Abstract
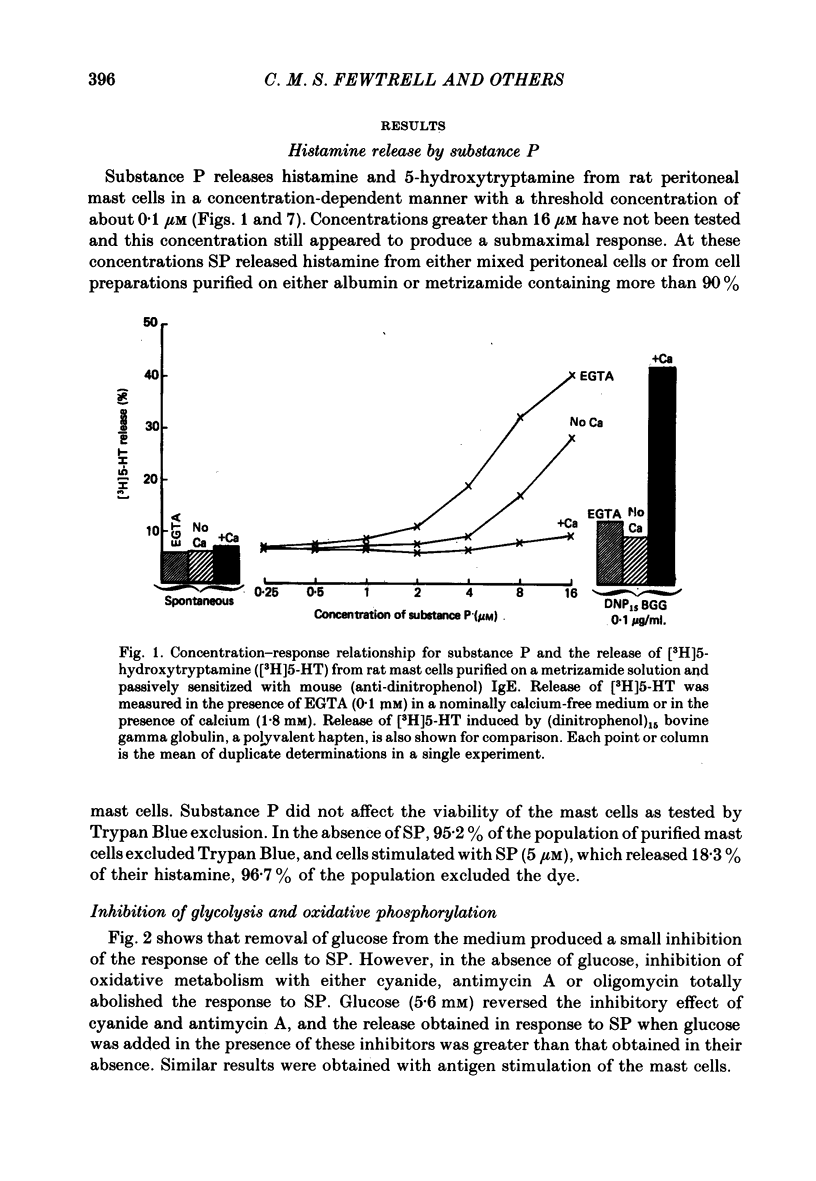
1. Substance P (SP) induces histamine release from isolated rat peritoneal mast cells at concentrations of 0·1-10 μM.
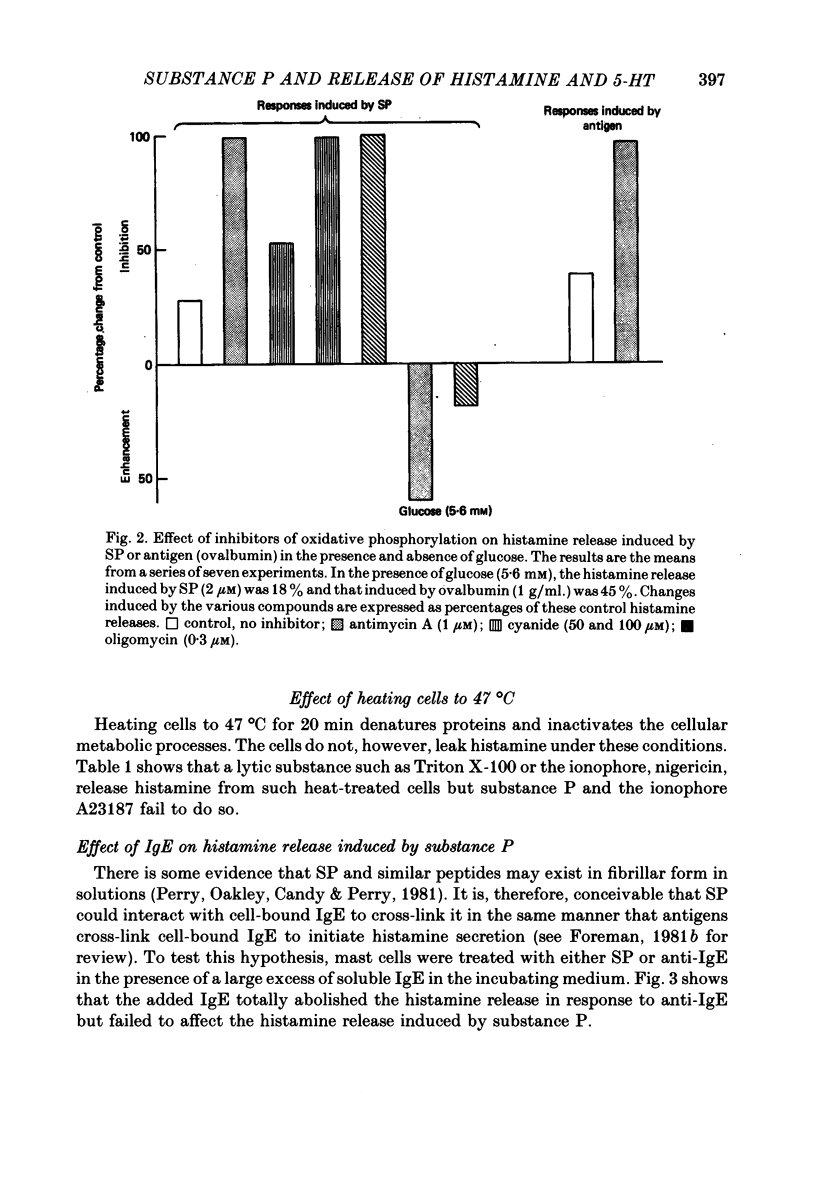
2. Inhibitors of glycolysis and oxidative