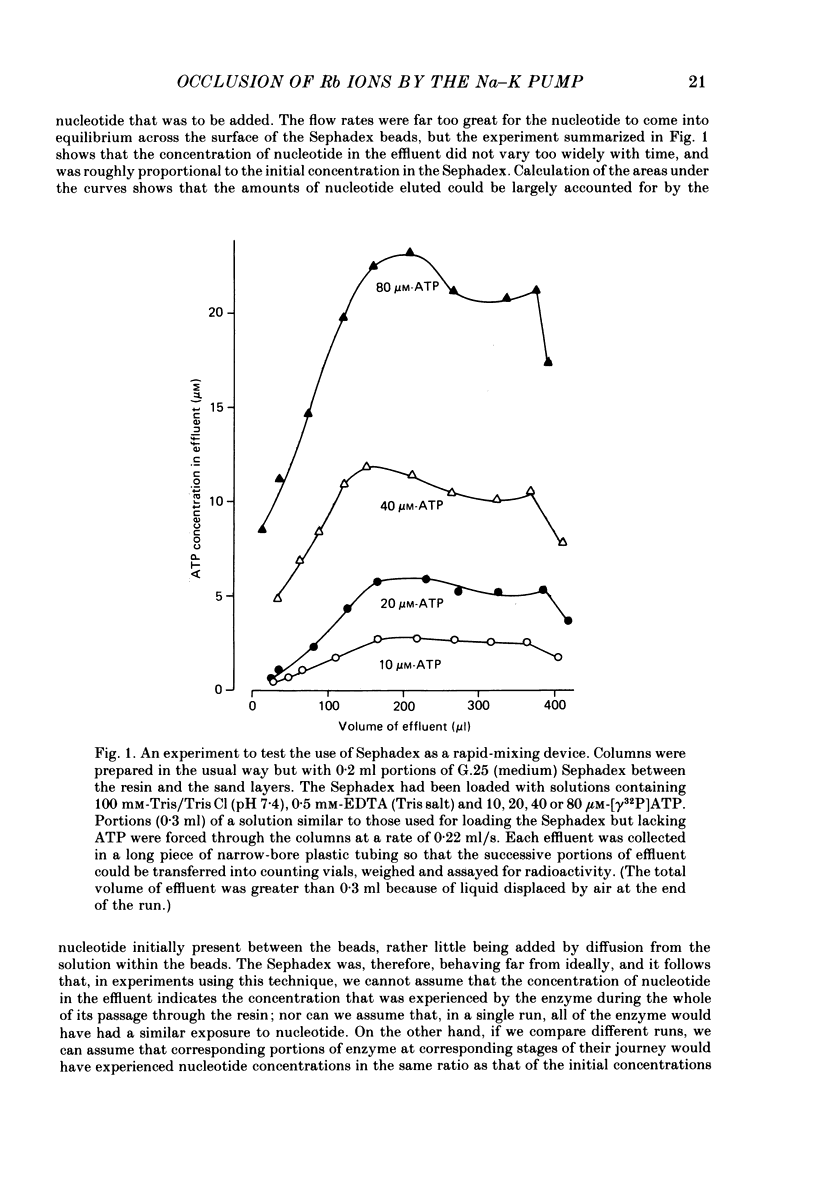
Abstract
1. The occlusion of rubidium ions by Na, K-ATPase has been investigated by suspending enzyme prepared from pig kidney outer medulla in media containing low concentrations of 86Rb, forcing the suspensions rapidly through small columns of cation-exchange resin, and measuring the amounts of radioactivity emerging from the columns.
2. When the suspension media contained 2 mM-ATP or ADP, or 15 mM-NaCl, the amounts of radioactivity emerging from the columns were greatly (and similarly) reduced, presumably because both nucleotides and sodium ions stabilized the enzyme in the E1 form. (See p. 19 for definition of E1 and E2). The extra radioactivity carried through the columns when nucleotides and sodium were absent was taken as a measure of the amount of rubidium occluded within the enzyme (in the E2 form) when it emerged from the resin.
