Abstract
1. The inhibition of the electrogenic sodium pump in guinea-pig ventricular muscle by cardiac glycosides was studied with a voltage-clamp technique.
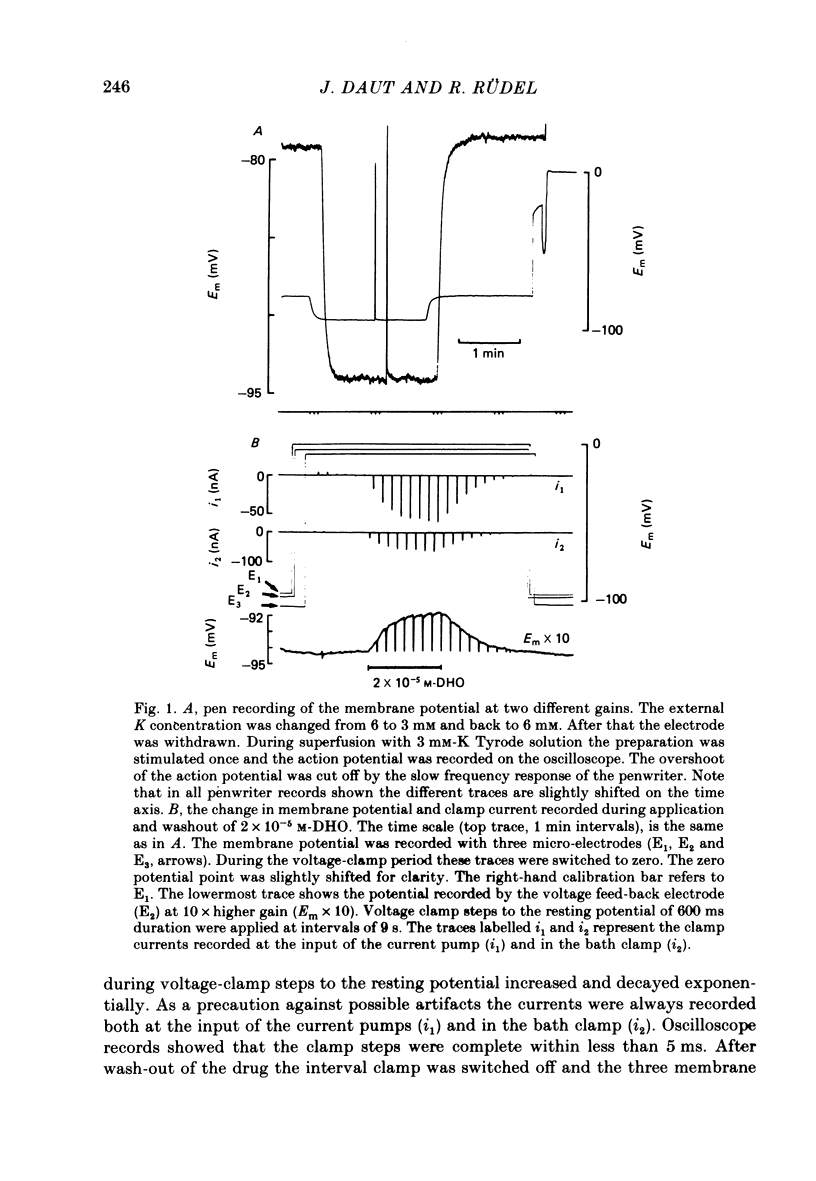
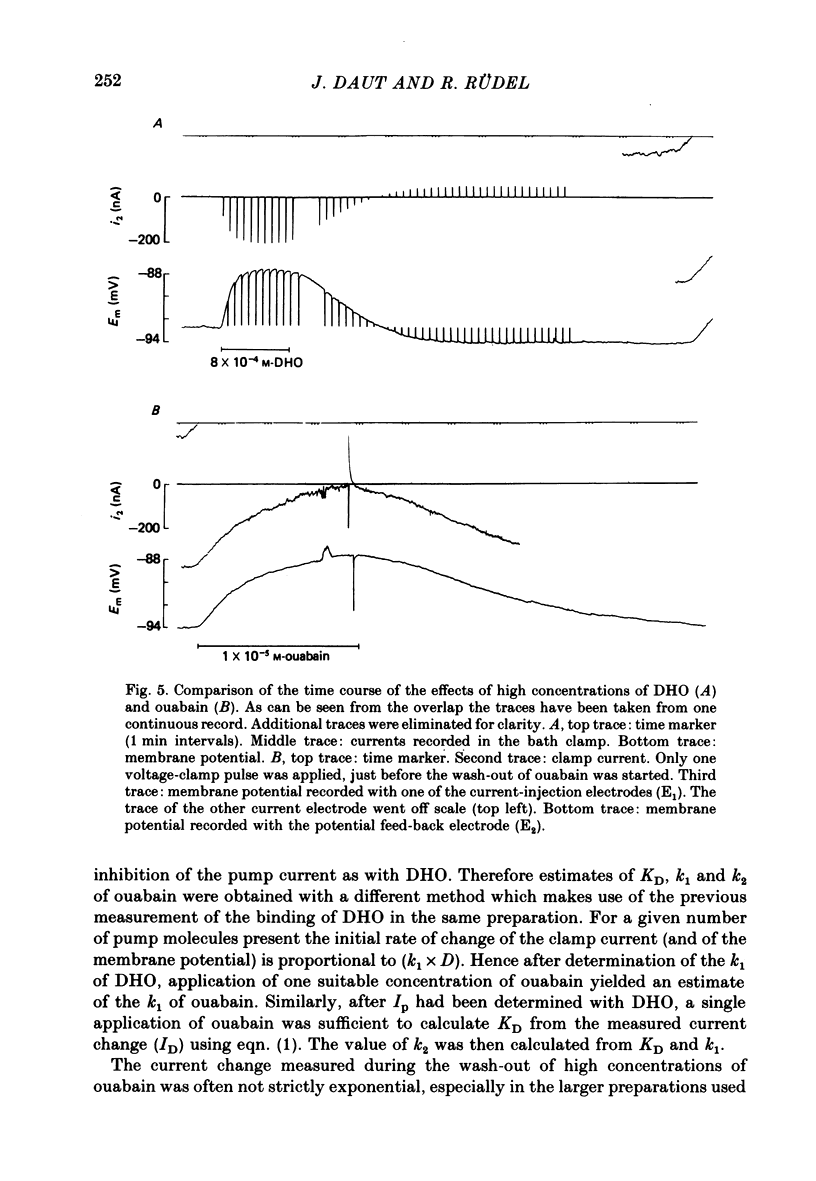
2. Superfusion of the preparation with dihydro-ouabain (DHO) produced a reversible depolarization of up to 7 mV. When the membrane