Abstract
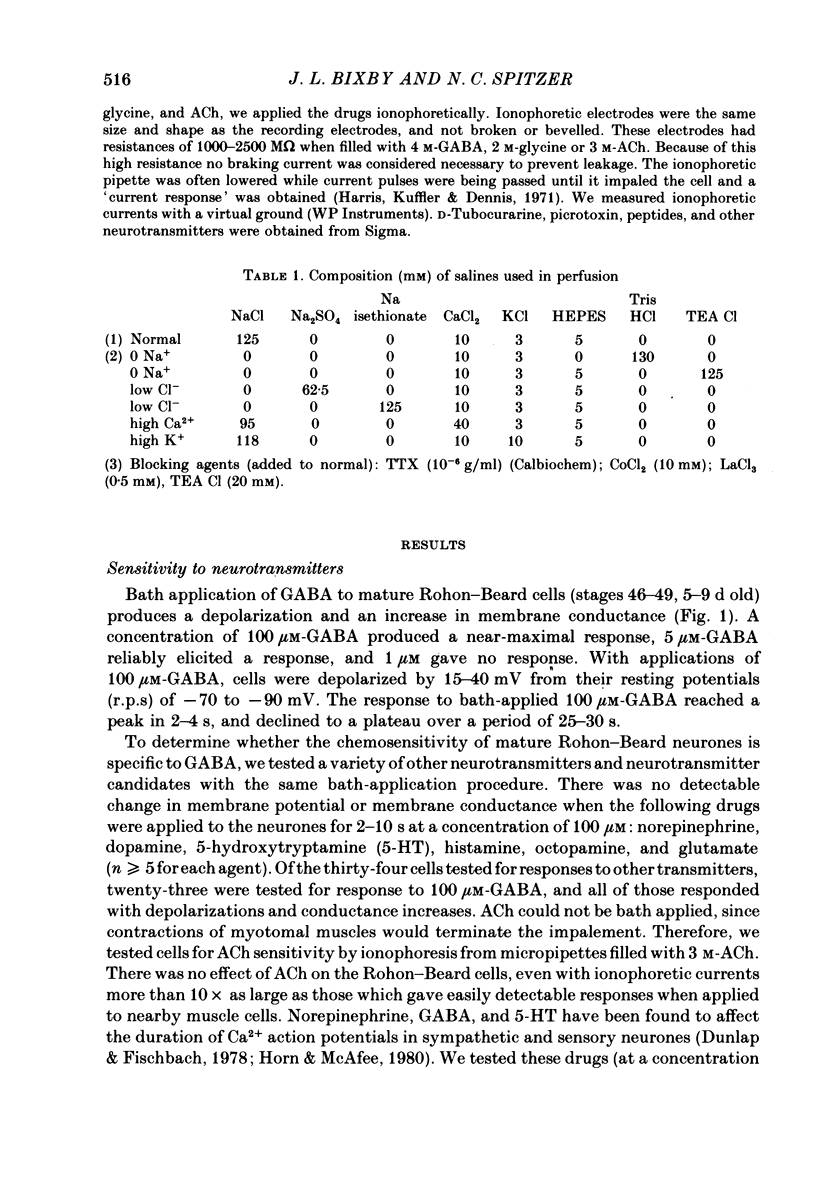
1. We have examined the onset and subsequent development of chemosensitivity in Rohon—Beard neurones from the Xenopus spinal cord. These cells become sensitive to bath-applied γ-aminobutyric acid (GABA) around stage 25 (early tailbud, about 1 d old), and remain so at least until stage 49 (9 d old). In contrast, a number of other neurotransmitter candidates tested caused no potential or conductance change during the same period.
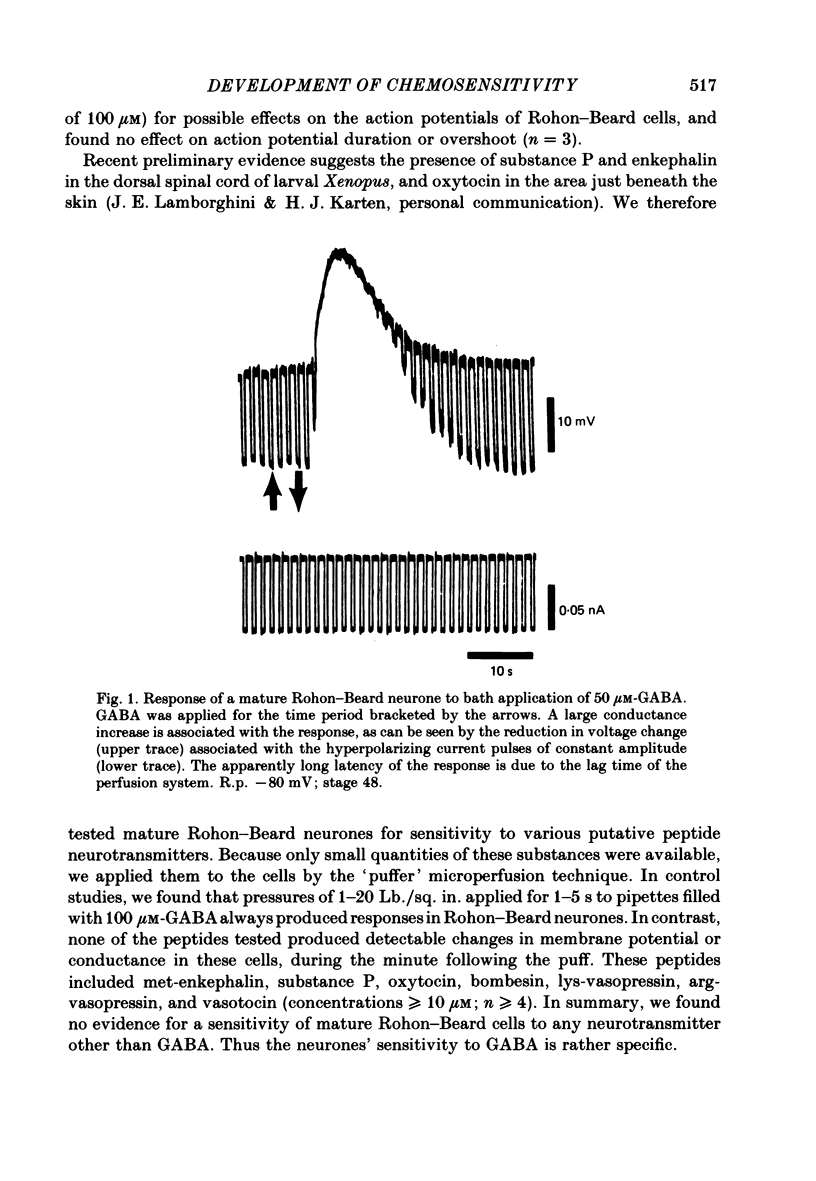
2. We examined ionophoretic dose—response relations of the cells at stage 26, a couple of hours after the first acquisition of GABA sensitivity. Sensitivities as high as 450 mV/nC were recorded. Comparable sensitivities were recorded between stages 46-49 (5-9 d old).
