Abstract
1. A voltage-clamp technique was developed for stable recording of small currents in guinea-pig ventricular muscle. Small cylindrical preparations were impaled with three micro-electrodes, one for measuring the feed-back potential and two for injecting current. 2. The longitudinal potential profile resulting from current injection at one point was measured. It agreed well with the theoretical predictions for a linear cable which is sealed at both ends ('healing over'), with a length constant (lambda) of 580 +/- 145 micron. 3. When the clamp current was injected symmetrically into each half of the preparation via two electronic current pumps a spatially homogeneous clamp could be achieved in preparations with a diameter of less than or equal to 250 micron and a length of less than or equal to 2 lambda. 4. The membrane capacity and the membrane resistance of the preparations at the resting potential were measured with small voltage-clamp pulses. Assuming a specific membrane capacity (Cm) of 1 microF/cm2 a specific membrane resistance (Rm) of 6.7 +/- 1.8 k omega cm2 was obtained in Tyrode solution containing 3 mM-K. 5. The total surface area was calculated from the measured capacity of the preparation assuming a Cm of 1 microF/cm2. The total cellular volume was estimated from optical measurement of the external dimensions of the preparation assuming an extracellular space of 25%. From these data the average surface/volume ratio of individual cells was calculated to be 7200 cm2/cm3. 6. From the measured electrical constants the specific resistance of the intracellular space (Ri) was calculated to be 200-250 omega cm. With small constant current pulses a membrane time constant of 6.6 +/- 1.3 ms was measured. 7. The influence of the extracellular potassium concentration ([K]o) on Rm was studied in the range 1.5-6 mM-[K]o. Rm was found to depend on [K]o less than predicted by the constant field theory.
Full text
PDF







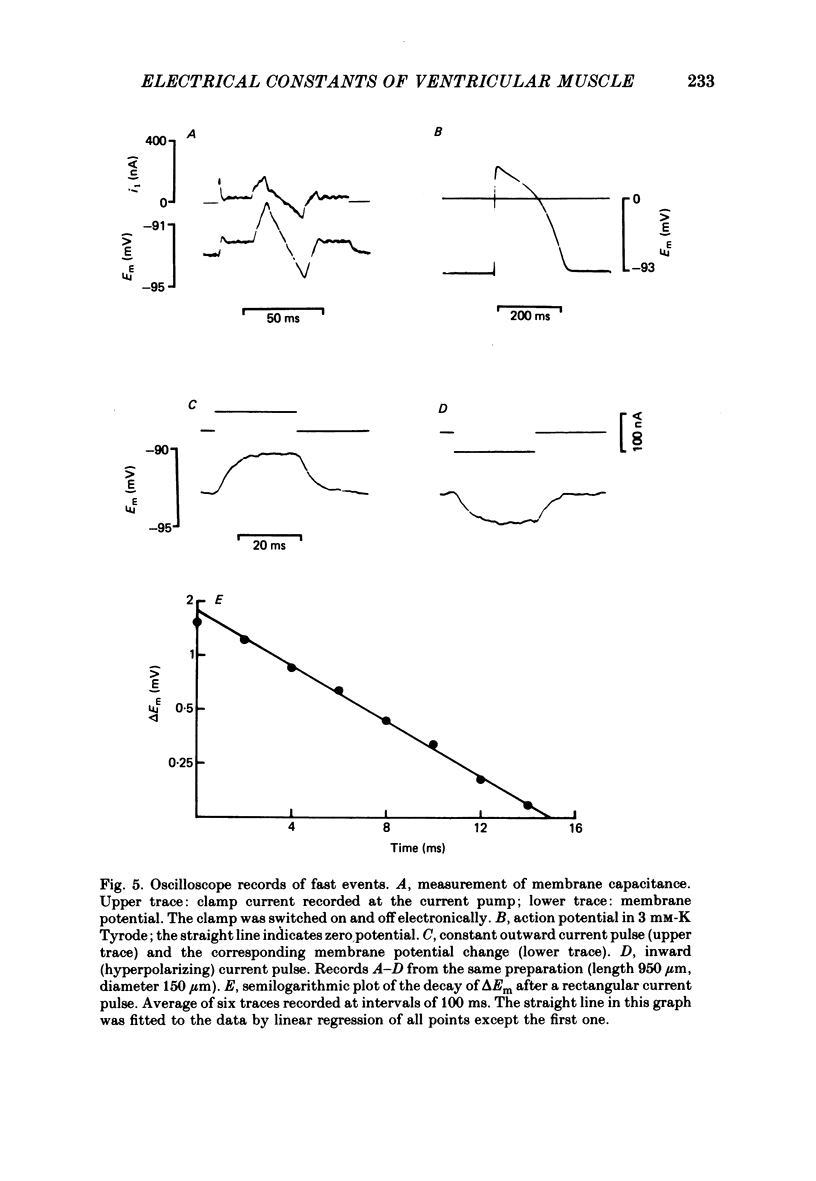








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Attwell D., Cohen I. The voltage clamp of multicellular preparations. Prog Biophys Mol Biol. 1977;31(3):201–245. doi: 10.1016/0079-6107(78)90009-3. [DOI] [PubMed] [Google Scholar]
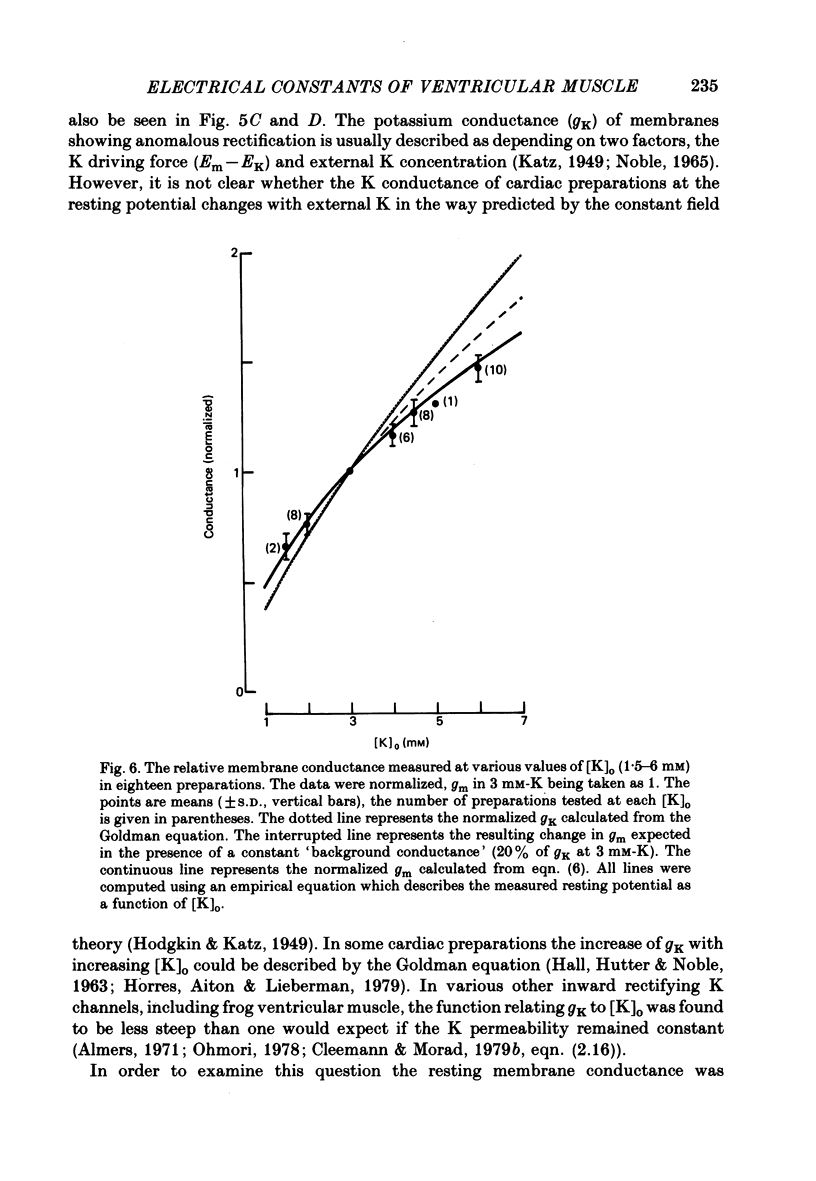
- Baumgarten C. M., Isenberg G. Depletion and accumulation of potassium in the extracellular clefts of cardiac Purkinje fibers during voltage clamp hyperpolarization and depolarization. Pflugers Arch. 1977 Mar 11;368(1-2):19–31. doi: 10.1007/BF01063450. [DOI] [PubMed] [Google Scholar]
- Beeler G. W., Jr, Reuter H. Voltage clamp experiments on ventricular myocarial fibres. J Physiol. 1970 Mar;207(1):165–190. doi: 10.1113/jphysiol.1970.sp009055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeler G. W., McGuigan J. A. Voltage clamping of multicellular myocardial preparations: capabilities and limitations of existing methods. Prog Biophys Mol Biol. 1978;34(3):219–254. doi: 10.1016/0079-6107(79)90019-1. [DOI] [PubMed] [Google Scholar]