potential was clamped to a constant value near the resting potential application of DHO produced a corresponding current change in the inward direction which reached a steady state in less than 1 min.
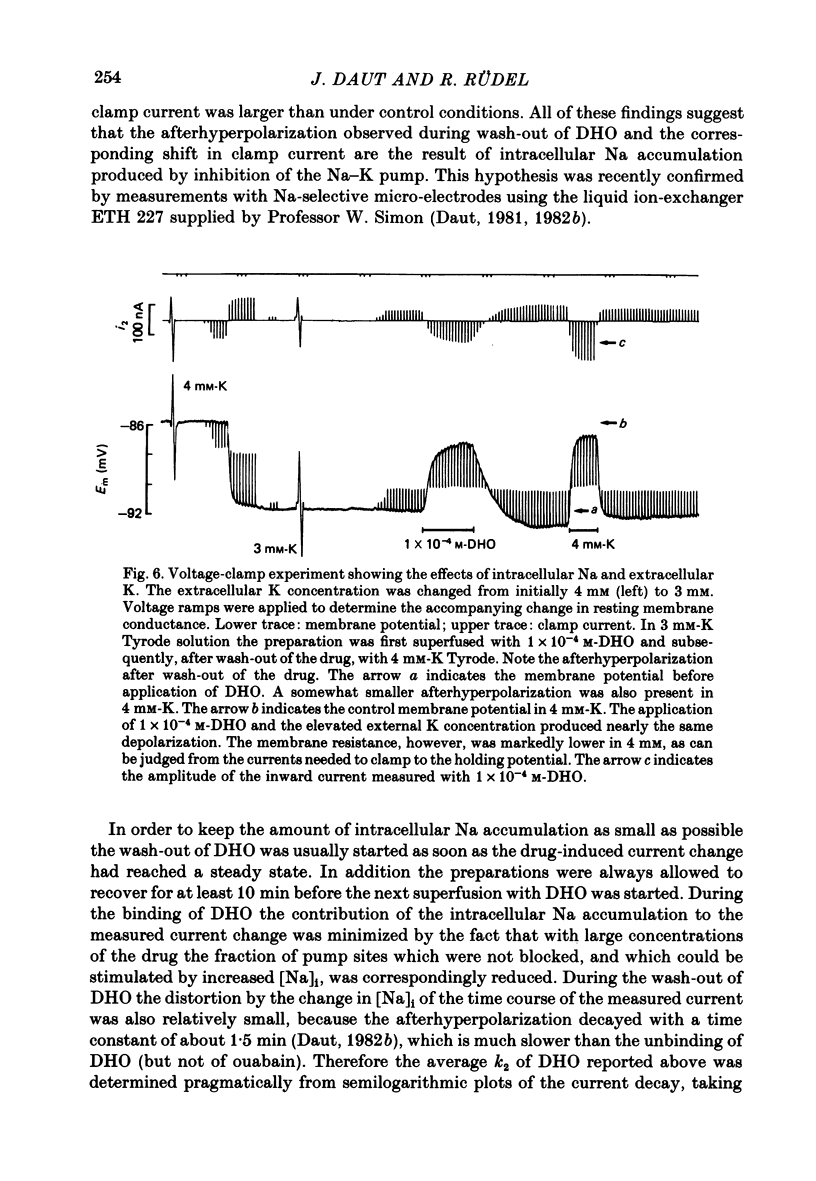
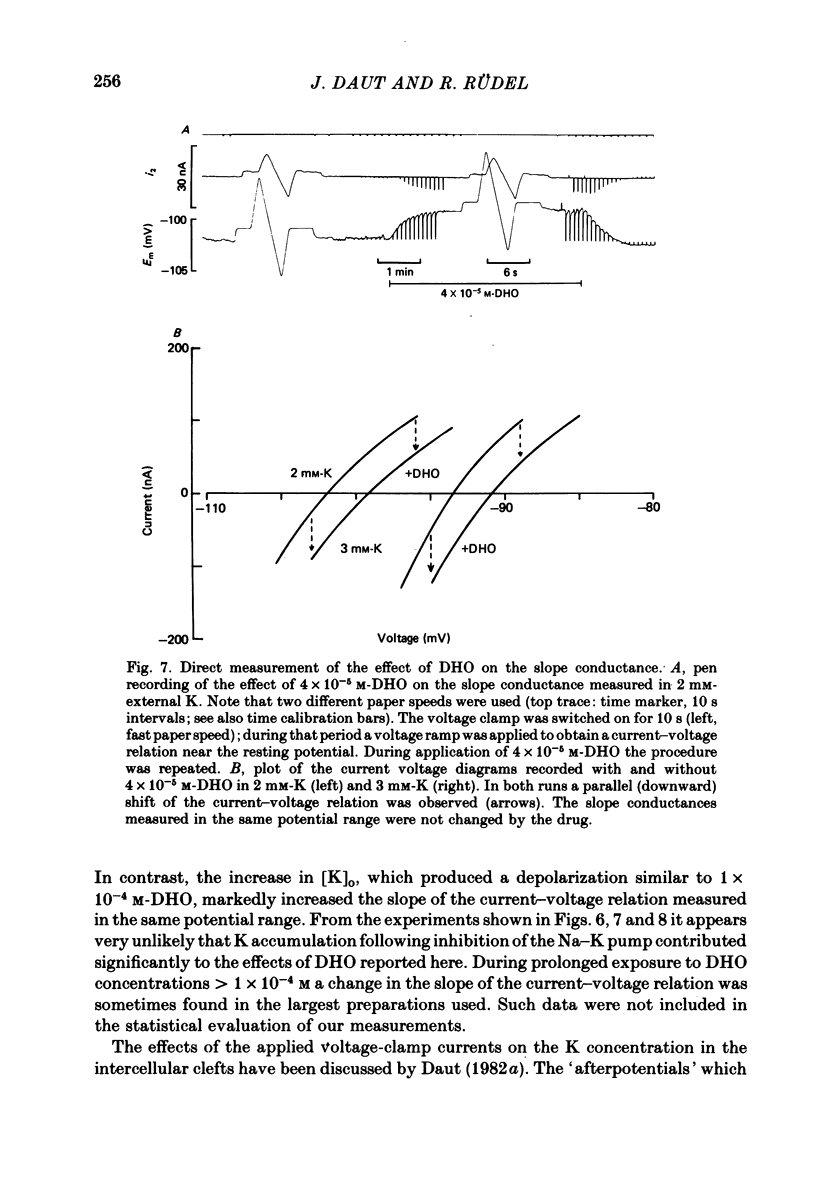
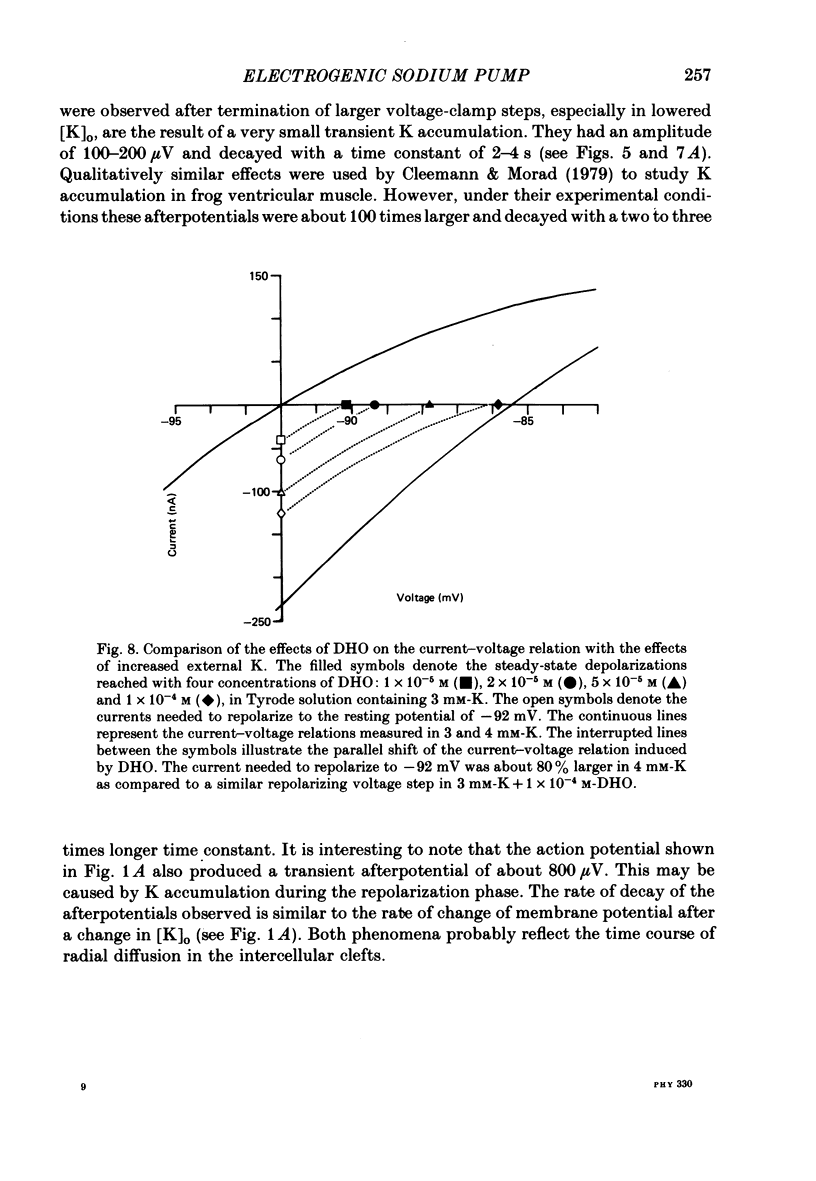