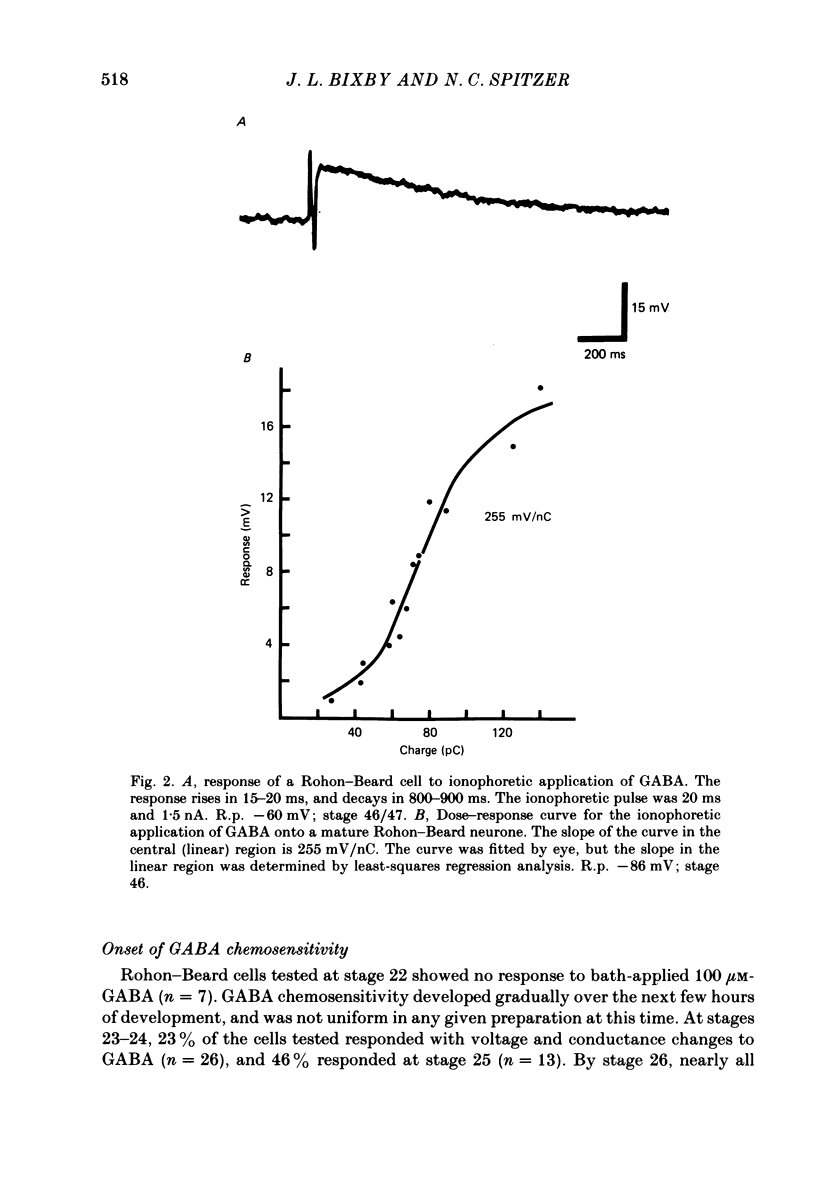
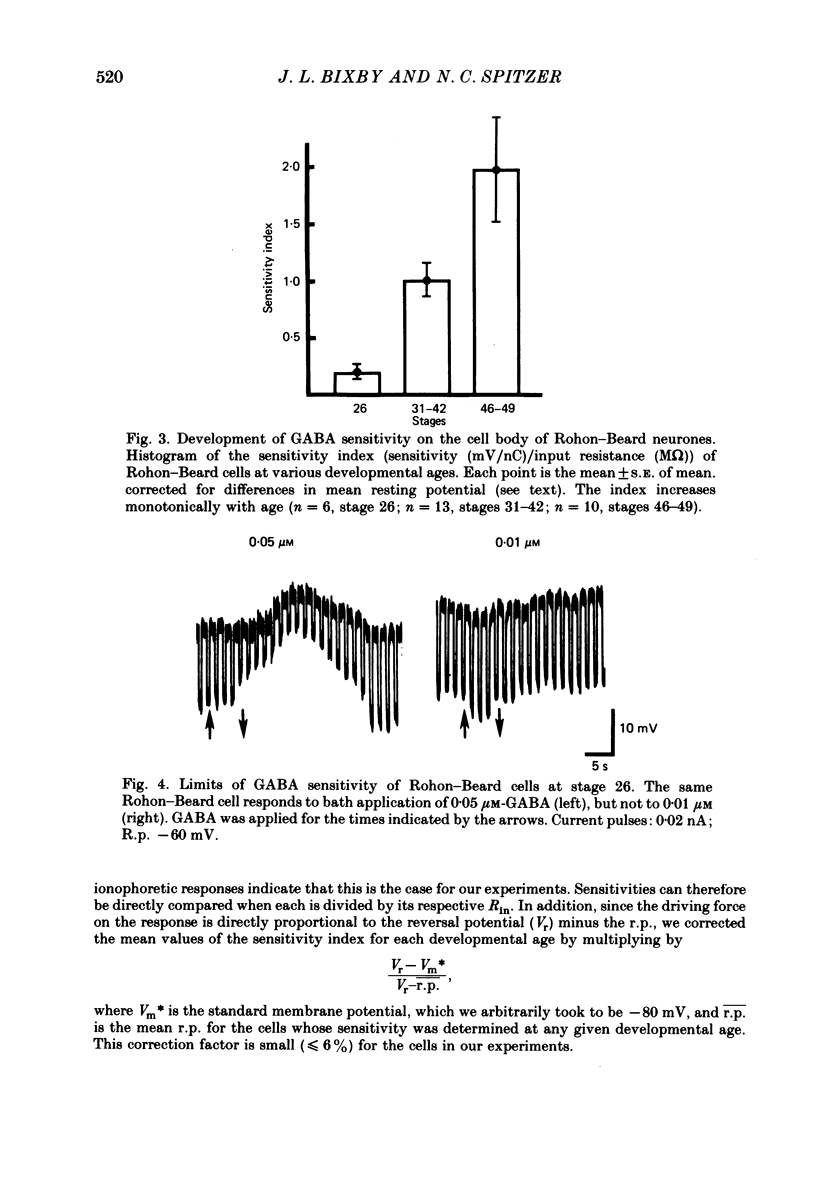
3. Measurements of ionophoretic sensitivities and input resistances during several periods from stage 26 to maturity show that the underlying conductance change for a given GABA dose is likely to