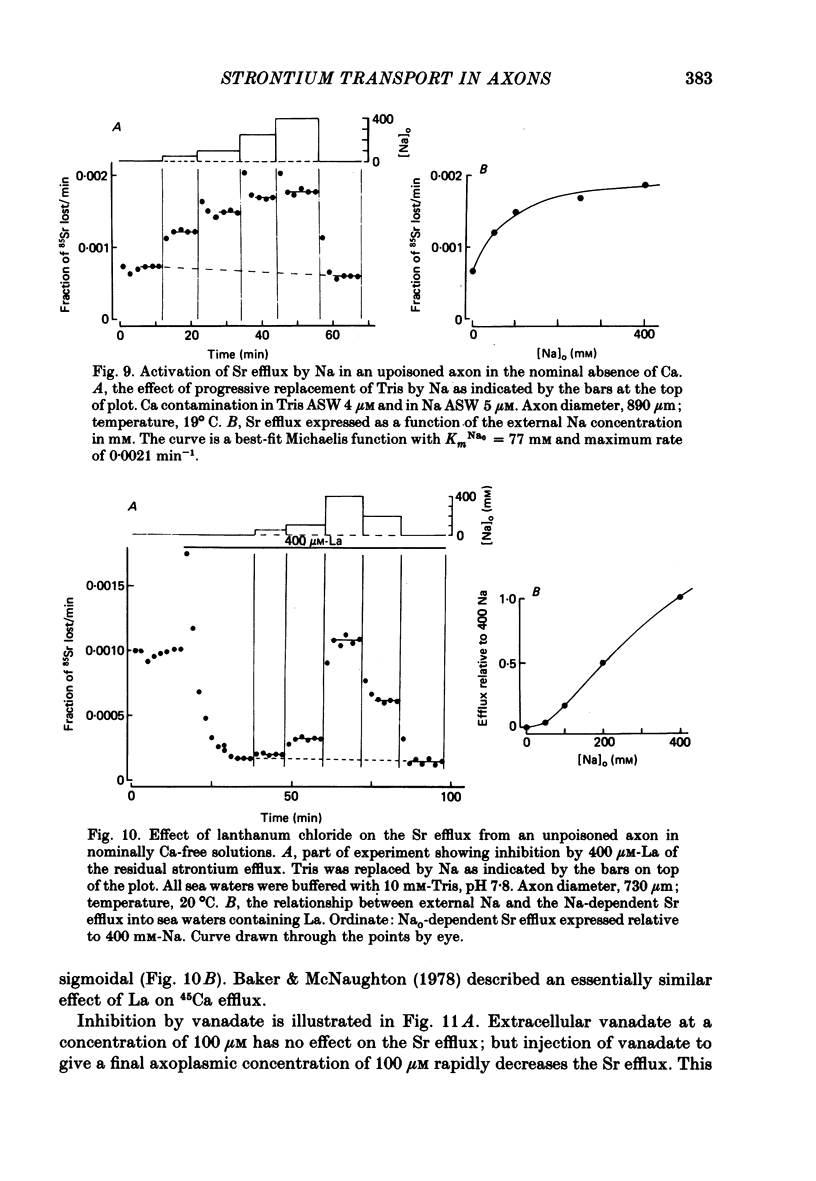
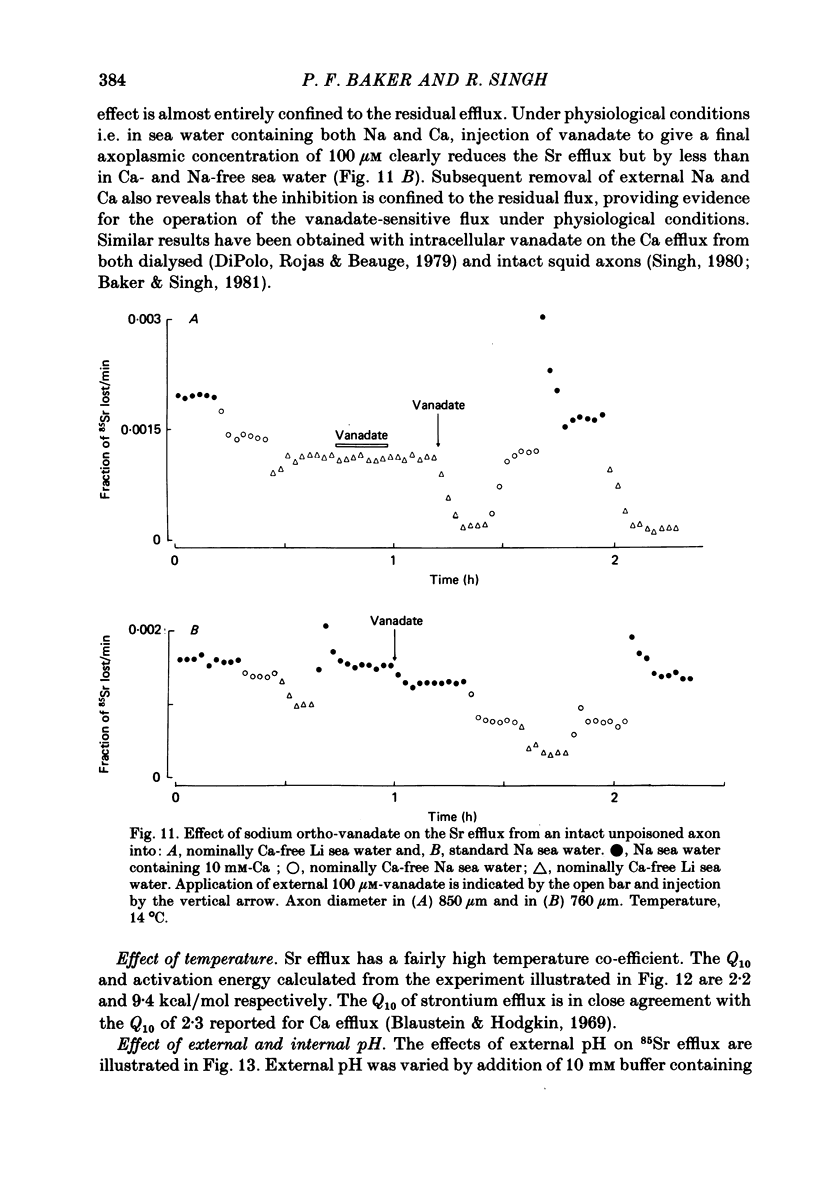
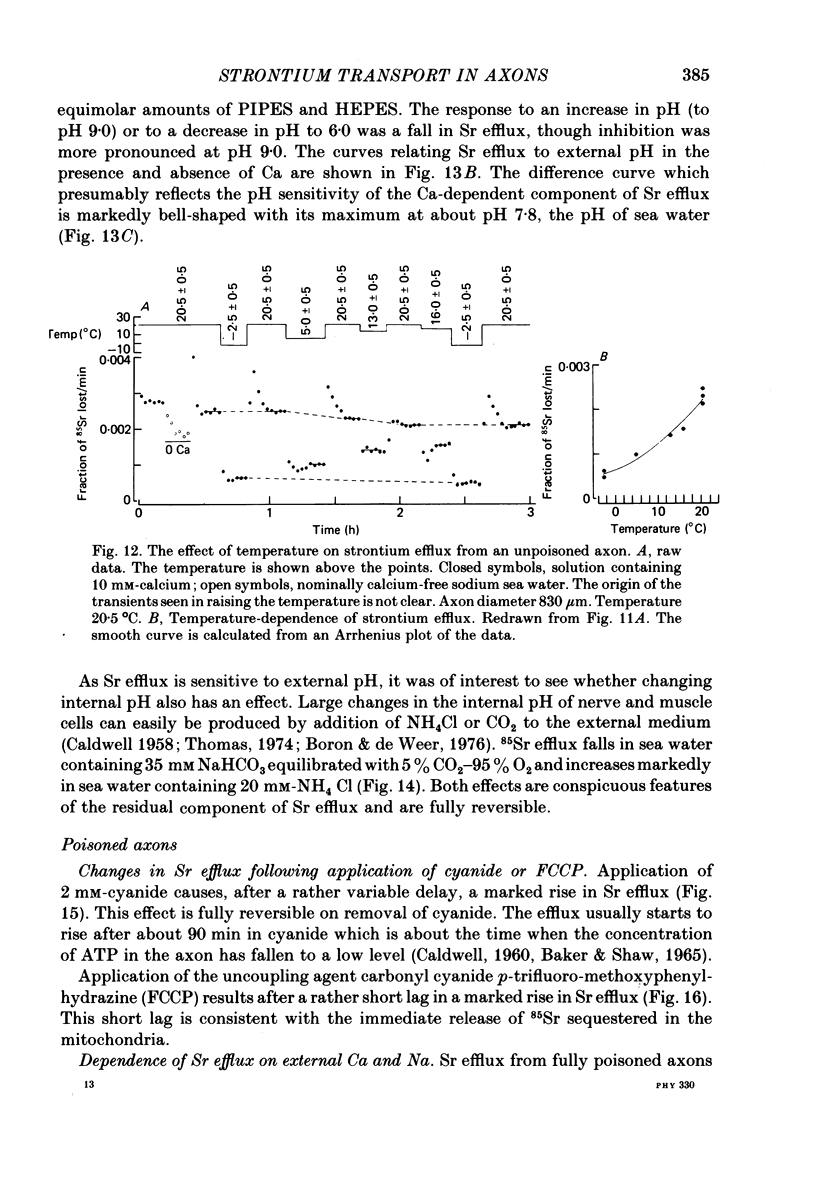
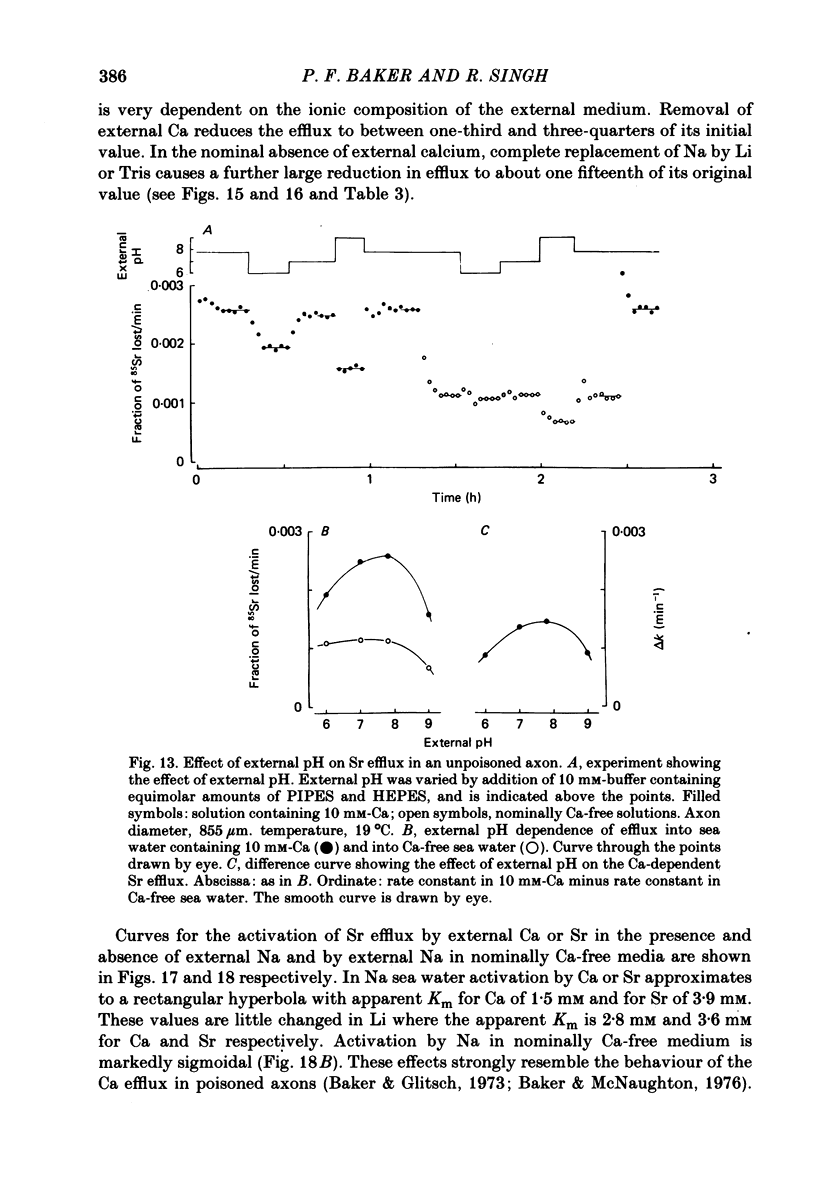
Abstract
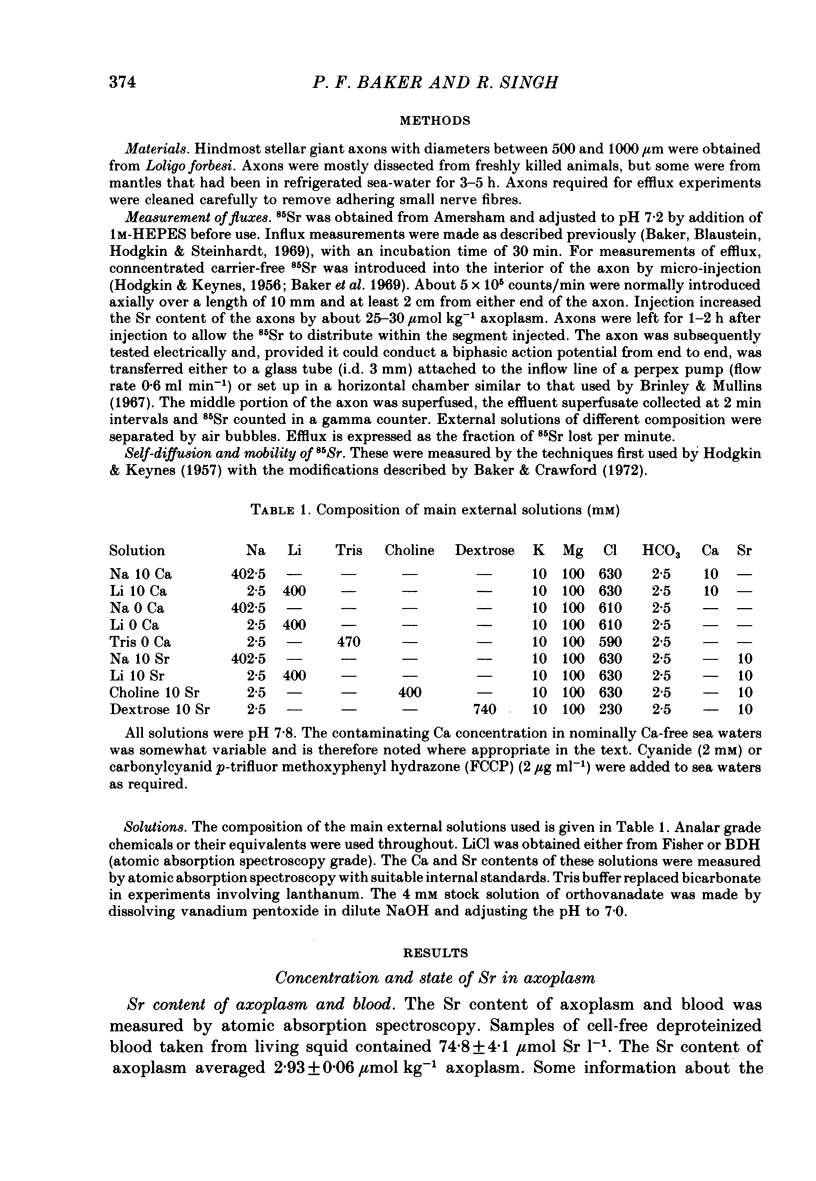
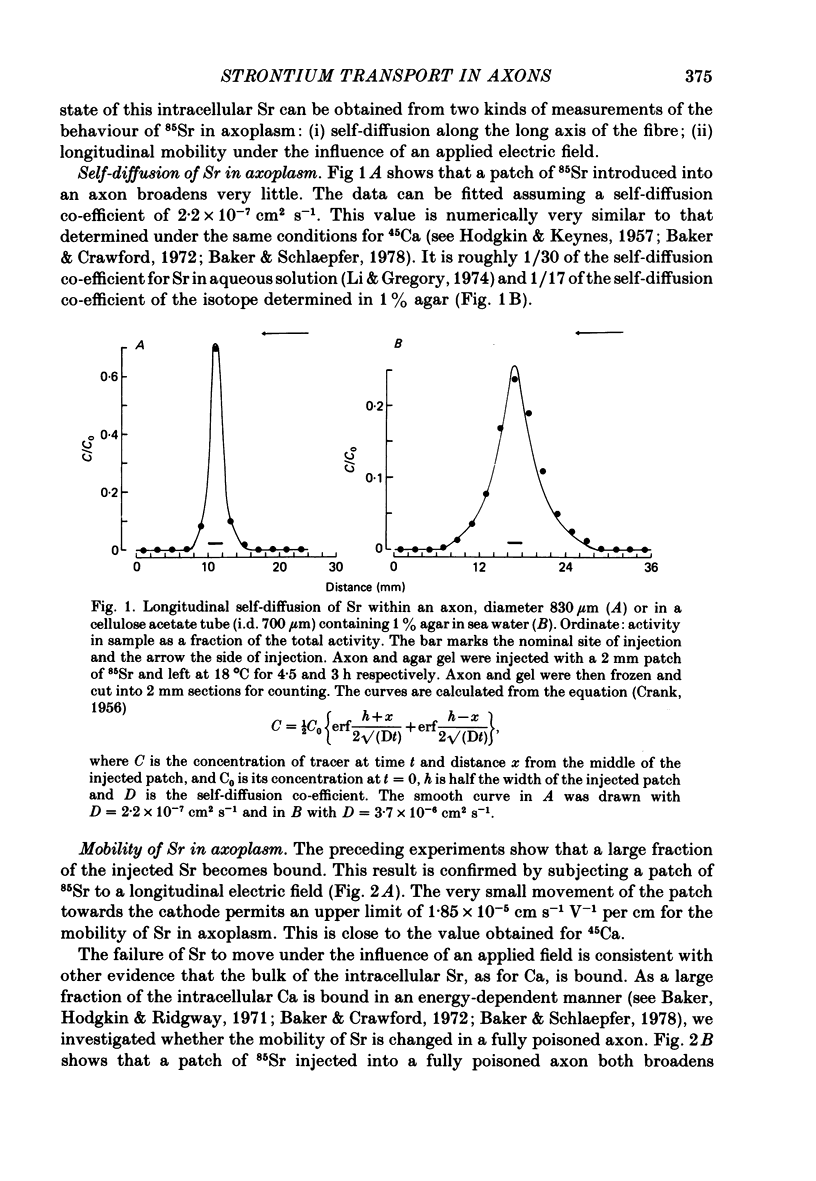
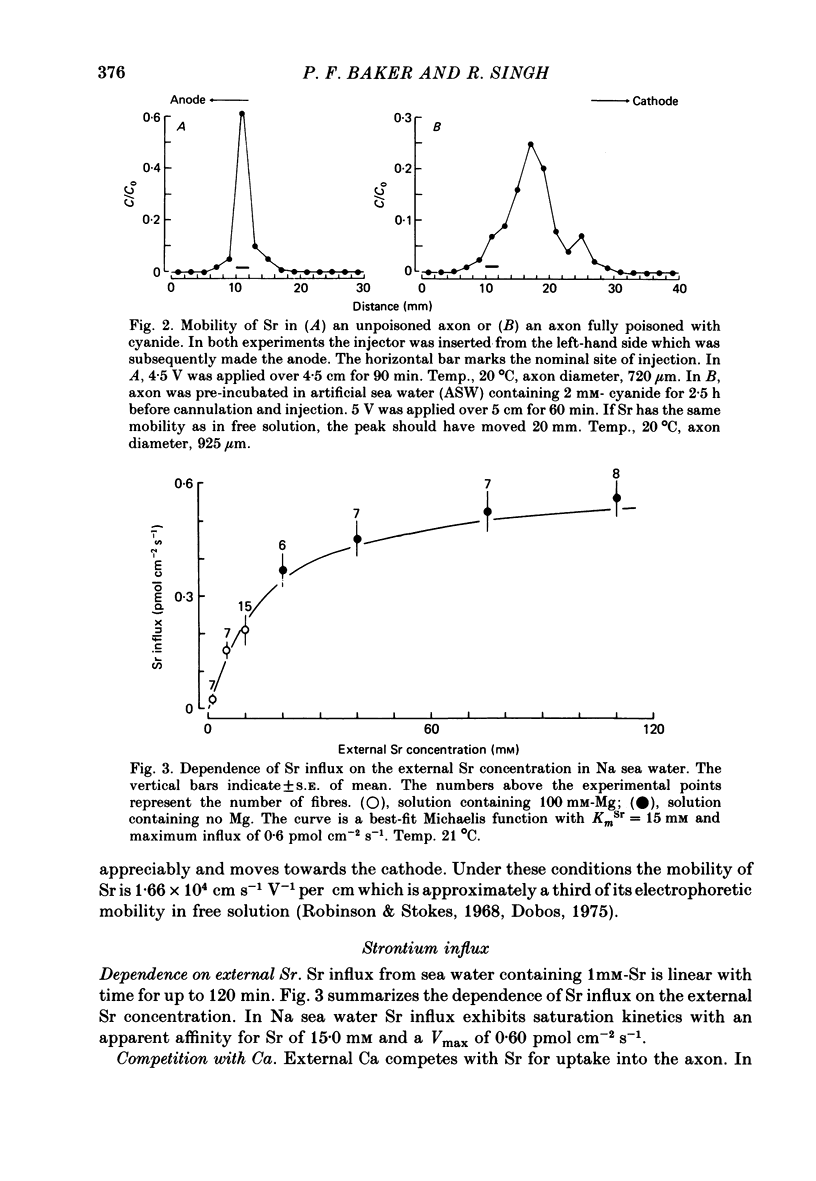
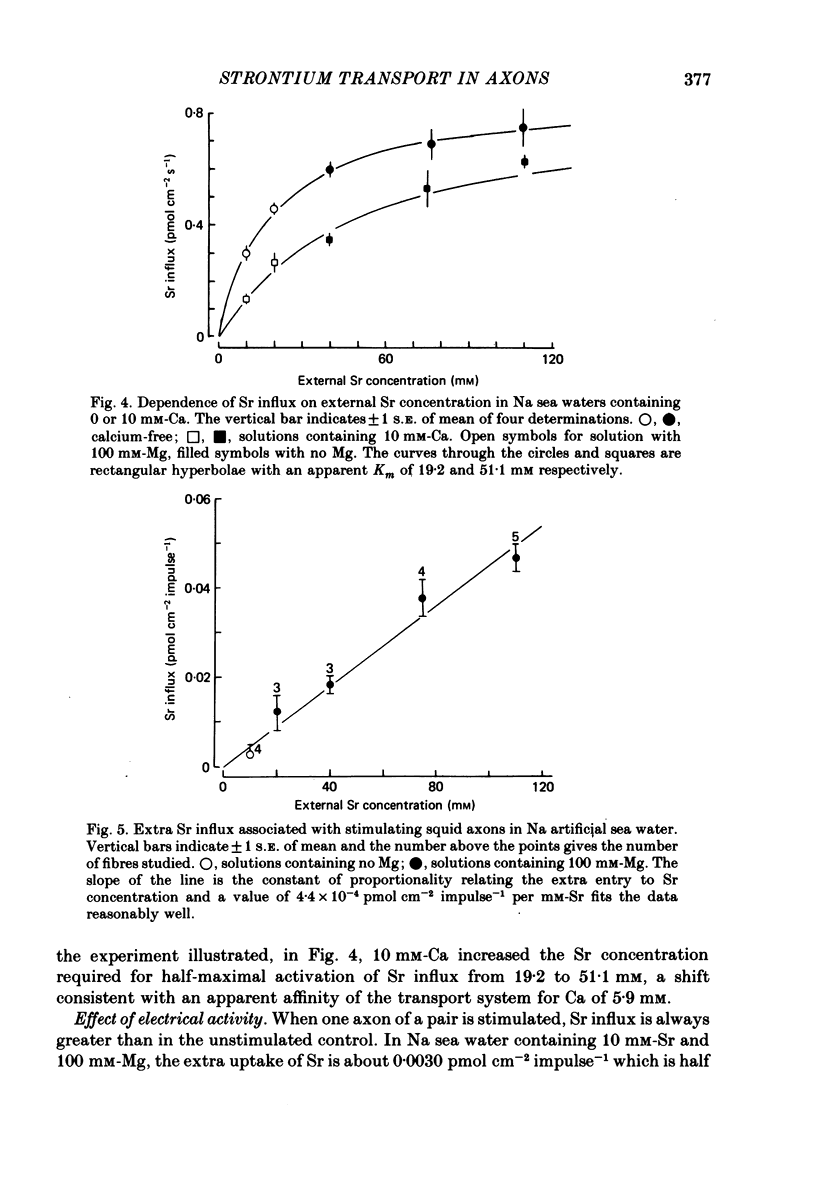
1. The strontium content of squid axons averages 2.9 mumol kg-1 axoplasm and squid blood contains 75 mumol Sr l-1. The bulk of the Sr inside axons is bound, possibly within mitochondria. Sr seems to be handled by squid axons in an essentially similar manner to Ca. 2. Sr uptake is increased by electrical activity, by lowering external Na, by depolarization with K and by poisoning with cyanide or FCCP. Uptake is unaffected by ouabain. 