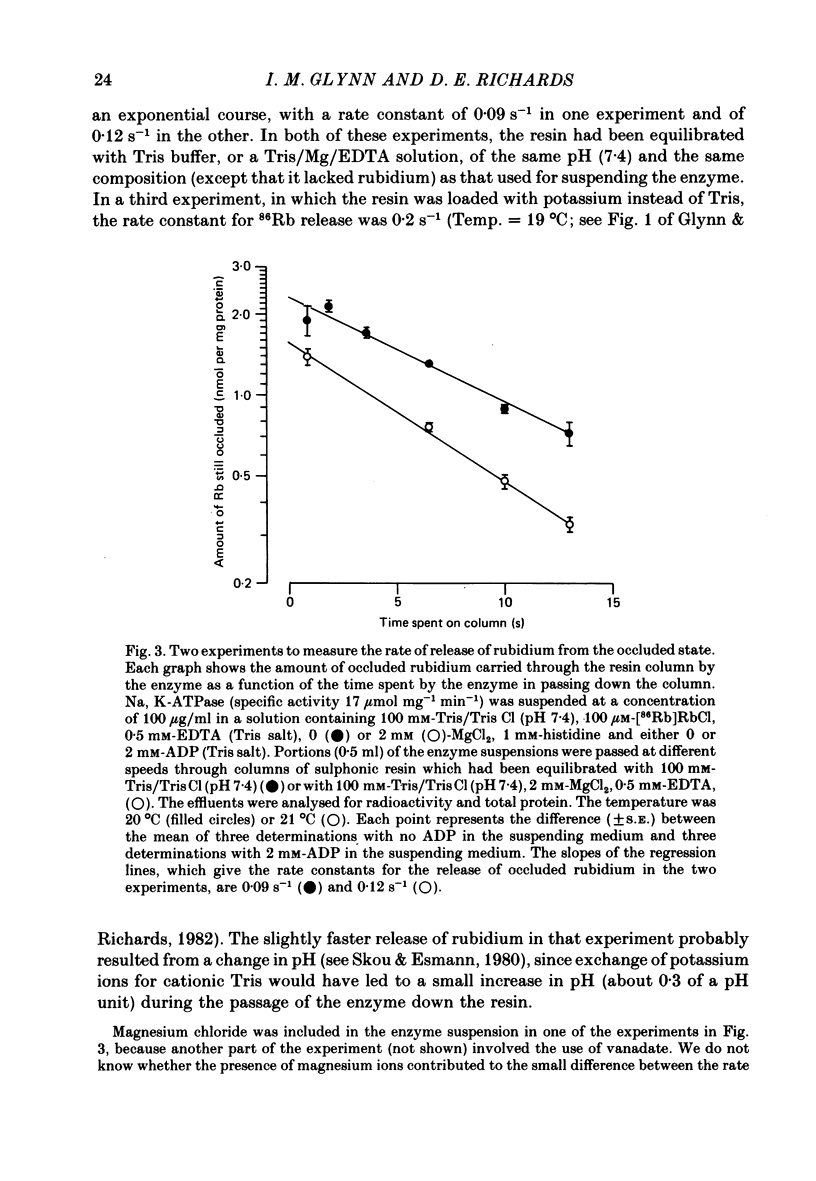
3. By varying the flow rate, and therefore the time spent by the enzyme on the resin, and relating this to the amount of radioactivity emerging from the columns, we have been able to estimate the rate constant for the conformational change (E2 → E1) that allows the occluded rubidium ions to escape. At 20 °C, and in the absence of nucleotides, it is about 0·1 S-1.