3. The drug-induced current change (ID) was found to be the result of a parallel shift of the current—voltage relation. The contributions of a change in extracellular K or intracellular Na to the measured ID were shown to be very small. From these findings and the results summarized below it was concluded that ID represents the blockage of the electrogenic pump current by DHO and that it is proportional to the number of drug molecules bound to the Na—K-ATPase in the intact cell.
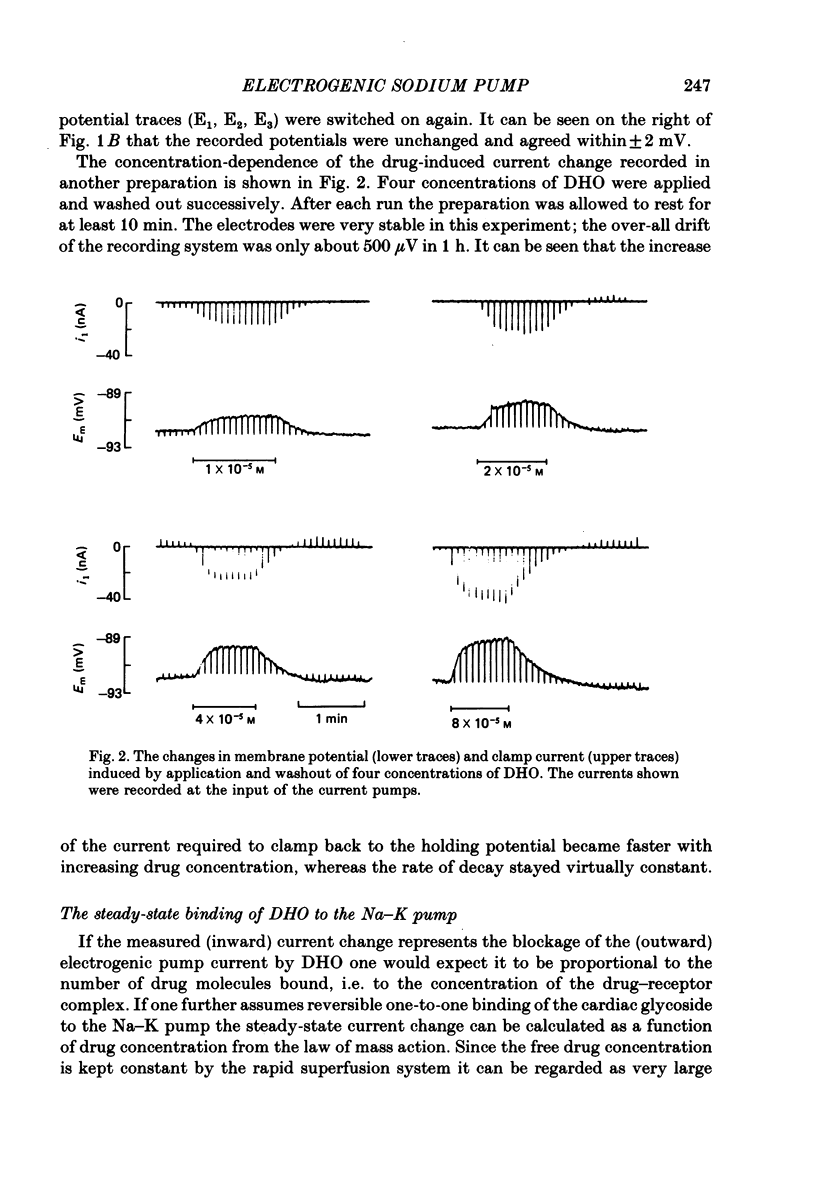
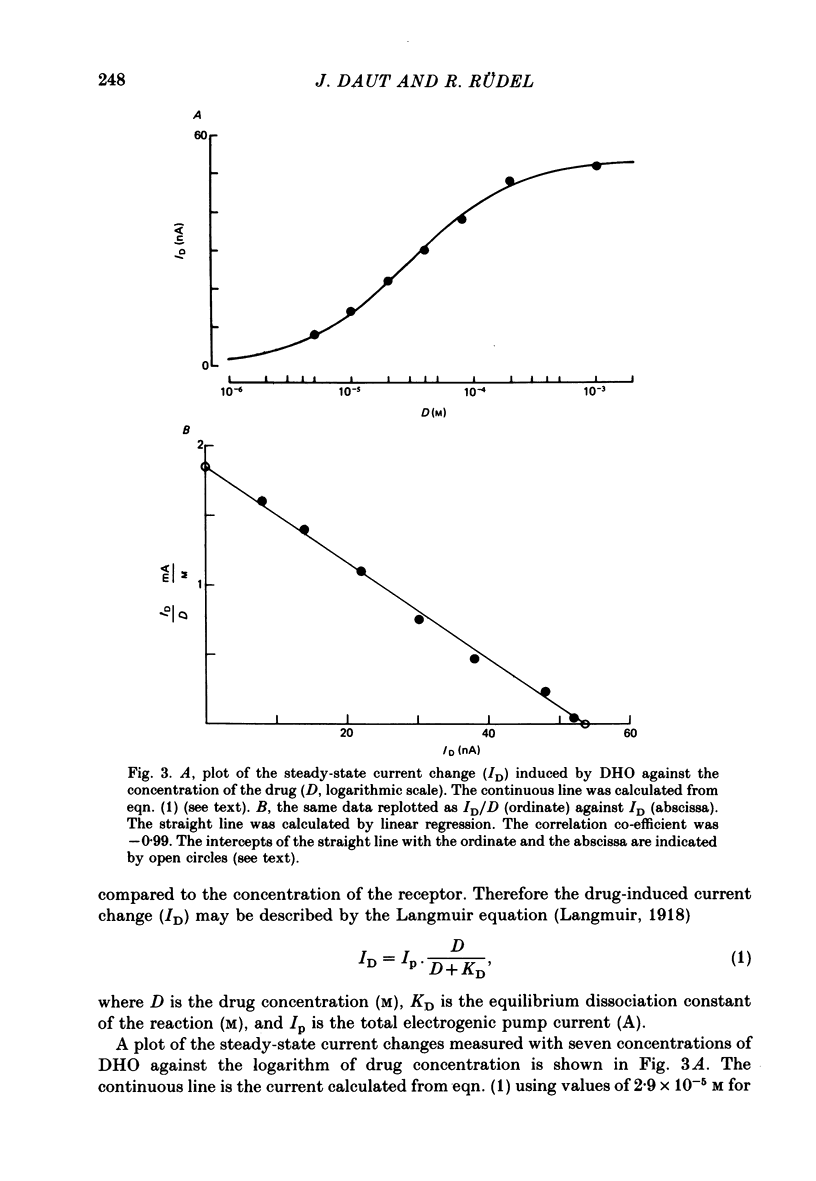
4. The dependence of ID on the concentration of DHO applied (5 × 10-6-8 × 10-4 M) was found to be consistent with the predictions of the law of mass action for reversible one-to-one binding of the drug to the Na—K pump under