Abstract
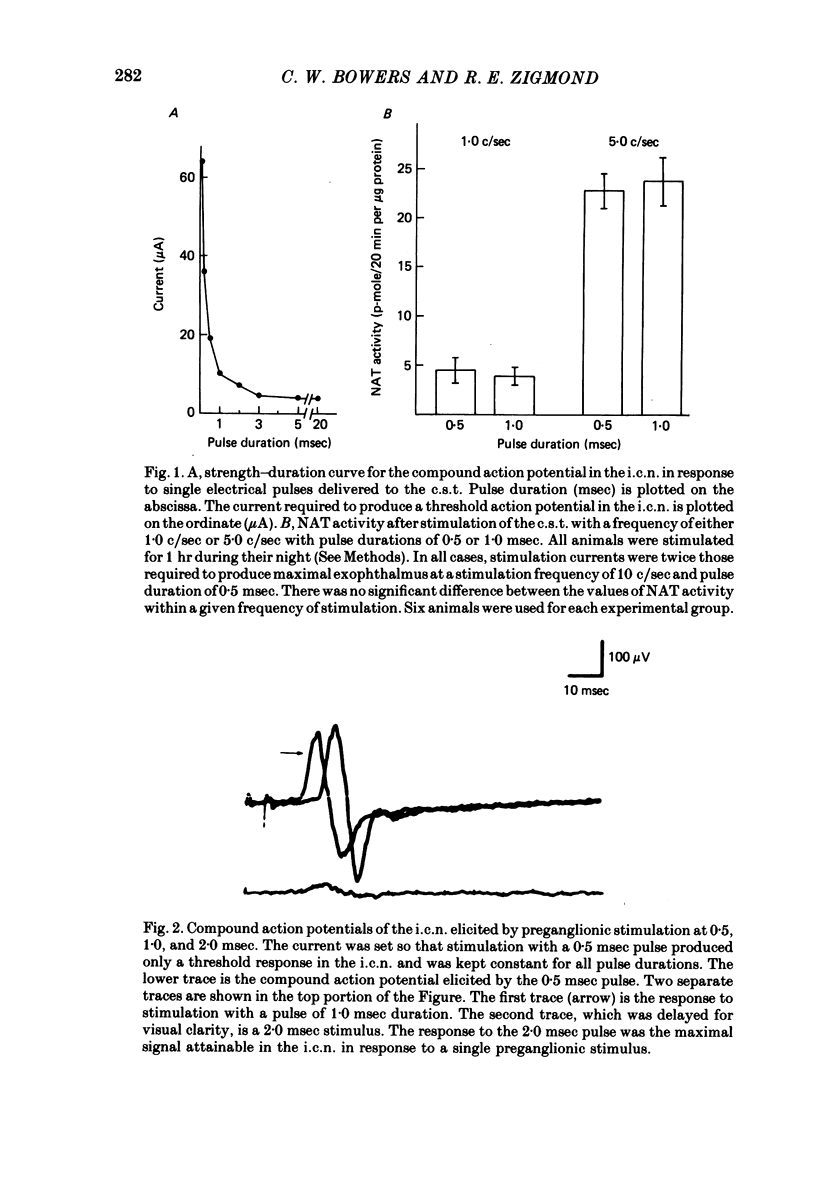
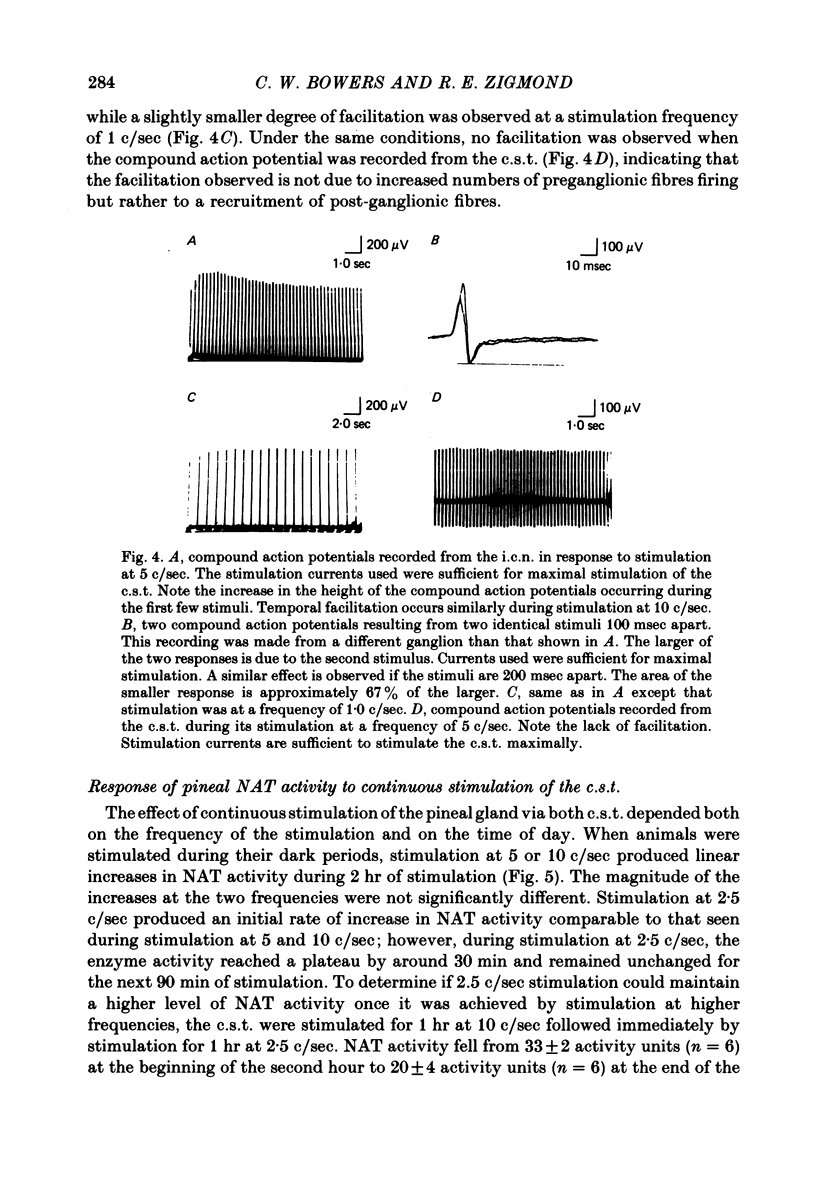
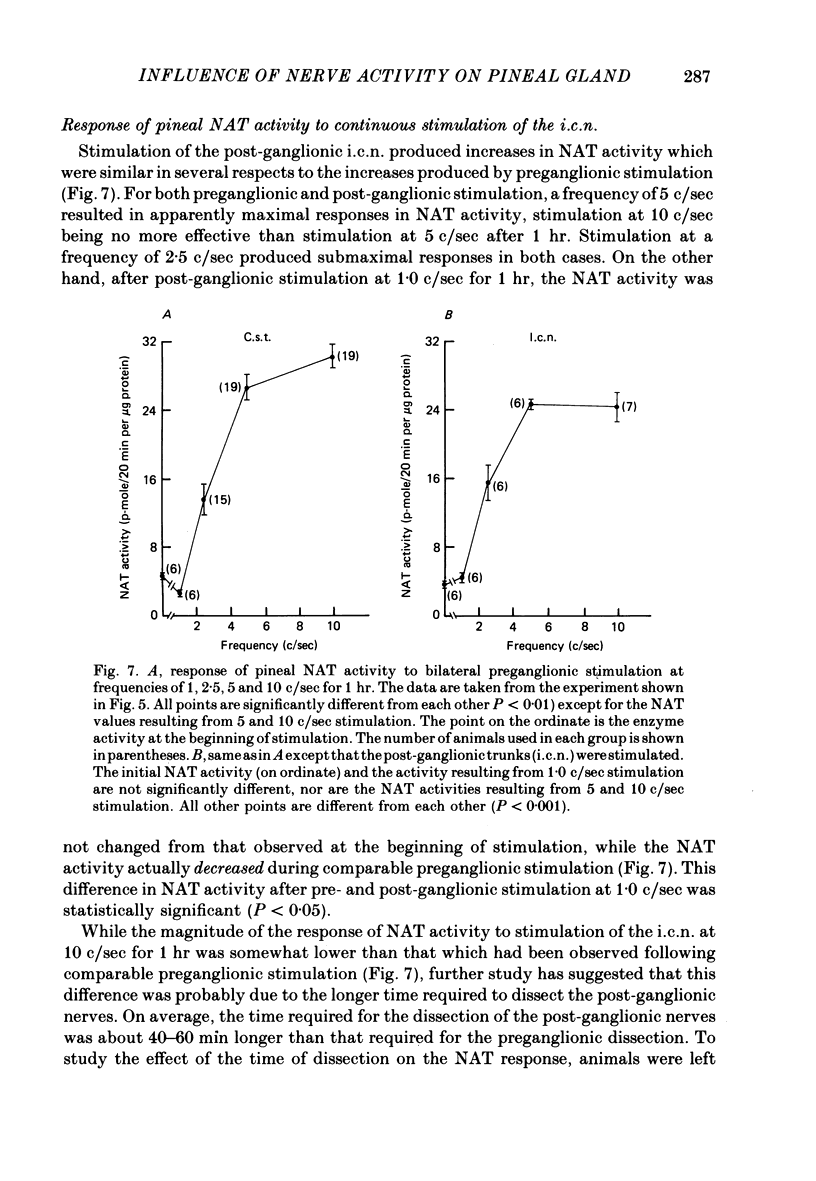
The activity of the pineal enzyme arylamine: N-acetyltransferase (NAT) was determined following direct stimulation of the preganglionic or post-ganglionic nerves of the superior cervical ganglia. 1. Stimulation of the preganglionic trunks at 10 c/sec during the day or night was sufficient to increase NAT activity approximately 50-fold, to levels comparable to those observed at night in the intact animal. The time course of this effect of nerve stimulation differed between day and night. 2. The responses of pineal NAT to certain frequencies of stimulation were similar for preganglionic and post-ganglionic stimulation. In both cases the responses to stimulation at 5 c/sec appeared to be maximal, 10 c/sec causing no further increase. However, at 10 c/sec, stimulation was more effective post-ganglionically than preganglionically. 3. Various patterns of preganglionic stimulation, having the same average frequency, differed in their ability to increase the activity of NAT. Some, though not all, of these differences between patterns were observed during post-ganglionic stimulation. 4. Unilateral stimulation of the preganglionic nerves produced an increase in NAT activity that was less than half the increase produced by bilateral stimulation, suggesting that the innervation from the two ganglia interact within the pineal gland. 5. These data indicate that changes in the firing rates of sympathetic nerves innervating the pineal gland, within the range of frequencies typically observed for sympathetic neurones, would be sufficient to account for the circadian rhythm in NAT activity observed in the intact rat. Changes in the over-all pattern of sympathetic activity, in addition to changes in the total number of stimuli, could play a significant role in the pineal response.
Full text
PDF














Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bowers C. W., Zigmond R. E. Electrical stimulation of the cerivcal sympathetic trunks mimics the effects of darkness on the activity of serotonin:N-acetyltransferase in the rat pineal. Brain Res. 1980 Mar 10;185(2):435–440. doi: 10.1016/0006-8993(80)91082-3. [DOI] [PubMed] [Google Scholar]