3. Sr efflux has a Q10 of 2.2. Efflux can be divided operationally into three components: one dependent on external Ca (or Sr), one dependent on external Na and a residual component that can be inhibited by external lanthanum or internal vanadate. 4. Poisoning an axon with cyanide or FCCP results in a large increase in Sr efflux which can be divided operationally into two components: one dependent on external Ca (or Sr) and one dependent on external Na.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker P. F., Blaustein M. P., Hodgkin A. L., Steinhardt R. A. The influence of calcium on sodium efflux in squid axons. J Physiol. 1969 Feb;200(2):431–458. doi: 10.1113/jphysiol.1969.sp008702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Crawford A. C. Mobility and transport of magnesium in squid giant axons. J Physiol. 1972 Dec;227(3):855–874. doi: 10.1113/jphysiol.1972.sp010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Glitsch H. G. Does metabolic energy participate directly in the Na+-dependent extrusion of Ca2+ -Ca2+ ions from squid giant axons? J Physiol. 1973 Aug;233(1):44P–46P. [PubMed] [Google Scholar]
- Baker P. F., Hodgkin A. L., Ridgway E. B. Depolarization and calcium entry in squid giant axons. J Physiol. 1971 Nov;218(3):709–755. doi: 10.1113/jphysiol.1971.sp009641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., McNaughton P. A. Kinetics and energetics of calcium efflux from intact squid giant axons. J Physiol. 1976 Jul;259(1):103–144. doi: 10.1113/jphysiol.1976.sp011457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., McNaughton P. A. The influence of extracellular calcium binding on the calcium efflux from squid axons. J Physiol. 