equilibrium conditions. From a Scatchard-type plot the equilibrium dissociation constant (KD) of DHO was determined to be 4·6 (±2·3) × 10-5 M.
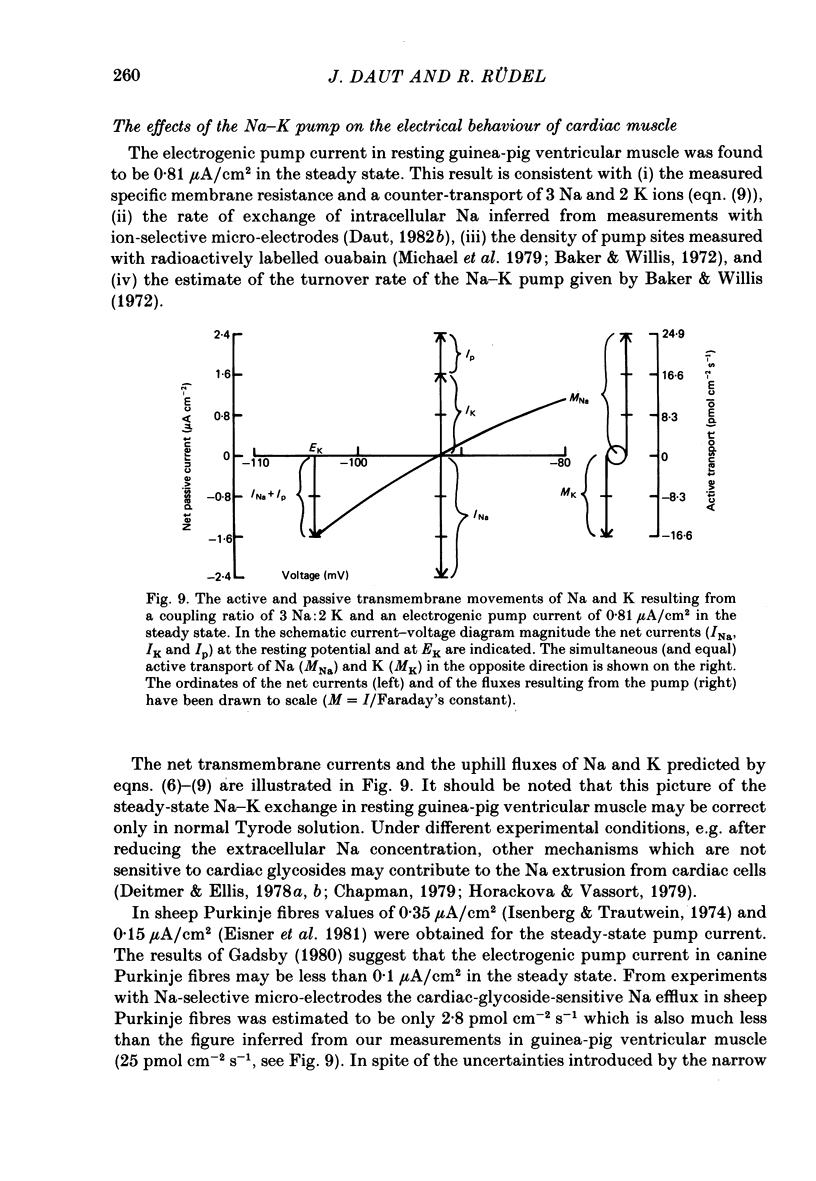
5. The steady-state pump current in the resting preparation was calculated to be 0·81±0·26 μA/cm2. It contributed 6·4±0·9 mV to the resting potential in Tyrode solution containing 3 mM-K.