increase steadily during this time. A `sensitivity index' (ionophoretic sensitivity/input resistance) was calculated, which is low at stage 26, higher at intermediate stages (stages 31-42), and highest for mature cells (stages 46-49; 5-9 d of development).
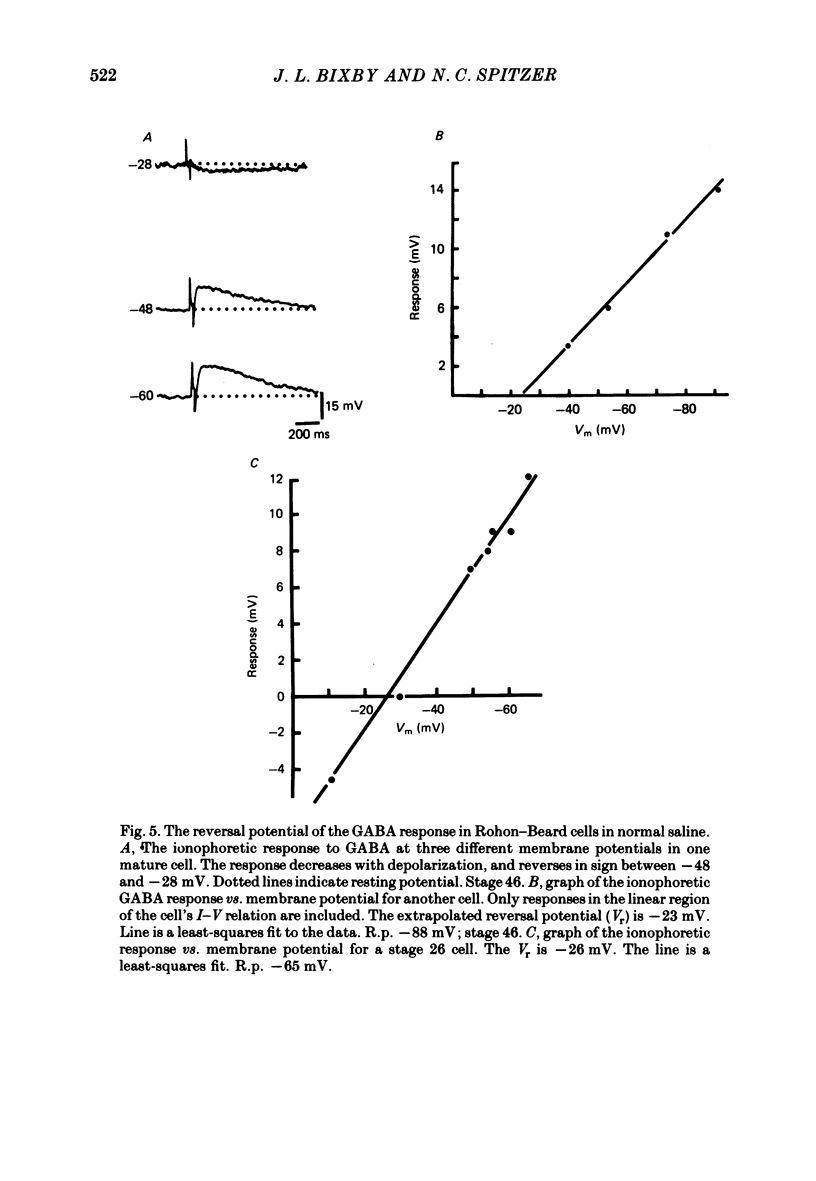
