neurons. J Comp Neurol. 1977 Nov 1;176(1):23–52. doi: 10.1002/cne.901760103. [DOI] [PubMed] [Google Scholar]
- Rodieck R. W. Visual pathways. Annu Rev Neurosci. 1979;2:193–225. doi: 10.1146/annurev.ne.02.030179.001205. [DOI] [PubMed] [Google Scholar]
- Schiller P. H., Malpeli J. G. Functional specificity of lateral geniculate nucleus laminae of the rhesus monkey. J Neurophysiol. 1978 May;41(3):788–797. doi: 10.1152/jn.1978.41.3.788. [DOI] [PubMed] [Google Scholar]
- Shapley R. M., Gordon J. The eel retina. Ganglion cell classes and spatial mechanisms. J Gen Physiol. 1978 Feb;71(2):139–155. doi: 10.1085/jgp.71.2.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapley R., Kaplan E., Soodak R. Spatial summation and contrast sensitivity of X and Y cells in the lateral geniculate nucleus of the macaque. Nature. 1981 Aug 6;292(5823):543–545. doi: 10.1038/292543a0. [DOI] [PubMed] [Google Scholar]
- Sherman S. M., Wilson J. R., Kaas J. H., Webb S. V. X- and Y-cells in the dorsal lateral geniculate nucleus of the owl monkey (Aotus trivirgatus). Science. 1976 Apr 30;192(4238):475–477. doi: 10.1126/science.816006. [DOI] [PubMed] [Google Scholar]
- So Y. T., Shapley R. Spatial properties of X and Y cells in the lateral geniculate nucleus of the cat and conduction veolcities of their inputs. Exp Brain Res. 1979 Aug 1;36(3):533–550. doi: 10.1007/BF00238521. [DOI] [PubMed] [Google Scholar]
- So Y. T., Shapley R. Spatial tuning of cells in and around lateral geniculate nucleus of the cat: X and Y relay cells and perigeniculate interneurons. J Neurophysiol. 1981 Jan;45(1):107–120. doi: 10.1152/jn.1981.45.1.107. [DOI] [PubMed] [Google Scholar]
- Sperling H. G., Crawford M. L., Espinoza S. Threshold spectral sensitivity of single neurons in the lateral geniculate nucleus and of performing monkeys. Mod Probl Ophthalmol. 1978;19:2–18. [PubMed] [Google Scholar]
- Tolhurst D. J. Separate channels for the analysis of the shape and the movement of moving visual stimulus. J Physiol. 1973 Jun;231(3):385–402. doi: 10.1113/jphysiol.1973.sp010239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victor J. D., Shapley R. M. The nonlinear pathway of Y ganglion cells in the cat retina. J Gen Physiol. 1979 Dec;74(6):671–689. doi: 10.1085/jgp.74.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesel T. N., Hubel D. H. Spatial and chromatic interactions in the lateral geniculate body of the rhesus monkey. J Neurophysiol. 1966 Nov;29(6):1115–1156. doi: 10.1152/jn.1966.29.6.1115. [DOI] [PubMed] [Google Scholar]
- de Monasterio F. M. Properties of ganglion cells with atypical receptive-field organization in retina of macaques. J Neurophysiol. 1978 Nov;41(6):1435–1449. doi: 10.1152/jn.1978.41.6.1435. [DOI] [PubMed] [Google Scholar]