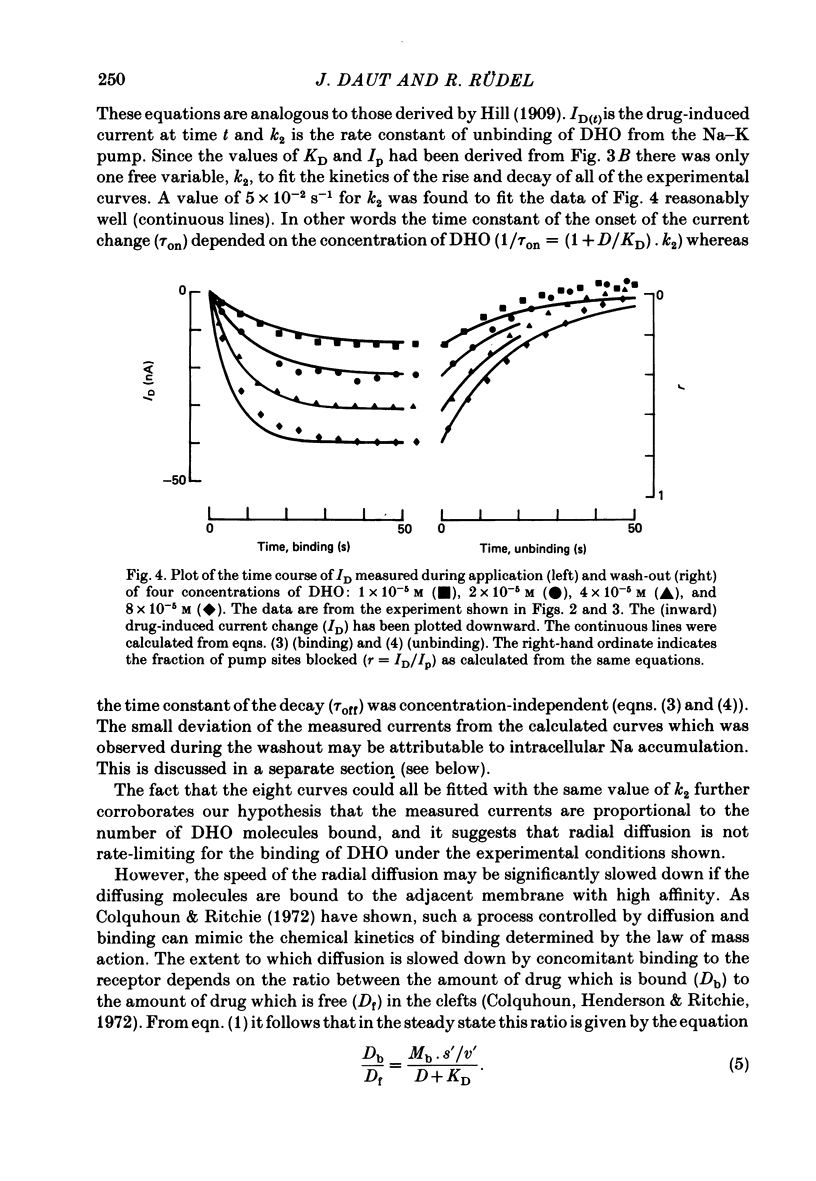
6. In the smallest preparations used the measured time course of the onset and decay of ID agreed with the chemical kinetics of binding and unbinding calculated for various DHO concentrations. The rate constant of unbinding (k2) was found to be 3·4 (±0·7) × 10-2 S-1 and the average rate constant of binding (k1) was 7·4 × 102 M-1 S-1.