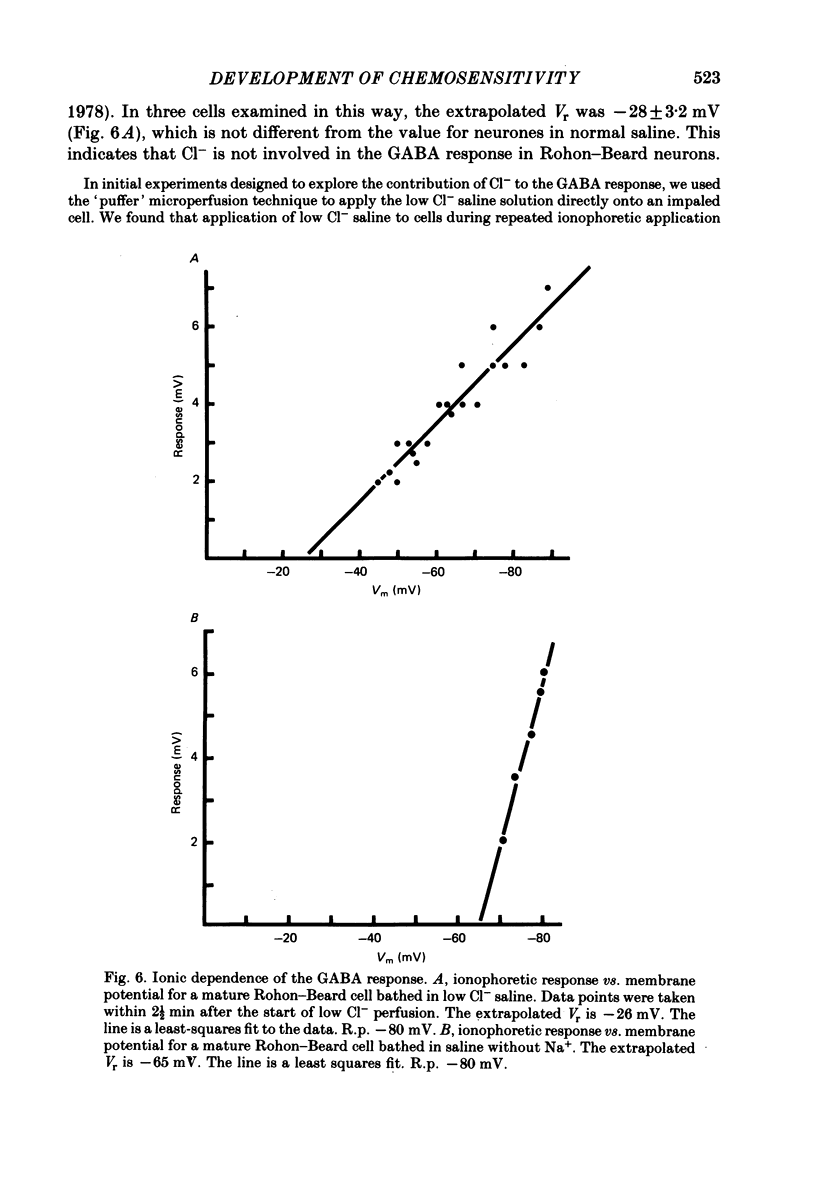
4. The reversal potential of the ionophoretic GABA response is the same at stage 26 (-30 mV) as it is in mature cells. Ion substitution experiments show that Na+ and K+, but not Cl- or Ca2+, are involved in the response.