phosphorylation prevent the release of histamine induced by SP.
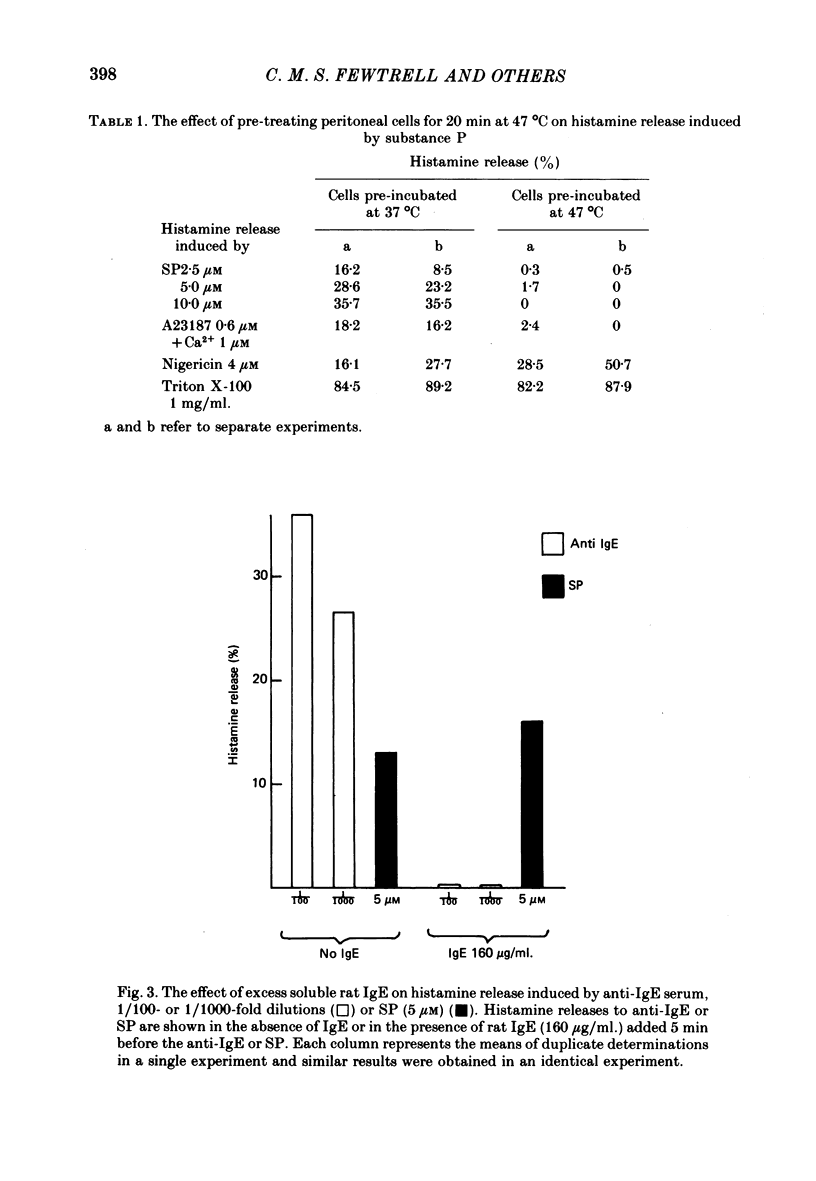
3. Cells heated to 47 °C for 20 min release histamine when treated with an agent causing cell lysis but fail to release in response to SP.