- Beeler G. W., Reuter H. Reconstruction of the action potential of ventricular myocardial fibres. J Physiol. 1977 Jun;268(1):177–210. doi: 10.1113/jphysiol.1977.sp011853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyett M. R., Coray A., McGuigan J. A. Cow ventricular muscle. I. The effect of the extracellular potassium concentration on the current-voltage relationship. II. Evidence for a time-dependent outward current. Pflugers Arch. 1980 Dec;389(1):37–44. doi: 10.1007/BF00587926. [DOI] [PubMed] [Google Scholar]
- Carmeliet E., Verdonck F. Reduction of potassium permeability by chloride substitution in cardiac cells. J Physiol. 1977 Feb;265(1):193–206. doi: 10.1113/jphysiol.1977.sp011712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman R. A., Fry C. H. An analysis of the cable properties of frog ventricular myocardium. J Physiol. 1978 Oct;283:263–282. doi: 10.1113/jphysiol.1978.sp012499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciani S., Krasne S., Miyazaki S., Hagiwara S. A model for anomalous rectification: electrochemical-potential-dependent gating of membrane channels. J Membr Biol. 1978 Dec 15;44(2):103–134. doi: 10.1007/BF01976035. [DOI] [PubMed] [Google Scholar]
- Cleemann L., Morad M. Extracellular potassium accumulation in voltage-clamped frog ventricular muscle. J Physiol. 1979 Jan;286:83–111. doi: 10.1113/jphysiol.1979.sp012608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleemann L., Morad M. Potassium currents in frog ventricular muscle: evidence from voltage clamp currents and extracellular K accumulation. J Physiol. 1979 Jan;286:113–143. doi: 10.1113/jphysiol.1979.sp012609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clerc L. Directional differences of impulse spread in trabecular muscle from mammalian heart. J Physiol. 1976 Feb;255(2):335–346. doi: 10.1113/jphysiol.1976.sp011283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen I., Daut J., Noble D. An analysis of the actions of low concentrations of ouabain on membrane currents in Purkinje fibres. J Physiol. 1976 Aug;260(1):75–103. doi: 10.1113/jphysiol.1976.sp011505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen I., Daut J., Noble D. The effects of potassium and temperature on the pace-maker current, iK2, in Purkinje fibres. J Physiol. 1976 Aug;260(1):55–74. doi: 10.1113/jphysiol.1976.sp011504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colatsky T. J., Tsien R. W. Electrical properties associated with wide intercellular clefts in rabbit Purkinje fibres. J Physiol. 