1978 Mar;276:127–150. doi: 10.1113/jphysiol.1978.sp012223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Schlaepfer W. W. Uptake and binding of calcium by axoplasm isolated from giant axons of Loligo and Myxicola. J Physiol. 1978 Mar;276:103–125. doi: 10.1113/jphysiol.1978.sp012222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Shaw T. I. A comparison of the phosphorus metabolism of intact squid nerve with that of the isolated axoplasm and sheath. J Physiol. 1965 Sep;180(2):424–438. doi: 10.1113/jphysiol.1965.sp007710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F. The regulation of intracellular calcium in giant axons of Loligo and Myxicola. Ann N Y Acad Sci. 1978 Apr 28;307:250–268. doi: 10.1111/j.1749-6632.1978.tb41956.x. [DOI] [PubMed] [Google Scholar]
- Baker P. F. Transport and metabolism of calcium ions in nerve. Prog Biophys Mol Biol. 1972;24:177–223. doi: 10.1016/0079-6107(72)90007-7. [DOI] [PubMed] [Google Scholar]
- Blaustein M. P., Hodgkin A. L. The effect of cyanide on the efflux of calcium from squid axons. J Physiol. 1969 Feb;200(2):497–527. doi: 10.1113/jphysiol.1969.sp008704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boron W. F., De Weer P. Intracellular pH transients in squid giant axons caused by CO2, NH3, and metabolic inhibitors. J Gen Physiol. 1976 Jan;67(1):91–112. doi: 10.1085/jgp.67.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinley F. J., Jr, Mullins L. J. Sodium extrusion by internally dialyzed squid axons. J Gen Physiol. 1967 Nov;50(10):2303–2331. doi: 10.1085/jgp.50.10.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CALDWELL P. C. Studies on the internal pH of large muscle and nerve fibres. J Physiol. 