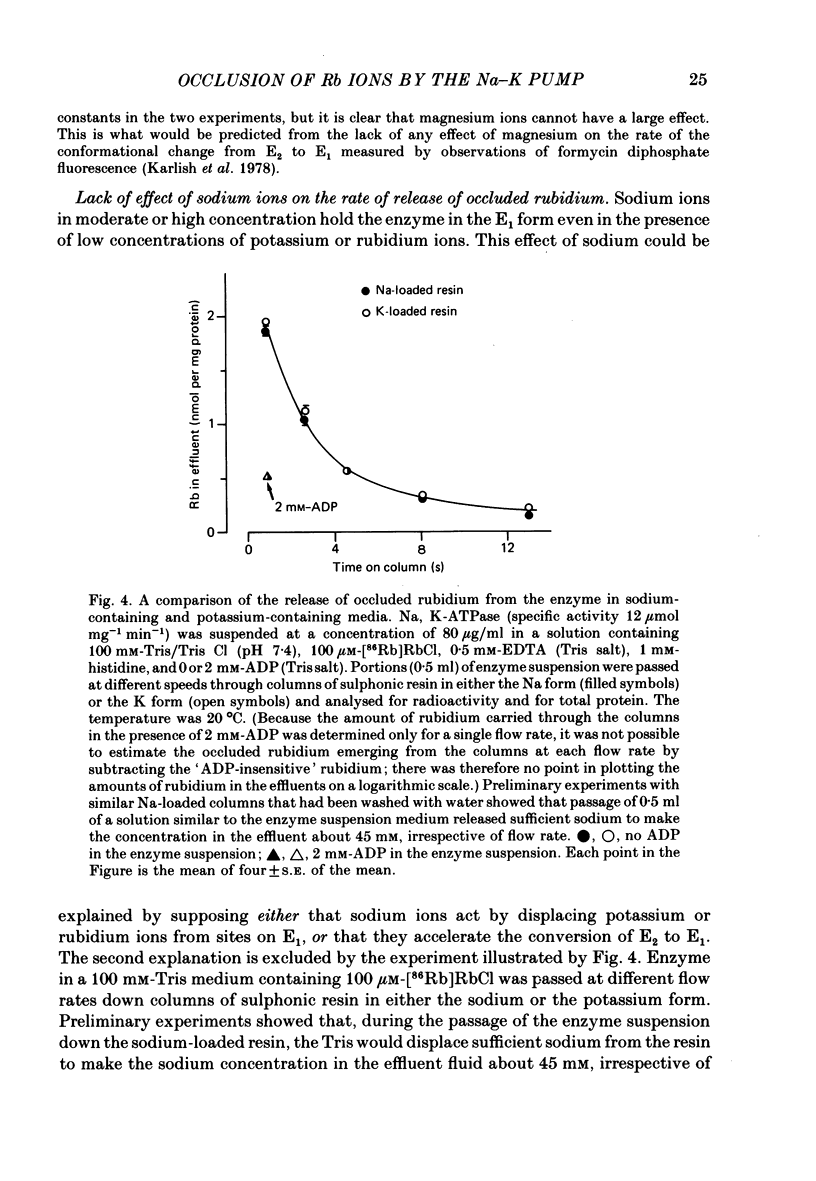
4. The rate constant for rubidium release was the same in a sodium-containing as in a potassium-containing medium. The opposite effects of sodium and potassium ions on the poise of the equilibrium between the E1 and the E2 forms of the enzyme must, therefore, be due solely to opposite