- Deguchi T., Axelrod J. Control of circadian change of serotonin N-acetyltransferase activity in the pineal organ by the beta--adrenergic receptor. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2547–2550. doi: 10.1073/pnas.69.9.2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deguchi T., Axelrod J. Sensitive assay for serotonin N-acetyltransferase activity in rat pineal. Anal Biochem. 1972 Nov;50(1):174–179. doi: 10.1016/0003-2697(72)90496-4. [DOI] [PubMed] [Google Scholar]
- Dunant Y., Dolivo M. Relations entre les potentiels synaptiques lents et l'excitabilité du ganglion. J Physiol (Paris) 1967 Jul-Aug;59(4):281–294. [PubMed] [Google Scholar]
- FOLKOW B. Impulse frequency in sympathetic vasomotor fibres correlated to the release and elimination of the transmitter. Acta Physiol Scand. 1952;25(1):49–76. doi: 10.1111/j.1748-1716.1952.tb00858.x. [DOI] [PubMed] [Google Scholar]
- FOLKOW B., LOFVING B., MELLANDER S. Quantitative aspects of the sympathetic neuro-hormonal control of the heart rate. Acta Physiol Scand. 1956 Nov 5;37(4):363–369. doi: 10.1111/j.1748-1716.1956.tb01372.x. [DOI] [PubMed] [Google Scholar]
- HUBEL D. H. Single unit activity in lateral geniculate body and optic tract of unrestrained cats. J Physiol. 1960 Jan;150:91–104. doi: 10.1113/jphysiol.1960.sp006375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IGGO A., VOGT M. Preganglionic sympathetic activity in normal and in reserpine-treated cats. J Physiol. 1960 Jan;150:114–133. doi: 10.1113/jphysiol.1960.sp006377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illnerová H., Vanecek J., Krecek J., Wetterberg L., Säf J. Effect of one minute exposure to light at night on rat pineal serotonin N-acetyltransferase and melatonin. J Neurochem. 1979 Feb;32(2):673–675. doi: 10.1111/j.1471-4159.1979.tb00407.x. [DOI] [PubMed] [Google Scholar]
- Jänig W., Schmidt R. F. Single unit responses in the cervical sympathetic trunk upon somatic nerve stimulation. Pflugers Arch. 1970;314(3):199–216. doi: 10.1007/BF00592246. [DOI] [PubMed] [Google Scholar]
- Jänig W., Szulczyk P. Functional properties of lumbar preganglionic neurones. Brain Res. 1980 Mar 17;186(1):115–131. doi: 10.1016/0006-8993(80)90259-0. [DOI] [PubMed] [Google Scholar]