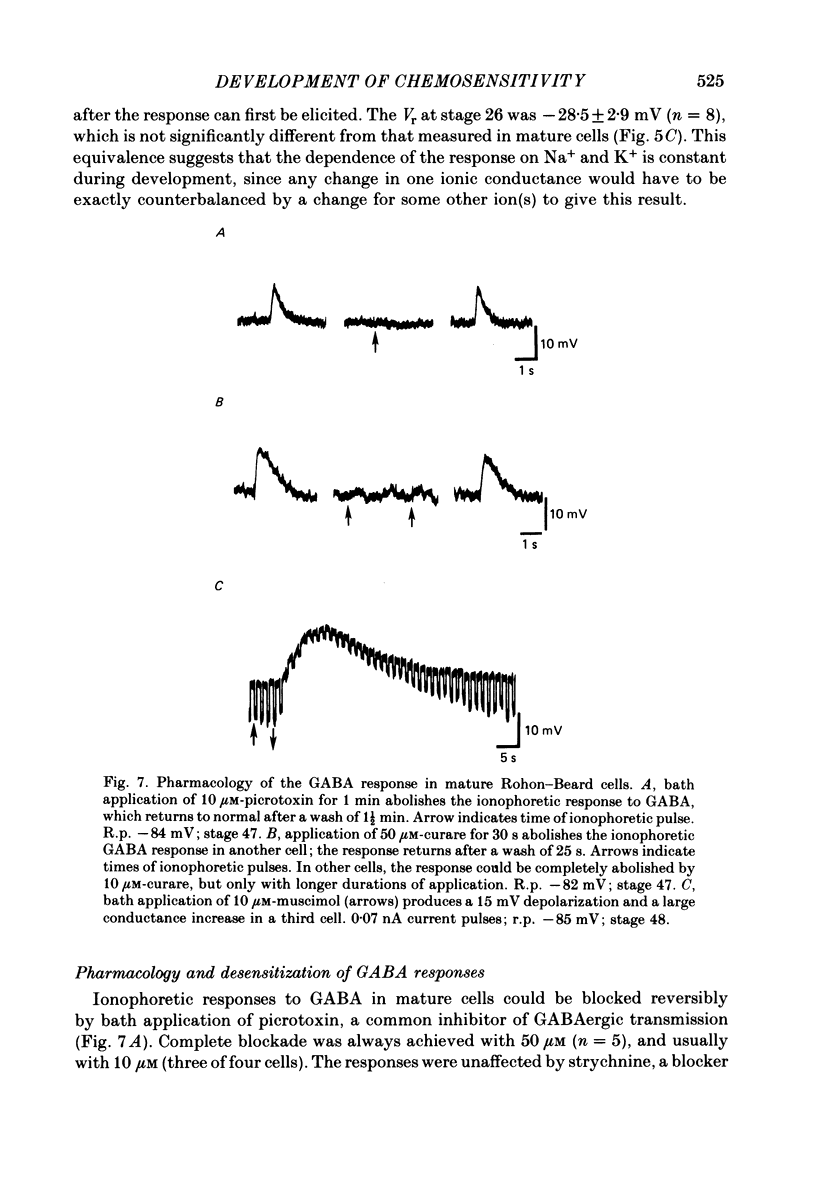
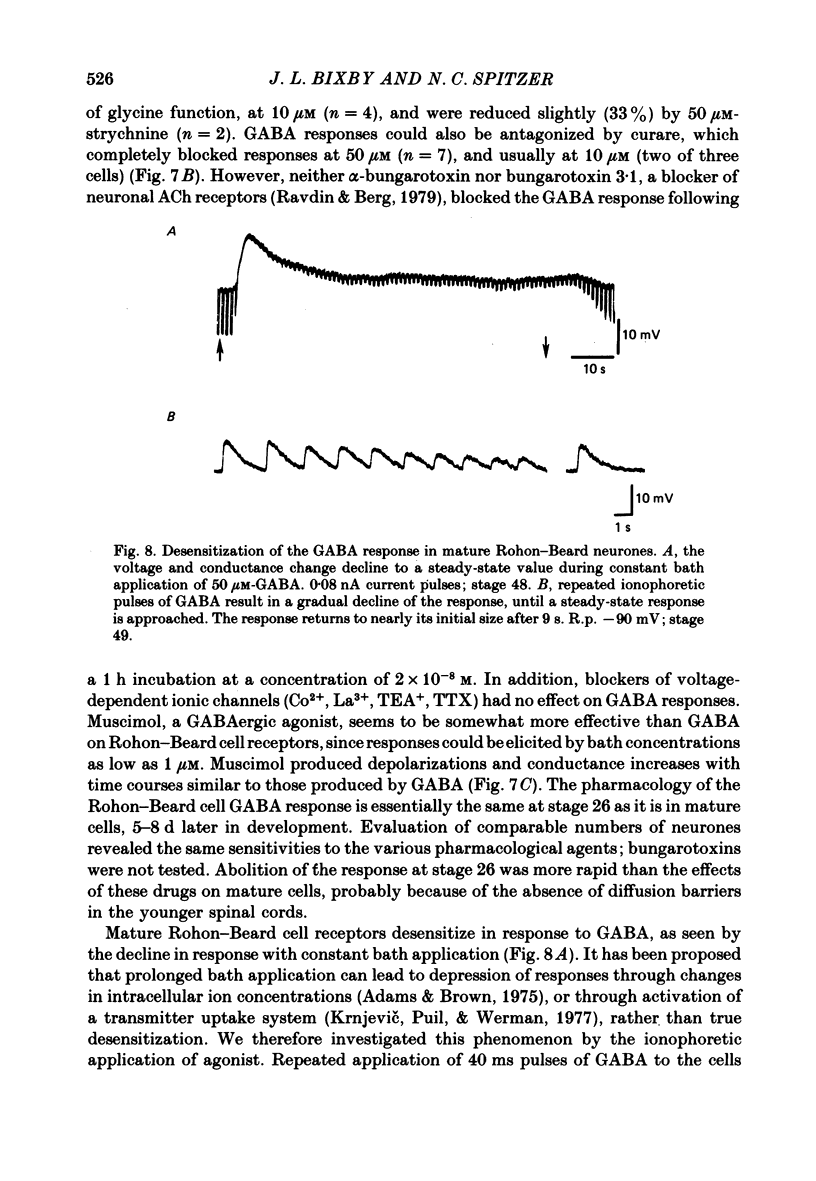
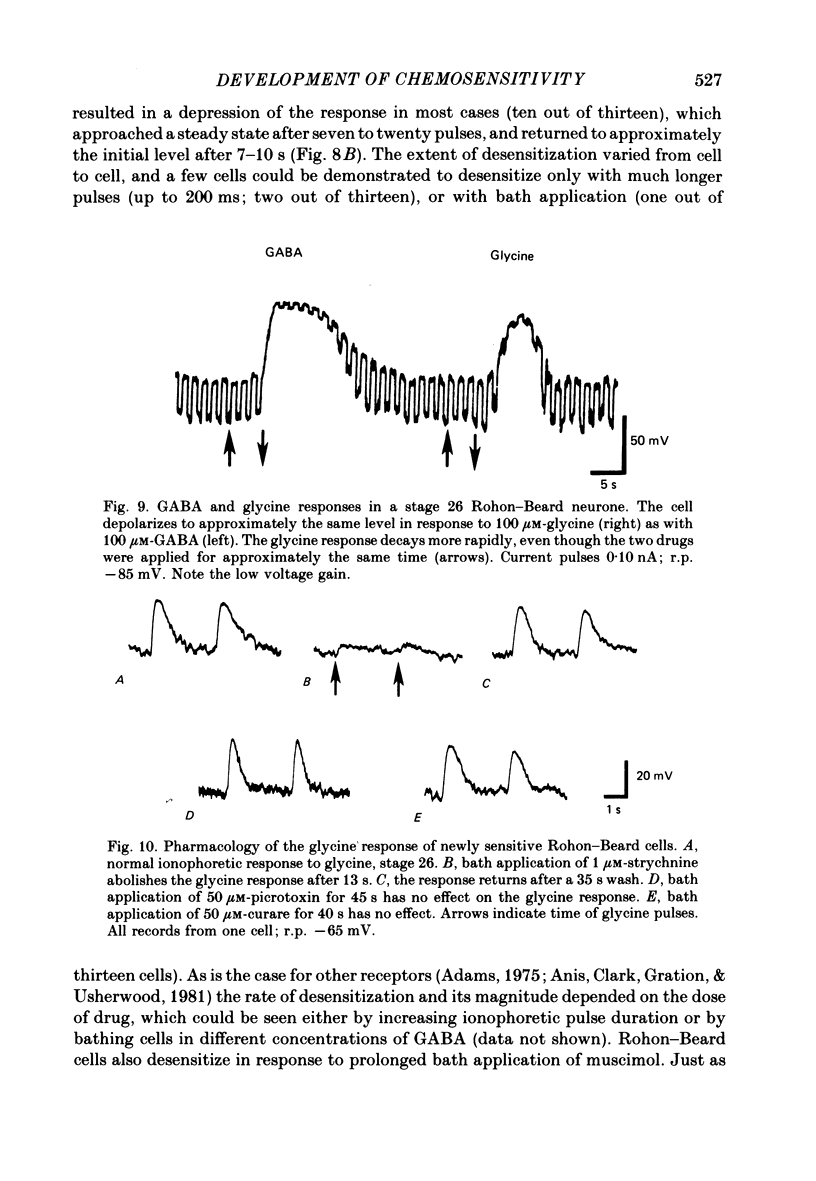
5. GABA responses at stage 26 are pharmacologically similar to those of mature cells. The responses are blocked by 10 μM-picrotoxin or curare, and muscimol