1979 May;290(2):227–252. doi: 10.1113/jphysiol.1979.sp012769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daut J., Rüdel R. The electrogenic sodium pump in guinea-pig ventricular muscle: inhibition of pump current by cardiac glycosides. J Physiol. 1982 Sep;330:243–264. doi: 10.1113/jphysiol.1982.sp014339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFrancesco D., McNaughton P. A. The effects of calcium on outward membrane currents in the cardiac Purkinje fibre. J Physiol. 1979 Apr;289:347–373. doi: 10.1113/jphysiol.1979.sp012741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyer F., Peper K. Iontophoretic application of acetylcholine: advantages of high resistance micropipettes in connection with an electronic current pump. Pflugers Arch. 1974 Apr 22;348(3):263–272. doi: 10.1007/BF00587417. [DOI] [PubMed] [Google Scholar]
- Dudel J., Peper K., Rüdel R., Trautwein W. Excitatory membrane current in heart muscle (Purkinje fibers). Pflugers Arch Gesamte Physiol Menschen Tiere. 1966;292(3):255–273. doi: 10.1007/BF00362740. [DOI] [PubMed] [Google Scholar]
- Dudel J., Peper K., Rüdel R., Trautwein W. The potassium component of membrane current in Purkinje fibers. Pflugers Arch Gesamte Physiol Menschen Tiere. 1967;296(4):308–327. doi: 10.1007/BF00362531. [DOI] [PubMed] [Google Scholar]
- Fozzard H. A., Lee C. O. Influence of changes in external potassium and chloride ions on membrane potential and intracellular potassium ion activity in rabbit ventricular muscle. J Physiol. 1976 Apr;256(3):663–689. doi: 10.1113/jphysiol.1976.sp011345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fray J. C., Laurens N. J. Mechanism by which albumin stimulates renin secretion in isolated kidneys and juxtaglomerular cells. J Physiol. 1981 Nov;320:31–39. doi: 10.1113/jphysiol.1981.sp013932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOERKE J., PAGE E. CAT HEART MUSCLE IN VITRO. VI. POTASSIUM EXCHANGE IN PAPILLARY MUSCLES. J Gen Physiol. 1965 May;48:933–948. doi: 10.1085/jgp.48.5.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HALL A. E., HUTTER O. F., NOBLE D. Current-voltage relations of Purkinje fibres in sodium-deficient solutions. J Physiol. 1963 Apr;166:225–240. doi: 10.1113/jphysiol.1963.sp007102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KATZ B. The effect of sodium ions on the electrical activity of giant axon of the squid. J Physiol. 1949 Mar 1;108(1):37–77. doi: 10.1113/jphysiol.1949.sp004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Takahashi K. The anomalous rectification and cation selectivity of the membrane of a starfish egg cell. J Membr Biol. 1974;18(1):61–80. doi: 10.1007/BF01870103. [DOI] [PubMed] [Google Scholar]