- KAPPERS J. A. The development, topographical relations and innervation of the epiphysis cerebri in the albino rat. Z Zellforsch Mikrosk Anat. 1960;52:163–215. doi: 10.1007/BF00338980. [DOI] [PubMed] [Google Scholar]
- Klein D. C., Weller J. L. Indole metabolism in the pineal gland: a circadian rhythm in N-acetyltransferase. Science. 1970 Sep 11;169(3950):1093–1095. doi: 10.1126/science.169.3950.1093. [DOI] [PubMed] [Google Scholar]
- Klein D. C., Weller J. L. Rapid light-induced decrease in pineal serotonin N-acetyltransferase activity. Science. 1972 Aug 11;177(4048):532–533. doi: 10.1126/science.177.4048.532. [DOI] [PubMed] [Google Scholar]
- Koss M. C., Rieger J. A. Differential transmission in the superior cervical ganglion of the cat. J Pharmacol Exp Ther. 1976 Dec;199(3):538–543. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Large B. J. Innervation both of peri-orbital structures and of the heart by the cervical sympathetic nerves in mouse, rat, guinea-pig, rabbit and cat. Br J Pharmacol. 1975 Jul;54(3):351–358. doi: 10.1111/j.1476-5381.1975.tb07575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomo T., Rosenthal J. Control of ACh sensitivity by muscle activity in the rat. J Physiol. 1972 Mar;221(2):493–513. doi: 10.1113/jphysiol.1972.sp009764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomo T., Westgaard R. H., Dahl H. A. Contractile properties of muscle: control by pattern of muscle activity in the rat. Proc R Soc Lond B Biol Sci. 1974 Aug 27;187(1086):99–103. doi: 10.1098/rspb.1974.0064. [DOI] [PubMed] [Google Scholar]
- Lomo T., Westgaard R. H. Further studies on the control of ACh sensitivity by muscle activity in the rat. J Physiol. 1975 Nov;252(3):603–626. doi: 10.1113/jphysiol.1975.sp011161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lømo T., Slater C. R. Control of junctional acetylcholinesterase by neural and muscular influences in the rat. J Physiol. 1980 Jun;303:191–202. doi: 10.1113/jphysiol.1980.sp013280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarley R. W., Hobson J. A. Discharge patterns of cat pontine brain stem neurons during desynchronized sleep. J Neurophysiol. 1975 Jul;38(4):751–766. doi: 10.1152/jn.1975.38.4.751. [DOI] [PubMed] [Google Scholar]