7. By comparing the effects of ouabain and DHO in the same preparation the following estimates of the chemical constants of ouabain binding to the Na—K pump were obtained: KD ≃ 1·5 × 10-6 M; k1 ≃ 4 × 103 M-1 S-1; k2 ≃ 6 × 10-3 S-1.
8. An analysis of the transmembrane movements of Na and K in the steady state showed that the measured pump current density is consistent with a counter-transport of 3 Na and 2 K ions.
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abercrombie R. F., de Weer P. Electric current generated by squid giant axon sodium pump: external K and internal ADP effects. Am J Physiol. 1978 Jul;235(1):C63–C68. doi: 10.1152/ajpcell.1978.235.1.C63. [DOI] [PubMed] [Google Scholar]
- Baker P. F., Willis J. S. Binding of the cardiac glycoside ouabain to intact cells. J Physiol. 1972 Jul;224(2):441–462. doi: 10.1113/jphysiol.1972.sp009904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeler G. W., Reuter H. Reconstruction of the action potential of ventricular myocardial fibres. J Physiol. 1977 Jun;268(1):177–210. doi: 10.1113/jphysiol.1977.sp011853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodemann H. H., Hoffman J. F. Comparison of the side-dependent effects of Na and K on orthophosphate-, UTP-, and ATP-promoted ouabain binding to reconstituted human red blood cell ghosts. J Gen Physiol. 1976 May;67(5):527–545. doi: 10.1085/jgp.67.5.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodemann H. H., Hoffman J. F. Effects of Mg and Ca on the side dependencies of Na and K on ouabain binding to red blood cell ghosts and the control of Na transport by internal Mg. J Gen Physiol. 1976 May;67(5):547–561. doi: 10.1085/jgp.67.5.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodemann H. H., Hoffman J. F. Side-dependent effects of internal versus external Na and K on ouabain binding to reconstituted human red blood cell ghosts. J Gen Physiol. 1976 May;67(5):497–525. doi: 10.1085/jgp.67.5.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeliet E., Verdonck F. Reduction of potassium permeability by chloride substitution in cardiac cells. J Physiol. 1977 Feb;265(1):193–206. doi: 10.1113/jphysiol.1977.sp011712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman R. A. Excitation-contraction coupling in cardiac muscle. Prog Biophys Mol Biol. 1979;35(1):1–52. doi: 10.1016/0079-6107(80)90002-4. [DOI] [PubMed] [Google Scholar]
- Cleemann L., Morad M. Extracellular potassium accumulation in voltage-clamped frog ventricular muscle. J Physiol. 1979 Jan;286:83–111. doi: 10.1113/jphysiol.1979.sp012608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen I., Daut J., Noble D. The effects of potassium and temperature on the pace-maker current, iK2, in Purkinje fibres. J Physiol. 1976 Aug;260(1):55–74. doi: 10.1113/jphysiol.1976.sp011504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D., Henderson R., Ritchie J. M. The binding of labelled tetrodotoxin to non-myelinated nerve fibres. J Physiol. 1972 Dec;227(1):95–126. doi: 10.1113/jphysiol.1972.sp010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D., Ritchie J. M. The kinetics of the interaction between tetrodotoxin and mammalian nonmyelinated nerve fibers. Mol Pharmacol. 1972 May;8(3):285–292. [PubMed] [Google Scholar]
- Daut J., Rüdel R. Cardiac glycoside binding to the Na/K-ATPase in the intact myocardial cell: electrophysiological measurement of chemical kinetics. J Mol Cell Cardiol. 1981 Aug;13(8):777–782. doi: 10.1016/0022-2828(81)90260-1. [DOI] [PubMed] [Google Scholar]
- Daut J. The passive electrical properties of guinea-pig ventricular muscle as examined with a voltage-clamp technique. J Physiol. 1982 Sep;330:221–242. doi: 10.1113/jphysiol.1982.sp014338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Pover A., Godfraind T. Interaction of ouabain with (Na+ + K+)ATPase from human heart and from guinea-pig heart. Biochem Pharmacol. 1979 Oct 15;28(20):3051–3056. doi: 10.1016/0006-2952(79)90612-9. [DOI] [PubMed] [Google Scholar]
- Deitmer J. W., Ellis D. Changes in the intracellular sodium activity of sheep heart Purkinje fibres produced by calcium and other divalent cations. J Physiol. 1978 Apr;277:437–453. doi: 10.1113/jphysiol.1978.sp012283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deitmer J. W., Ellis D. The intracellular sodium activity of cardiac Purkinje fibres during inhibition and re-activation of the Na-K pump. J Physiol. 1978 Nov;284:241–259. doi: 10.1113/jphysiol.1978.sp012539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisner D. A., Lederer W. J., Vaughan-Jones R. D. The dependence of sodium pumping and tension on intracellular sodium activity in voltage-clamped sheep Purkinje fibres. J Physiol. 1981 Aug;317:163–187. doi: 10.1113/jphysiol.1981.sp013819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis D. The effects of external cations and ouabain on the intracellular sodium activity of sheep heart Purkinje fibres. J Physiol. 