4. SP does not release histamine by interacting with cell-bound IgE.
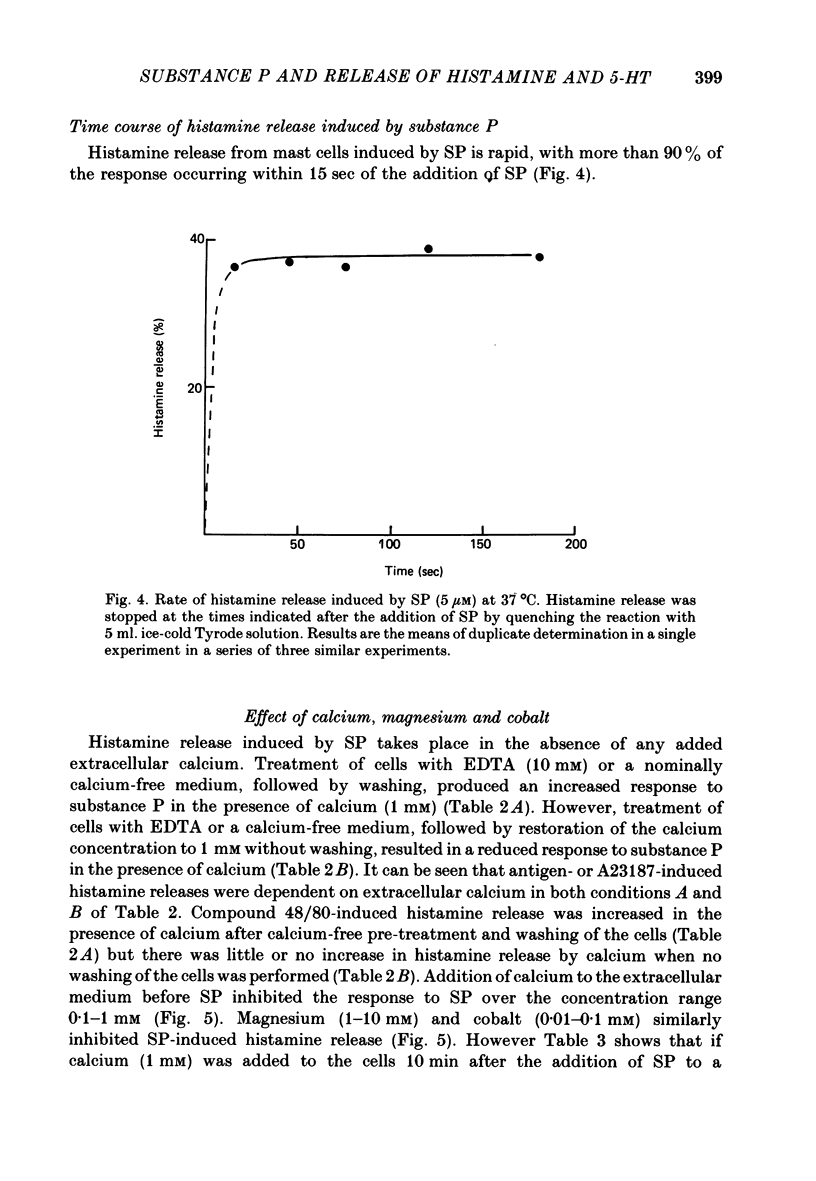
5. Histamine release by SP is rapid, with more than 90% of the response occurring within 1 min of the addition of the peptide to mast cells at 37 °C.
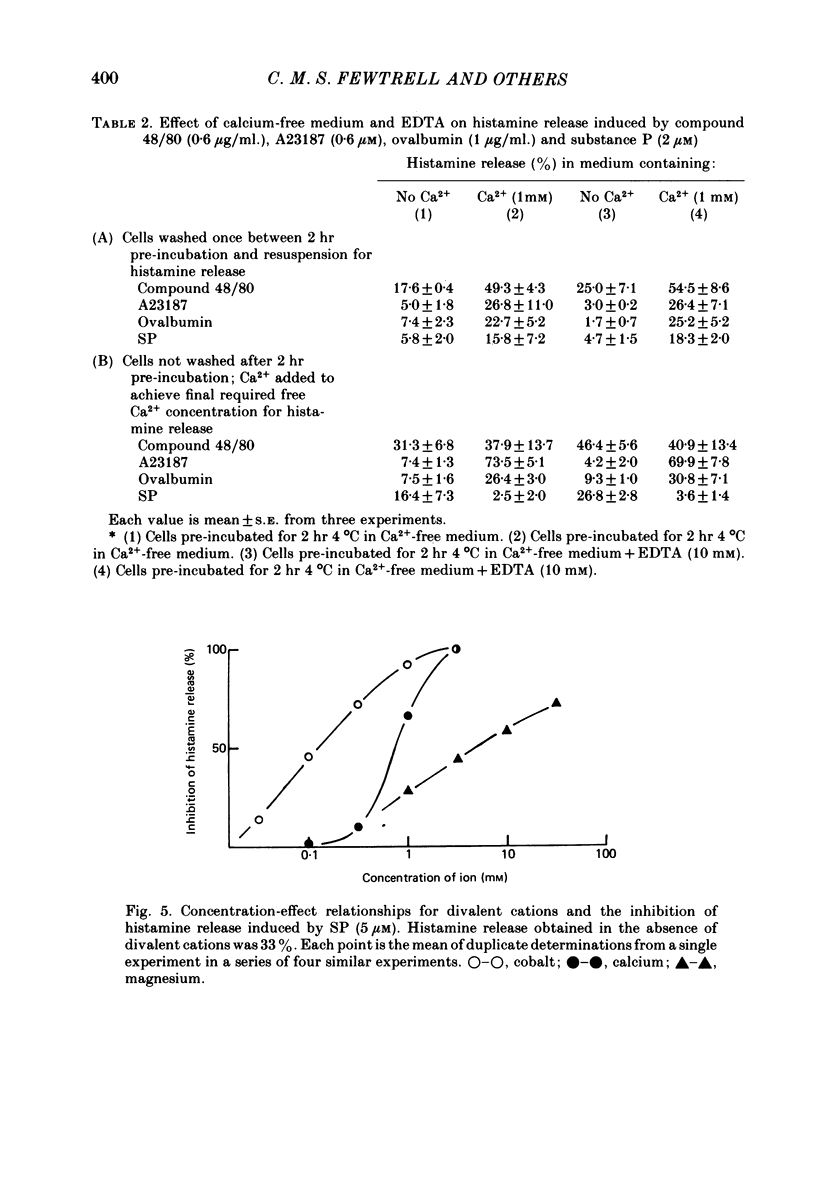
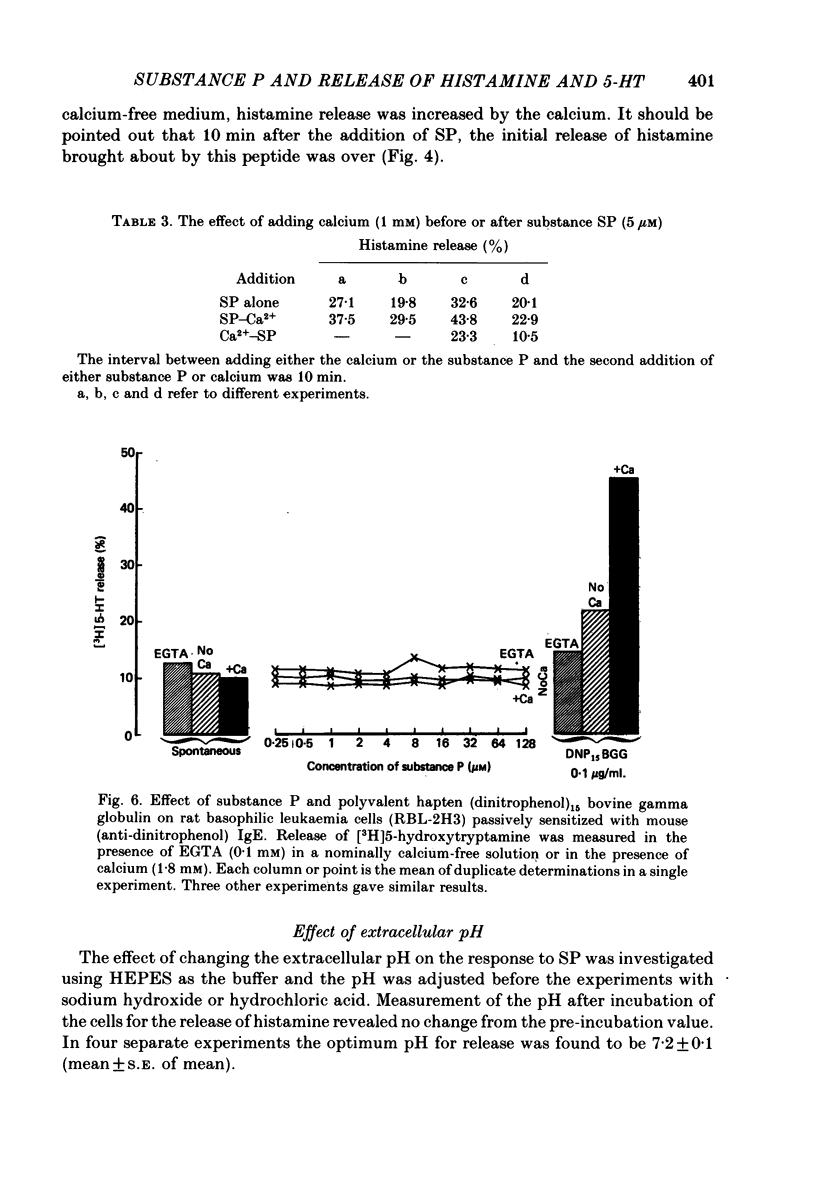
6. Substance P, unlike antigen—antibody or compound 48/80, does not show enhanced release of histamine when calcium (0·1-1 mM) is present in the extracellular medium but calcium increases the response to SP when the ion is added after the peptide. Extracellular calcium (0·1-1 mM), magnesium (1-10 mM) and cobalt (0·01-0·1 mM) all inhibit SP-induced histamine release when added before the peptide. Pre-treatment of the cells with EDTA (10 mM) and washing in calcium-free medium inhibits the histamine release induced by SP.