- Morrissey J. J., Lovenberg W. Synthesis of RNA in the pineal gland during N-acetyltransferase induction. Biochem Pharmacol. 1978 Feb 15;27(4):551–555. doi: 10.1016/0006-2952(78)90393-3. [DOI] [PubMed] [Google Scholar]
- Murrin L. C., Roth R. H. Dopaminergic neurons: effects of electrical stimulation on dopamine biosynthesis. Mol Pharmacol. 1976 May;12(3):463–475. [PubMed] [Google Scholar]
- OWMAN C. SYMPATHETIC NERVES PROBABLY STORING TWO TYPES OF MONOAMINES IN THE RAT PINEAL GLAND. Int J Neuropharmacol. 1964 Apr;3:105–112. doi: 10.1016/0028-3908(64)90052-8. [DOI] [PubMed] [Google Scholar]
- Parfitt A., Weller J. L., Klein D. C., Sakai K. K., Marks B. H. Blockade by ouabain or elevated potassium ion concentration of the adrenergic and adenosine cyclic 3',5'-monophosphate-induced stimulation of pineal serotonin N-acetyltransferase activity. Mol Pharmacol. 1975 May;11(3):241–255. [PubMed] [Google Scholar]
- Passatore M., Pettorossi V. E. Efferent fibers in the cervical sympathetic nerve influenced by light. Exp Neurol. 1976 Jul;52(1):66–82. doi: 10.1016/0014-4886(76)90201-6. [DOI] [PubMed] [Google Scholar]
- ROSE J. E., MOUNTCASTLE V. B. Activity of single neurons in the tactile thalamic region of the cat in response to a transient peripheral stimulus. Bull Johns Hopkins Hosp. 1954 May;94(5):238–282. [PubMed] [Google Scholar]
- Ralph C. L., Binkley S., MacBride S. E., Klein D. C. Regulation of pineal rhythms in chickens: effects of blinding, constant light, constant dark, and superior cervical ganglionectomy. Endocrinology. 1975 Dec;97(6):1373–1378. doi: 10.1210/endo-97-6-1373. [DOI] [PubMed] [Google Scholar]
- Romero J. A., Axelrod J. Regulation of sensitivity to beta-adrenergic stimulation in induction of pineal N-acetyltransferase. Proc Natl Acad Sci U S A. 1975 May;72(5):1661–1665. doi: 10.1073/pnas.72.5.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero J. A., Zatz M., Axelrod J. Beta-adrenergic stimulation of pineal N-acetyltransferase: adenosine 3':5'-cyclic monophosphate stimulates both RNA and protein synthesis. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2107–2111. doi: 10.1073/pnas.72.6.2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin L. L., Schuetze S. M., Weill C. L., Fischbach G. D. Regulation of acetylcholinesterase appearance at neuromuscular junctions in vitro. Nature. 1980 Jan 17;283(5744):264–267. doi: 10.1038/283264a0. [DOI] [PubMed] [Google Scholar]