is an agonist in concentrations as low as 1 μM.
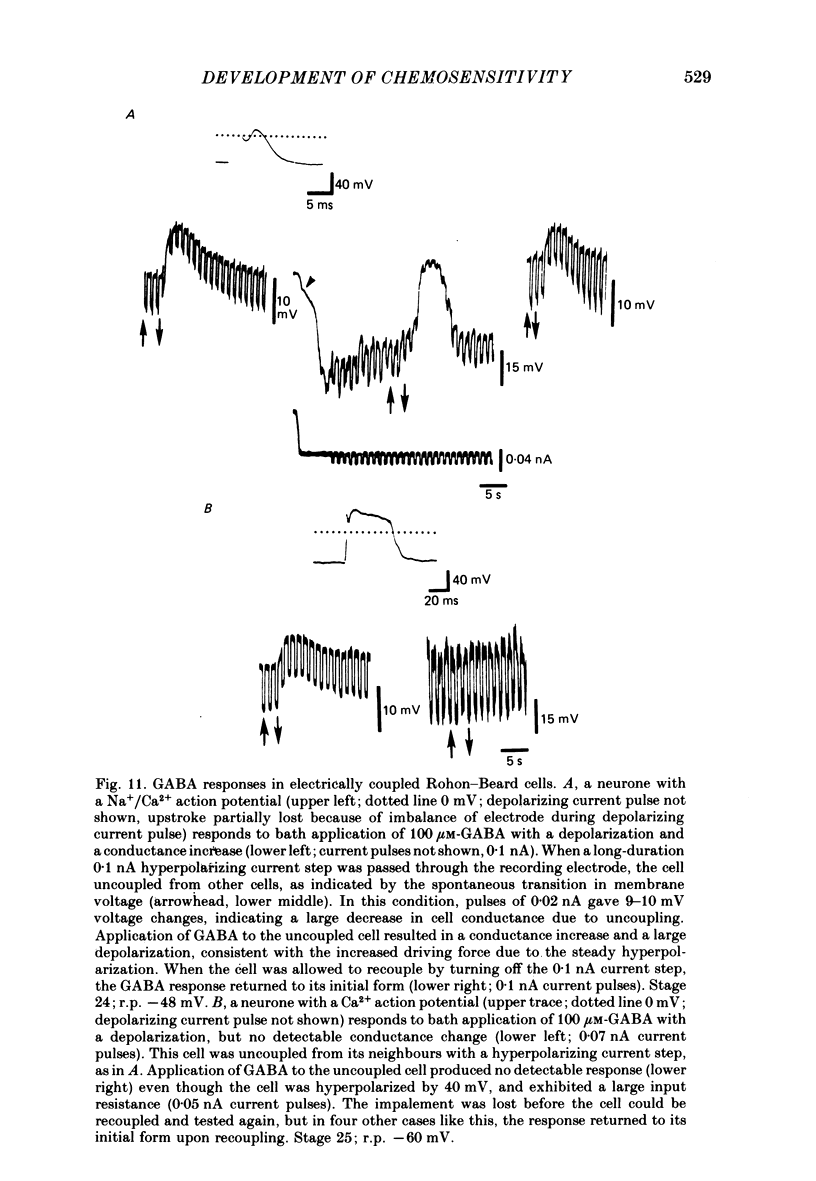
6. GABA responses at stage 26 desensitize in a manner similar to that seen for mature cells, either with prolonged bath application of GABA or with repetitive ionophoretic application.