1958 Jun 18;142(1):22–62. doi: 10.1113/jphysiol.1958.sp005998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CALDWELL P. C. The phosphorus metabolism of squid axons and its relationship to the active transport of sodium. J Physiol. 1960 Jul;152:545–560. doi: 10.1113/jphysiol.1960.sp006508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crankshaw D. J., Janis R. A., Daniel E. E. The effects of Ca2+ antagonists on Ca2+ accumulation by subcellular fractions of rat myometrium. Can J Physiol Pharmacol. 1977 Oct;55(5):1028–1032. doi: 10.1139/y77-141. [DOI] [PubMed] [Google Scholar]
- DiPolo R., Rojas H. R., Beaugé L. Vanadate inhibits uncoupled Ca efflux but not Na--Ca exchange in squid axons. Nature. 1979 Sep 20;281(5728):229–230. doi: 10.1038/281228a0. [DOI] [PubMed] [Google Scholar]
- Foreman J. C., Hallett M. B., Mongar J. L. Movement of strontium ions into mast cells and its relationship to the secretory response. J Physiol. 1977 Sep;271(1):233–251. doi: 10.1113/jphysiol.1977.sp011998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KEYNES R. D. Experiments on the injection of substances into squid giant axons by means of a microsyringe. J Physiol. 1956 Mar 28;131(3):592–616. doi: 10.1113/jphysiol.1956.sp005485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KEYNES R. D. Movements of labelled calcium in squid giant axons. J Physiol. 1957 Sep 30;138(2):253–281. doi: 10.1113/jphysiol.1957.sp005850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddas R. A., Landis C. A., Putney J. W., Jr Relationship between calcium release and potassium release in rat parotid gland. J Physiol. 1979 Jun;291:457–465. doi: 10.1113/jphysiol.1979.sp012825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meiri U., Rahamimoff R. Activation of transmitter release by strontium and calcium ions at the neuromuscular junction. J Physiol. 1971 Jul;215(3):709–726. doi: 10.1113/jphysiol.1971.sp009493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papworth D. G., Patrick G. The kinetics of influx of calcium and strontium into rat intestine in vitro. J Physiol. 1970 Nov;210(4):999–1020. doi: 10.1113/jphysiol.1970.sp009254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas R. C. Intracellular pH of snail neurones measured with a new pH-sensitive glass mirco-electrode. J Physiol. 1974 Apr;238(1):159–180. doi: 10.1113/jphysiol.1974.sp010516. [DOI] [PMC free article] [PubMed] [Google Scholar]