7. Histamine release induced by SP was optimum at an extracellular pH of 7·2.
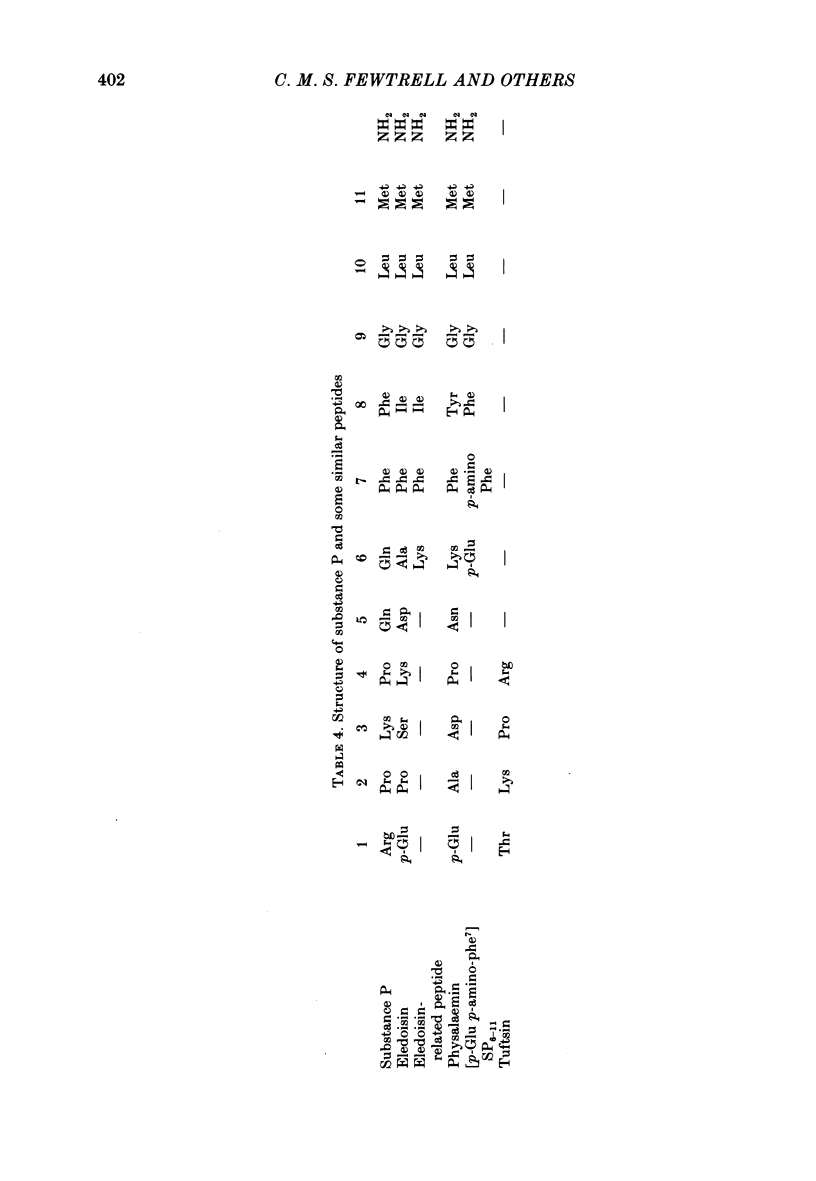
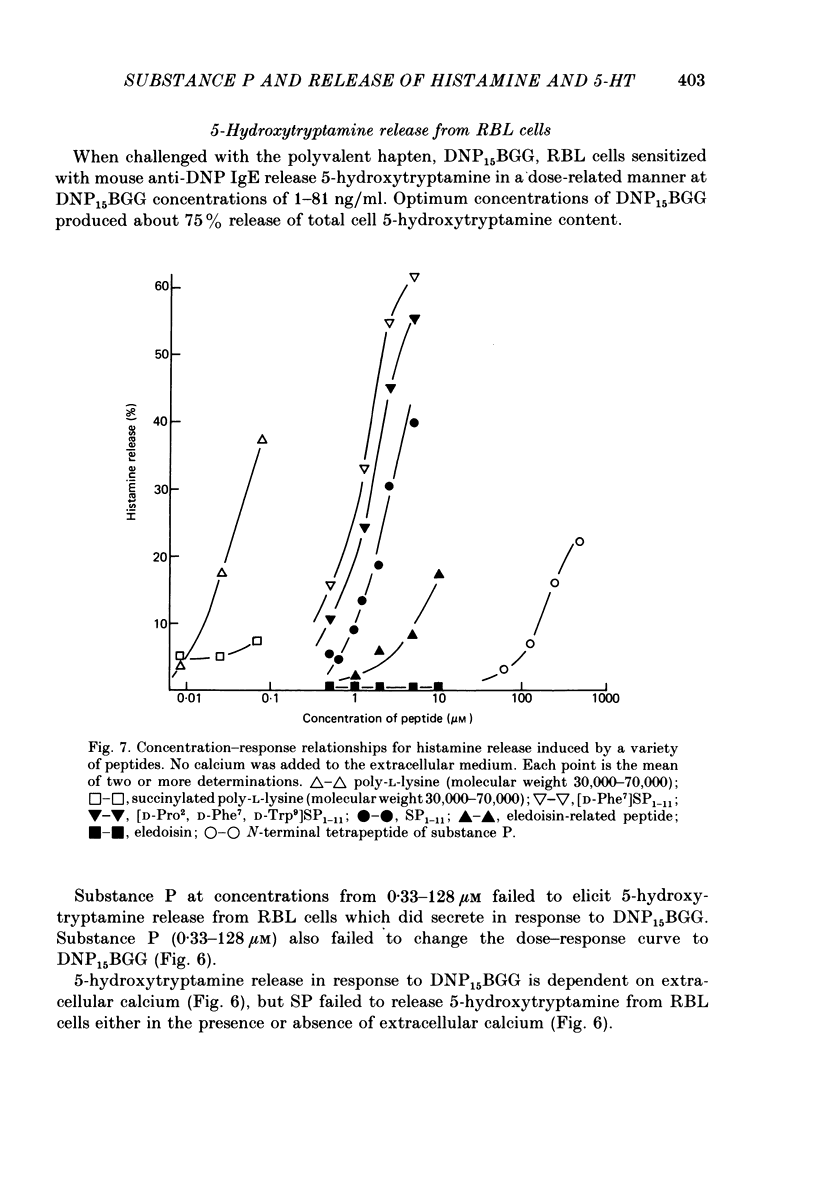
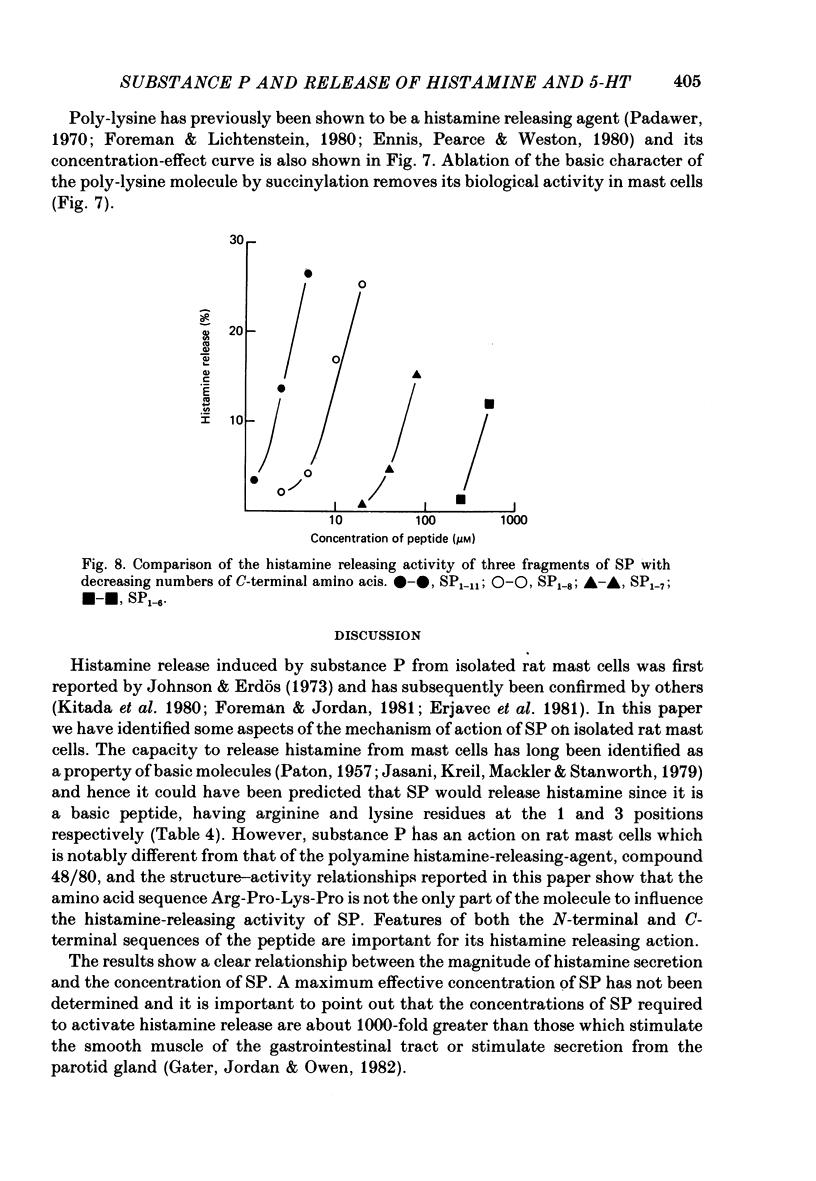
8. A number of peptides structurally related to SP were examined for histamine-releasing activity. At the concentrations tested, the N-terminal dipeptides Lys-Pro and Arg-Pro, tuftsin, physalaemin, eledoisin, SP3-11, SP4-11 and [p-Glu6, p-amino Phe7]-SP6-11 were all found to be inactive. The relative activities of the other peptides were: [Formula: see text]
9. Rat basophilic leukaemia cells (RBL-2H3) fail to respond to SP at concentrations which activate rat mast cells. Release of 5-hydroxytryptamine by immunological activation of RBL cells is not changed by the presence of SP.