- Hagiwara S., Yoshii M. Effects of internal potassium and sodium on the anomalous rectification of the starfish egg as examined by internal perfusion. J Physiol. 1979 Jul;292:251–265. doi: 10.1113/jphysiol.1979.sp012849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B., Schwarz W. Potassium channels as multi-ion single-file pores. J Gen Physiol. 1978 Oct;72(4):409–442. doi: 10.1085/jgp.72.4.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horres C. R., Aiton J. F., Lieberman M. Potassium permeability of embryonic avian heart cells in tissue culture. Am J Physiol. 1979 Mar;236(3):C163–C170. doi: 10.1152/ajpcell.1979.236.3.C163. [DOI] [PubMed] [Google Scholar]
- Horres C. R., Lieberman M. Compartmental analysis of potassium efflux from growth-oriented heart cells. J Membr Biol. 1977 Jun 15;34(4):331–350. doi: 10.1007/BF01870307. [DOI] [PubMed] [Google Scholar]
- Johnson E. A., Lieberman M. Heart: excitation and contraction. Annu Rev Physiol. 1971;33:479–532. doi: 10.1146/annurev.ph.33.030171.002403. [DOI] [PubMed] [Google Scholar]
- Kamiyama A., Matsuda K. Electrophysiological properties of the canine ventricular fiber. Jpn J Physiol. 1966 Aug 15;16(4):407–420. doi: 10.2170/jjphysiol.16.407. [DOI] [PubMed] [Google Scholar]
- Kass R. S., Siegelbaum S. A., Tsien R. W. Three-micro-electrode voltage clamp experiments in calf cardiac Purkinje fibres: is slow inward current adequately measured? J Physiol. 1979 May;290(2):201–225. doi: 10.1113/jphysiol.1979.sp012768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister R. E., Noble D., Tsien R. W. Reconstruction of the electrical activity of cardiac Purkinje fibres. J Physiol. 1975 Sep;251(1):1–59. doi: 10.1113/jphysiol.1975.sp011080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald T. F., Trautwein W. The potassium current underlying delayed rectification in cat ventricular muscle. J Physiol. 1978 Jan;274:217–246. doi: 10.1113/jphysiol.1978.sp012144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuigan J. A. Some limitations of the double sucrose gap, and its use in a study of the slow outward current in mammalian ventricular muscle. J Physiol. 1974 Aug;240(3):775–806. doi: 10.1113/jphysiol.1974.sp010634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirolli M., Talbott S. R. The geometrical factors determining the electrotonic properties of a molluscan neurone. J Physiol. 1972 Dec;227(1):19–34. doi: 10.1113/jphysiol.1972.sp010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noma A., Irisawa H. Membrane currents in the rabbit sinoatrial node cell as studied by the double microelectrode method. Pflugers Arch. 1976 Jun 29;364(1):45–52. doi: 10.1007/BF01062910. [DOI] [PubMed] [Google Scholar]