effects of these ions on the rate of conversion of E1 to E2.
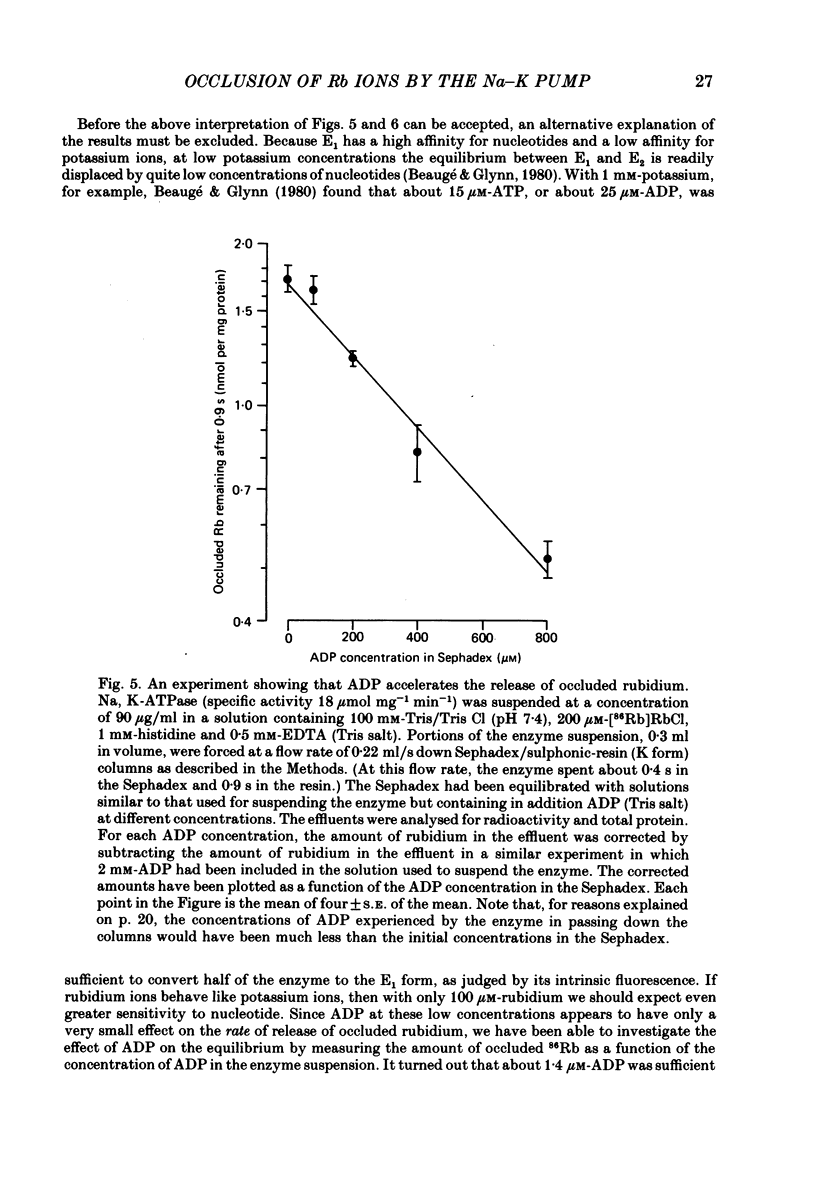
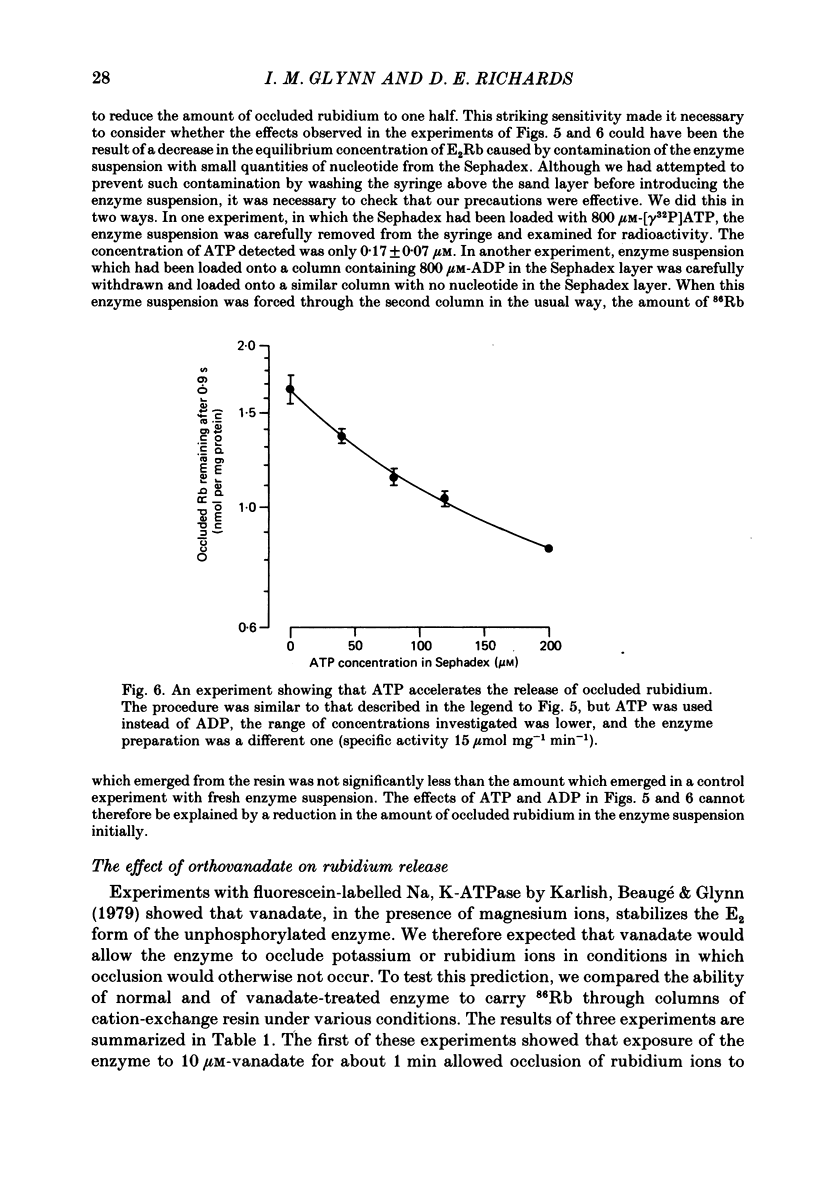
5. The rate constant for rubidium release was greatly increased by ATP and by ADP. Both nucleotides appeared to act at low-affinity sites and without phosphorylating the enzyme.