10. The mechanism of action of SP on mast cells and the nature of the SP receptor on mast cells is discussed in relation to SP receptors in other cell types.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown C. L., Hanley M. R. The effects of substance P and related peptides on alpha-amylase release from rat parotid gland slices. Br J Pharmacol. 1981 Jun;73(2):517–523. doi: 10.1111/j.1476-5381.1981.tb10451.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chahl L. A. The effect of putative peptide neurotransmitters on cutaneous vascular permeability in the rat. Naunyn Schmiedebergs Arch Pharmacol. 1979 Nov;309(2):159–163. doi: 10.1007/BF00501224. [DOI] [PubMed] [Google Scholar]
- Chakravarty N. Further observations on the inhibition of histamine release by 2-deoxyglucose. Acta Physiol Scand. 1968 Apr;72(4):425–432. doi: 10.1111/j.1748-1716.1968.tb03867.x. [DOI] [PubMed] [Google Scholar]
- Chipkin R. E., Stewart J. M., Sweeney V. E., Harris K., Williams R. In vitro activities of some synthetic substance P analogs. Arch Int Pharmacodyn Ther. 1979 Aug;240(2):193–202. [PubMed] [Google Scholar]
- Cuello A. C., Del Fiacco M., Paxinos G. The central and peripheral ends of the substance P-containing sensory neurones in the rat trigeminal system. Brain Res. 1978 Sep 8;152(3):499–500. doi: 10.1016/0006-8993(78)91105-8. [DOI] [PubMed] [Google Scholar]
- Engberg G., Svensson T. H., Rosell S., Folkders K. A synthetic peptide as an antagonist of substance P. Nature. 1981 Sep 17;293(5829):222–223. doi: 10.1038/293222a0. [DOI] [PubMed] [Google Scholar]
- Ennis M., Pearce F. L., Weston P. M. Some studies on the release of histamine from mast cells stimulated with polylysine. Br J Pharmacol. 1980 Oct;70(2):329–334. doi: 10.1111/j.1476-5381.1980.tb07940.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erjavec F., Lembeck F., Florjanc-Irman T., Skofitsch G., Donnerer J., Saria A., Holzer P. Release of histamine by substance P. Naunyn Schmiedebergs Arch Pharmacol. 1981 Aug;317(1):67–70. doi: 10.1007/BF00506259. [DOI] [PubMed] [Google Scholar]
- Foreman J. C., Hallett M. B., Mongar J. L. The relationship between histamine secretion and 45calcium uptake by mast cells. J Physiol. 1977 Sep;271(1):193–214. doi: 10.1113/jphysiol.1977.sp011996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foreman J. C., Lichtenstein L. M. Induction of histamine secretion by polycations. Biochim Biophys Acta. 1980 May 22;629(3):587–603. doi: 10.1016/0304-4165(80)90164-6. [DOI] [PubMed] [Google Scholar]
- Foreman J. C., Mongar J. L., Gomperts B. D. Calcium ionophores and movement of calcium ions following the physiological stimulus to a secretory process. Nature. 1973 Oct 5;245(5423):249–251. doi: 10.1038/245249a0. [DOI] [PubMed] [Google Scholar]
- Foreman J. C., Mongar J. L. The action of lanthanum and manganese on anaphylactic histamine secretion. Br J Pharmacol. 1973 Jul;48(3):527–537. doi: 10.1111/j.1476-5381.1973.tb08359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foreman J. C., Mongar J. L. The role of the alkaline earth ions in anaphylactic histamine secretion. J Physiol. 1972 Aug;224(3):753–769. doi: 10.1113/jphysiol.1972.sp009921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foreman J. C. The pharmacological control of immediate hypersensitivity. Annu Rev Pharmacol Toxicol. 1981;21:63–81. doi: 10.1146/annurev.pa.21.040181.000431. [DOI] [PubMed] [Google Scholar]
- Gater P. R., Jordan C. C., Owen D. G. Relative activities of substance P-related peptides in the guinea-pig ileum and rat parotid gland, in vitro. Br J Pharmacol. 1982 Feb;75(2):341–351. doi: 10.1111/j.1476-5381.1982.tb08792.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzer P., Saria A., Skofitsch G., Lembeck F. Increase in tissue concentrations of histamine and 5-hydroxytryptamine following capsaicin treatment of newborn rats. Life Sci. 1981 Sep 14;29(11):1099–1105. doi: 10.1016/0024-3205(81)90197-1. [DOI] [PubMed] [Google Scholar]