- TUTTLE R. S., KELTS A. P. PERIODIC VERSUS CONTINUOUS STIMULATION OF CAT SYMPATHETIC PREGANGLIONIC NERVES ON HEART RATE AND CONTRACTION OF THE NICTITATING MEMBRANE. Life Sci. 1964 May;3:421–430. doi: 10.1016/0024-3205(64)90202-4. [DOI] [PubMed] [Google Scholar]
- Taylor A. N., Wilson R. W. Electrophysiological evidence for the action of light on the pineal gland in the rat. Experientia. 1970 Mar 15;26(3):267–269. doi: 10.1007/BF01900087. [DOI] [PubMed] [Google Scholar]
- Volkman P. H., Heller A. Pineal N-acetyltransferase activity: effect of sympathetic stimulation. Science. 1971 Aug 27;173(3999):839–840. doi: 10.1126/science.173.3999.839. [DOI] [PubMed] [Google Scholar]
- Weiner N., Rabadjija M. The effect of nerve stimulation on the synthesis and metabolism of norepinephrine in the isolated guinea-pig hypogastric nerve-vas deferens preparation. J Pharmacol Exp Ther. 1968 Mar;160(1):61–71. [PubMed] [Google Scholar]
- Wilkinson M., Arendt J., Bradtke J., de Ziegler D. Determination of a dark-induced increase of pineal N-acetyl transferase activity and simultaneous radioimmunoassay of melatonin in pineal, serum and pituitary tissue of the male rat. J Endocrinol. 1977 Feb;72(2):243–244. doi: 10.1677/joe.0.0720243. [DOI] [PubMed] [Google Scholar]
- Zatz M., Romero J. A., Axelrod J. Diurnal variation in the requirement for RNA synthesis in the induction of pineal N-acetyltransferase. Biochem Pharmacol. 1976 Apr 15;25(8):903–906. doi: 10.1016/0006-2952(76)90312-9. [DOI] [PubMed] [Google Scholar]
- Zigmond R. E., Baldwin C., Bowers C. W. Rapid recovery of function after partial denervation of the rat pineal gland suggests a novel mechanism for neural plasticity. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3959–3963. doi: 10.1073/pnas.78.6.3959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigmond R. E., Chalazonitis A. Long-term effects of preganglionic nerve stimulation on tyrosine hydroxylase activity in the rat superior cervical ganglion. Brain Res. 1979 Mar 23;164:137–152. doi: 10.1016/0006-8993(79)90011-8. [DOI] [PubMed] [Google Scholar]