7. Nearly half of the cells tested at stage 26 respond to glycine, in concentrations as low as 5 μM. This sensitivity is absent by 3½ d of development.
8. The responses of Rohon—Beard neurones to GABA are similar to those of other cells in that they involve a conductance increase, are mimicked by muscimol, and are blocked by picrotoxin. These responses are different in that they do not involve Cl- and are blocked by low concentrations of curare.
9. Many of the characteristics of GABA receptors, i.e. the reversal potential, desensitization, and pharmacology, are constant during development. However, the sensitivity of the cells to GABA and the spectrum of transmitters to which they are sensitive appear to change.
Full text
PDF













Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. R. A study of desensitization using voltage clamp. Pflugers Arch. 1975 Oct 28;360(2):135–144. doi: 10.1007/BF00580536. [DOI] [PubMed] [Google Scholar]
- Adams P. R., Brown D. A. Actions of gamma-aminobutyric acid on sympathetic ganglion cells. J Physiol. 1975 Aug;250(1):85–120. doi: 10.1113/jphysiol.1975.sp011044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen P., Dingledine R., Gjerstad L., Langmoen I. A., Laursen A. M. Two different responses of hippocampal pyramidal cells to application of gamma-amino butyric acid. J Physiol. 1980 Aug;305:279–296. doi: 10.1113/jphysiol.1980.sp013363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baccaglini P. I., Spitzer N. C. Developmental changes in the inward current of the action potential of Rohon-Beard neurones. J Physiol. 1977 Sep;271(1):93–117. doi: 10.1113/jphysiol.1977.sp011992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker J. L., Nicoll R. A. The pharmacology and ionic dependency of amino acid responses in the frog spinal cord. J Physiol. 1973 Jan;228(2):259–277. doi: 10.1113/jphysiol.1973.sp010085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker J. L., Ransom B. R. Amino acid pharmacology of mammalian central neurones grown in tissue culture. J Physiol. 1978 Jul;280:331–354. doi: 10.1113/jphysiol.1978.sp012387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. V., Goodenough D. A. Gap junctions, electrotonic coupling, and intercellular communication. Neurosci Res Program Bull. 1978 Sep;16(3):1–486. [PubMed] [Google Scholar]
- Bevan S., Steinbach J. H. The distribution of alpha-bungarotoxin binding sites of mammalian skeletal muscle developing in vivo. J Physiol. 1977 May;267(1):195–213. doi: 10.1113/jphysiol.1977.sp011808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackshaw S. E., Warner A. E. Low resistance junctions between mesoderm cells during development of trunk muscles. J Physiol. 1976 Feb;255(1):209–230. doi: 10.1113/jphysiol.1976.sp011276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackshaw S., Warner A. Onset of acetylcholine sensitivity and endplate activity in developing myotome muscles of Xenopus. Nature. 1976 Jul 15;262(5565):217–218. doi: 10.1038/262217a0. [DOI] [PubMed] [Google Scholar]
- Brookes N., Werman R. Discrete states of responsiveness of a locust muscle gamma-aminobutyric acid receptor: the influence of extracellular ion concentrations. Neuroscience. 1980;5(10):1669–1680. doi: 10.1016/0306-4522(80)90086-x. [DOI] [PubMed] [Google Scholar]
- Burden S. Development of the neuromuscular junction in the chick embryo: the number, distribution, and stability of acetylcholine receptors. Dev Biol. 1977 Jun;57(2):317–329. doi: 10.1016/0012-1606(77)90218-4. [DOI] [PubMed] [Google Scholar]
- Choi D. W., Fischbach G. D. GABA conductance of chick spinal cord and dorsal root ganglion neurons in cell culture. J Neurophysiol. 1981 Apr;45(4):605–620. doi: 10.1152/jn.1981.45.4.605. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Dreyer F., Sheridan R. E. The actions of tubocurarine at the frog neuromuscular junction. J Physiol. 1979 Aug;293:247–284. doi: 10.1113/jphysiol.1979.sp012888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIAMOND J., MILEDI R. A study of foetal and new-born rat muscle fibres. J Physiol. 1962 Aug;162:393–408. doi: 10.1113/jphysiol.1962.sp006941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis M. J., Ziskind-Conhaim L., Harris A. J. Development of neuromuscular junctions in rat embryos. Dev Biol. 1981 Jan 30;81(2):266–279. doi: 10.1016/0012-1606(81)90290-6. [DOI] [PubMed] [Google Scholar]
- Diamond J., Roper S. Analysis of Mauthner cell responses to iontophoretically delivered pulses of GABA, glycine and L-glutamate. J Physiol. 1973 Jul;232(1):113–128. doi: 10.1113/jphysiol.1973.sp010259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichter M. A. Physiological identification of GABA as the inhibitory transmitter for mammalian cortical neurons in cell culture. Brain Res. 1980 May 19;190(1):111–121. doi: 10.1016/0006-8993(80)91163-4. [DOI] [PubMed] [Google Scholar]
- Dunlap K., Fischbach G. D. Neurotransmitters decrease the calcium ocmponent of sensory neurone action potentials. Nature. 1978 Dec 21;276(5690):837–839. doi: 10.1038/276837a0. [DOI] [PubMed] [Google Scholar]