- Hägermark O., Hökfelt T., Pernow B. Flare and itch induced by substance P in human skin. J Invest Dermatol. 1978 Oct;71(4):233–235. doi: 10.1111/1523-1747.ep12515092. [DOI] [PubMed] [Google Scholar]
- Johnson A. R., Erdös E. G. Release of histamine from mast cells by vasoactive peptides. Proc Soc Exp Biol Med. 1973 Apr;142(4):1252–1256. doi: 10.3181/00379727-142-37219. [DOI] [PubMed] [Google Scholar]
- Kitada C., Ashida Y., Maki Y., Fujino M., Hirai Y., Yasuhara T., Nakajima T., Takeyama M., Koyama K., Yajima H. Synthesis of granuliberin-R and various fragment peptides and their histamine-releasing activities. Chem Pharm Bull (Tokyo) 1980 Mar;28(3):887–892. doi: 10.1248/cpb.28.887. [DOI] [PubMed] [Google Scholar]
- Lembeck F., Donnerer J. Postocclusive cutaneous vasodilatation mediated by substance P. Naunyn Schmiedebergs Arch Pharmacol. 1981 Apr;316(2):165–171. doi: 10.1007/BF00505312. [DOI] [PubMed] [Google Scholar]
- Lembeck F., Holzer P. Substance P as neurogenic mediator of antidromic vasodilation and neurogenic plasma extravasation. Naunyn Schmiedebergs Arch Pharmacol. 1979 Dec;310(2):175–183. doi: 10.1007/BF00500282. [DOI] [PubMed] [Google Scholar]
- Mazurek N., Pecht I., Teichberg V. I., Blumberg S. The role of the N-terminal tetrapeptide in the histamine releasing action of substance P. Neuropharmacology. 1981 Nov;20(11):1025–1027. doi: 10.1016/0028-3908(81)90091-5. [DOI] [PubMed] [Google Scholar]
- Mongar J. L., Svec P. The effect of phospholipids on anaphylactic histamine release. Br J Pharmacol. 1972 Dec;46(4):741–752. doi: 10.1111/j.1476-5381.1972.tb06899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PATON W. D. Histamine release by compounds of simple chemical structure. Pharmacol Rev. 1957 Jun;9(2):269–328. [PubMed] [Google Scholar]
- Pearce F. L., Ennis M., Truneh A., White J. R. Role of intra- and extracellular calcium in histamine release from rat peritoneal mast cells. Agents Actions. 1981 Apr;11(1-2):51–54. doi: 10.1007/BF01991455. [DOI] [PubMed] [Google Scholar]
- Perry E. K., Oakley A. E., Candy J. M., Perry R. H. Properties and possible significance of substance P and insulin fibrils. Neurosci Lett. 1981 Sep 25;25(3):321–325. doi: 10.1016/0304-3940(81)90412-2. [DOI] [PubMed] [Google Scholar]
- Peterson C. Histamine release induced by compound 48-80 from isolated rat cells: dependence on endogenous ATP. Acta Pharmacol Toxicol (Copenh) 1974 May;34(5):356–367. doi: 10.1111/j.1600-0773.1974.tb03532.x. [DOI] [PubMed] [Google Scholar]
- Rosell S., Olgart L., Gazelius B., Panopoulos P., Folkers K., Hörig J. Inhibition of antidromic and substance P-induced vasodilatation by a substance P antagonist. Acta Physiol Scand. 1981 Mar;111(3):381–382. doi: 10.1111/j.1748-1716.1981.tb06752.x. [DOI] [PubMed] [Google Scholar]
- Rossi G., Newman S. A., Metzger H. Assay and partial characterization of the solubilized cell surface receptor for immunoglobulin E. J Biol Chem. 1977 Jan 25;252(2):704–711. [PubMed] [Google Scholar]
- SHORE P. A., BURKHALTER A., COHN V. H., Jr A method for the fluorometric assay of histamine in tissues. J Pharmacol Exp Ther. 1959 Nov;127:182–186. [PubMed] [Google Scholar]
- Siraganian R. P., Kulczycki A., Jr, Mendoza G., Metzger H. Ionophore A-23187 induced histamine release from rat mast cells and rat basophil leukemia (RBL-1) cells. J Immunol. 1975 Dec;115(6):1599–1602. [PubMed] [Google Scholar]
- Stanworth D. R., Kings M., Roy P. D., Moran J. M., Moran D. M. Synthetic peptides comprising sequences of the human immunoglobulin E heavy chain capable of releasing histamine. Biochem J. 1979 Jun 15;180(3):665–668. doi: 10.1042/bj1800665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taurog J. D., Mendoza G. R., Hook W. A., Siraganian R. P., Metzger H. Noncytotoxic IgE-mediated release of histamine and serotonin from murine mastocytoma cells. J Immunol. 1977 Nov;119(5):1757–1761. [PubMed] [Google Scholar]
- UVNAS B., THON I. L. Evidence for enzymatic histamine release from isolated rat mast cells. Exp Cell Res. 1961 Feb;23:45–57. doi: 10.1016/0014-4827(61)90062-3. [DOI] [PubMed] [Google Scholar]