1977 Dec;273(1):211–240. doi: 10.1113/jphysiol.1977.sp012090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadsby D. C. Activation of electrogenic Na+/K+ exchange by extracellular K+ in canine cardiac Purkinje fibers. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4035–4039. doi: 10.1073/pnas.77.7.4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadsby D. C., Cranefield P. F. Direct measurement of changes in sodium pump current in canine cardiac Purkinje fibers. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1783–1787. doi: 10.1073/pnas.76.4.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadsby D. C., Cranefield P. F. Electrogenic sodium extrusion in cardiac Purkinje fibers. J Gen Physiol. 1979 Jun;73(6):819–837. doi: 10.1085/jgp.73.6.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glitsch H. G., Grabowski W., Thielen J. Activation of the electrogenic sodium pump in guinea-pig atria by external potassium ions. J Physiol. 1978 Mar;276:515–524. doi: 10.1113/jphysiol.1978.sp012250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glitsch H. G., Reuter H., Scholz H. The effect of the internal sodium concentration on calcium fluxes in isolated guinea-pig auricles. J Physiol. 1970 Jul;209(1):25–43. doi: 10.1113/jphysiol.1970.sp009153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn I. M., Karlish S. J. The sodium pump. Annu Rev Physiol. 1975;37:13–55. doi: 10.1146/annurev.ph.37.030175.000305. [DOI] [PubMed] [Google Scholar]
- Hill A. V. The mode of action of nicotine and curari, determined by the form of the contraction curve and the method of temperature coefficients. J Physiol. 1909 Dec 23;39(5):361–373. doi: 10.1113/jphysiol.1909.sp001344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horackova M., Vassort G. Sodium-calcium exchange in regulation of cardiac contractility. Evidence for an electrogenic, voltage-dependent mechanism. J Gen Physiol. 1979 Apr;73(4):403–424. doi: 10.1085/jgp.73.4.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isenberg G., Trautwein W. The effect of dihydro-ouabain and lithium-ions on the outward current in cardiac Purkinje fibers. Evidence for electrogenicity of active transport. Pflugers Arch. 1974;350(1):41–54. doi: 10.1007/BF00586737. [DOI] [PubMed] [Google Scholar]
- Karlish S. J., Pick U. Sidedness of the effects of sodium and potassium ions on the conformational state of the sodium-potassium pump. J Physiol. 1981 Mar;312:505–529. doi: 10.1113/jphysiol.1981.sp013641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. O., Kang D. H., Sokol J. H., Lee K. S. Relation between intracellular Na ion activity and tension of sheep cardiac Purkinje fibers exposed to dihydro-ouabain. Biophys J. 1980 Feb;29(2):315–330. doi: 10.1016/S0006-3495(80)85135-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister R. E., Noble D., Tsien R. W. Reconstruction of the electrical activity of cardiac Purkinje fibres. J Physiol. 1975 Sep;251(1):1–59. doi: 10.1113/jphysiol.1975.sp011080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael L. H., Schwartz A., Wallick E. T. Nature of the transport adenosine triphosphatase-digitalis complex: XIV. Inotropy and cardiac glycoside interaction with Na+,K+-ATPase of isolated cat papillary muscles. Mol Pharmacol. 1979 Jul;16(1):135–146. [PubMed] [Google Scholar]
- Noma A., Irisawa H. Contribution of an electrogenic sodium pump to the membrane potential in rabbit sinoatrial node cells. Pflugers Arch. 1975 Aug 12;358(4):289–301. doi: 10.1007/BF00580527. [DOI] [PubMed] [Google Scholar]
- PAGE E. Cat heart muscle in vitro. III. The extracellular space. J Gen Physiol. 1962 Nov;46:201–213. doi: 10.1085/jgp.46.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAGE E., STORN S. R. CAT HEART MUSCLE IN VITRO. 8. ACTIVE TRANSPORT OF SODIUM IN PAPILLARY MUSCLES. J Gen Physiol. 1965 May;48:957–972. doi: 10.1085/jgp.48.5.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Ceretti E., Nguyen T. A., Schanne O. F., Caille J. P. An electrogenic component of resting potential in rabbit ventricular muscle? Am J Physiol. 1981 Jan;240(1):C28–C34. doi: 10.1152/ajpcell.1981.240.1.C28. [DOI] [PubMed] [Google Scholar]
- Vaughan-Jones R. D. Non-passive chloride distribution in mammalian heart muscle: micro-electrode measurement of the intracellular chloride activity. J Physiol. 1979 Oct;295:83–109. doi: 10.1113/jphysiol.1979.sp012956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan-Jones R. D. Regulation of chloride in quiescent sheep-heart Purkinje fibres studied using intracellular chloride and pH-sensitive micro-electrodes. J Physiol. 1979 Oct;295:111–137. doi: 10.1113/jphysiol.1979.sp012957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WINEGRAD S., SHANES A. M. Calcium flux and contractility in guinea pig atria. J Gen Physiol. 1962 Jan;45:371–394. doi: 10.1085/jgp.45.3.371. [DOI] [PMC free article] [PubMed] [Google Scholar]