- Gallagher J. P., Higashi H., Nishi S. Characterization and ionic basis of GABA-induced depolarizations recorded in vitro from cat primary afferent neurones. J Physiol. 1978 Feb;275:263–282. doi: 10.1113/jphysiol.1978.sp012189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman C. S., Bate M., Spitzer N. C. Embryonic development of identified neurons: origin and transformation of the H cell. J Neurosci. 1981 Jan;1(1):94–102. doi: 10.1523/JNEUROSCI.01-01-00094.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman C. S., Spitzer N. C. Embryonic development of identified neurones: differentiation from neuroblast to neurone. Nature. 1979 Jul 19;280(5719):208–214. doi: 10.1038/280208a0. [DOI] [PubMed] [Google Scholar]
- Goodman C. S., Spitzer N. C. The development of electrical properties of identified neurones in grasshopper embryos. J Physiol. 1981;313:385–403. doi: 10.1113/jphysiol.1981.sp013672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris A. J., Kuffler S. W., Dennis M. J. Differential chemosensitivity of synaptic and extrasynaptic areas on the neuronal surface membrane in parasympathetic neurons of the frog, tested by microapplication of acetylcholine. Proc R Soc Lond B Biol Sci. 1971 Apr 27;177(1049):541–553. doi: 10.1098/rspb.1971.0046. [DOI] [PubMed] [Google Scholar]
- Horn J. P., McAfee D. A. Alpha-drenergic inhibition of calcium-dependent potentials in rat sympathetic neurones. J Physiol. 1980 Apr;301:191–204. doi: 10.1113/jphysiol.1980.sp013198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATZ B., THESLEFF S. On the factors which determine the amplitude of the miniature end-plate potential. J Physiol. 1957 Jul 11;137(2):267–278. doi: 10.1113/jphysiol.1957.sp005811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krnjević K., Puil E., Werman R. GABA and glycine actions on spinal motoneurons. Can J Physiol Pharmacol. 1977 Jun;55(3):658–669. doi: 10.1139/y77-090. [DOI] [PubMed] [Google Scholar]
- Lamborghini J. E. Rohon-beard cells and other large neurons in Xenopus embryos originate during gastrulation. J Comp Neurol. 1980 Jan 15;189(2):323–333. doi: 10.1002/cne.901890208. [DOI] [PubMed] [Google Scholar]
- Nishi S., Minota S., Karczmar A. G. Primary afferent neurones: the ionic mechanism of GABA-mediated depolarization. Neuropharmacology. 1974 Mar;13(3):215–219. doi: 10.1016/0028-3908(74)90110-5. [DOI] [PubMed] [Google Scholar]
- Ohmori H., Sasaki S. Development of neuromuscular transmission in a larval tunicate. J Physiol. 1977 Jul;269(2):221–254. doi: 10.1113/jphysiol.1977.sp011900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter D. D., Furshpan E. J., Lennox E. S. Connections between cells of the developing squid as revealed by electrophysiological methods. Proc Natl Acad Sci U S A. 1966 Feb;55(2):328–336. doi: 10.1073/pnas.55.2.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravdin P. M., Berg D. K. Inhibition of neuronal acetylcholine sensitivity by alpha-toxins from Bungarus multicinctus venom. Proc Natl Acad Sci U S A. 1979 Apr;76(4):2072–2076. doi: 10.1073/pnas.76.4.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie A. K., Fambrough D. M. Electrophysiological properties of the membrane and acetylcholine receptor in developing rat and chick myotubes. J Gen Physiol. 1975 Sep;66(3):327–355. doi: 10.1085/jgp.66.3.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer N. C. The ionic basis of the resting potential and a slow depolarizing response in Rohon-Beard neurones of Xenopus tadpoles. J Physiol. 1976 Feb;255(1):105–135. doi: 10.1113/jphysiol.1976.sp011272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer N. C. Voltage- and stage-dependent uncoupling of Rohon-Beard neurones during embryonic development of Xenopus tadpoles. J Physiol. 1982 Sep;330:145–162. doi: 10.1113/jphysiol.1982.sp014334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKEUCHI A., TAKEUCHI N. LOCALIZED ACTION OF GAMMA-AMINOBUTYRIC ACID ON THE CRAYFISH MUSCLE. J Physiol. 1965 Mar;177:225–238. doi: 10.1113/jphysiol.1965.sp007588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKEUCHI A., TAKEUCHI N. On the permeability of end-plate membrane during the action of transmitter. J Physiol. 1960 Nov;154:52–67. doi: 10.1113/jphysiol.1960.sp006564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THESLEFT S. The mode of neuromuscular block caused by acetylcholine, nicotine, decamethonium and succinylcholine. Acta Physiol Scand. 1955 Oct 27;34(2-3):218–231. doi: 10.1111/j.1748-1716.1955.tb01242.x. [DOI] [PubMed] [Google Scholar]
- Yarowsky P. J., Carpenter D. O. Receptors for gamma-aminobutyric acid (GABA) on Aplysia neurons. Brain Res. 1978 Apr 7;144(1):75–94. doi: 10.1016/0006-8993(78)90436-5. [DOI] [PubMed] [Google Scholar]
- Ziskind L., Dennis M. J. Depolarising effect of curare on embryonic rat muscles. Nature. 1978 Dec 7;276(5688):622–623. doi: 10.1038/276622a0. [DOI] [PubMed] [Google Scholar]