- Ohmori H. Inactivation kinetics and steady-state current noise in the anomalous rectifier of tunicate egg cell membranes. J Physiol. 1978 Aug;281:77–99. doi: 10.1113/jphysiol.1978.sp012410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAGE E. Cat heart muscle in vitro. III. The extracellular space. J Gen Physiol. 1962 Nov;46:201–213. doi: 10.1085/jgp.46.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page E. Quantitative ultrastructural analysis in cardiac membrane physiology. Am J Physiol. 1978 Nov;235(5):C147–C158. doi: 10.1152/ajpcell.1978.235.5.C147. [DOI] [PubMed] [Google Scholar]
- Reuter H. Properties of two inward membrane currents in the heart. Annu Rev Physiol. 1979;41:413–424. doi: 10.1146/annurev.ph.41.030179.002213. [DOI] [PubMed] [Google Scholar]
- Sakamoto Y., Goto M. A study of the membrane constants in the dog myocardium. Jpn J Physiol. 1970 Feb 15;20(1):30–41. doi: 10.2170/jjphysiol.20.30. [DOI] [PubMed] [Google Scholar]
- Sakamoto Y. Membrane characteristics of the canine papillary muscle fiber. J Gen Physiol. 1969 Dec;54(6):765–781. doi: 10.1085/jgp.54.6.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer J. R., Johnson E. A. Cardiac muscle. A comparative study of Purkinje fibers and ventricular fibers. J Cell Biol. 1968 Mar;36(3):497–526. doi: 10.1083/jcb.36.3.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka I., Sasaki Y. On the electrotonic spread in cardiac muscle of the mouse. J Gen Physiol. 1966 Jul;49(6):1089–1110. doi: 10.1085/jgp.0491089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tille J. Electrotonic interaction between muscle fibers in the rabbit ventricle. J Gen Physiol. 1966 Sep;50(1):189–202. doi: 10.1085/jgp.50.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trautwein W., McDonald T. F. Current-voltage relations in ventricular muscle preparations from different species. Pflugers Arch. 1978 Apr 25;374(1):79–89. doi: 10.1007/BF00585700. [DOI] [PubMed] [Google Scholar]
- Vaughan-Jones R. D. Non-passive chloride distribution in mammalian heart muscle: micro-electrode measurement of the intracellular chloride activity. J Physiol. 1979 Oct;295:83–109. doi: 10.1113/jphysiol.1979.sp012956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan-Jones R. D. Regulation of chloride in quiescent sheep-heart Purkinje fibres studied using intracellular chloride and pH-sensitive micro-electrodes. J Physiol. 1979 Oct;295:111–137. doi: 10.1113/jphysiol.1979.sp012957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEIDMANN S. The electrical constants of Purkinje fibres. J Physiol. 1952 Nov;118(3):348–360. doi: 10.1113/jphysiol.1952.sp004799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidmann S. Electrical constants of trabecular muscle from mammalian heart. J Physiol. 1970 Nov;210(4):1041–1054. doi: 10.1113/jphysiol.1970.sp009256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidmann S. The diffusion of radiopotassium across intercalated disks of mammalian cardiac muscle. J Physiol. 1966 Nov;187(2):323–342. doi: 10.1113/jphysiol.1966.sp008092. [DOI] [PMC free article] [PubMed] [Google Scholar]