6. Orthovanadate, in the presence of magnesium ions, stabilized the enzyme in the occluded-rubidium (E2Rb) form.
7. Ouabain, in the presence of magnesium ions, prevented the occlusion of rubidium ions.
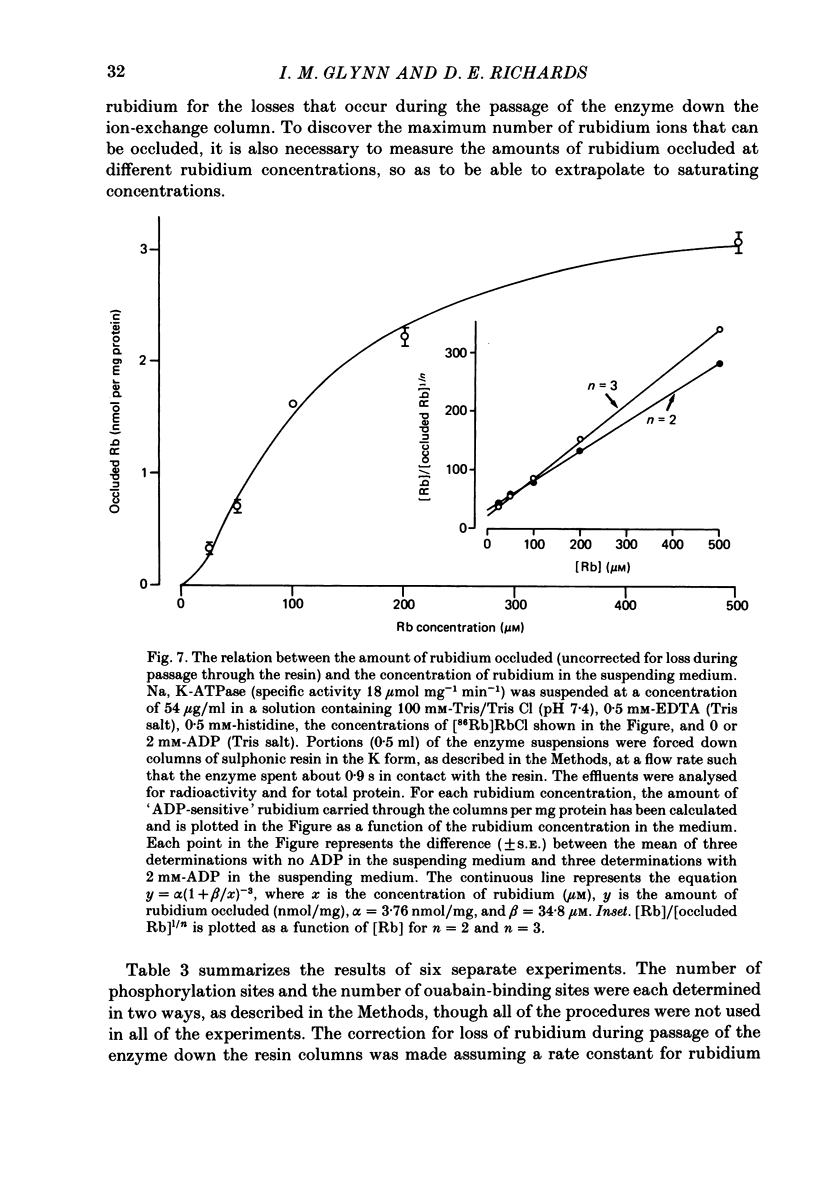
8. We have measured the amount of rubidium occluded by the enzyme as a function of rubidium concentration, and estimate that at saturating rubidium concentrations about three rubidium ions can be occluded per phosphorylation site (or per ouabain-binding site).
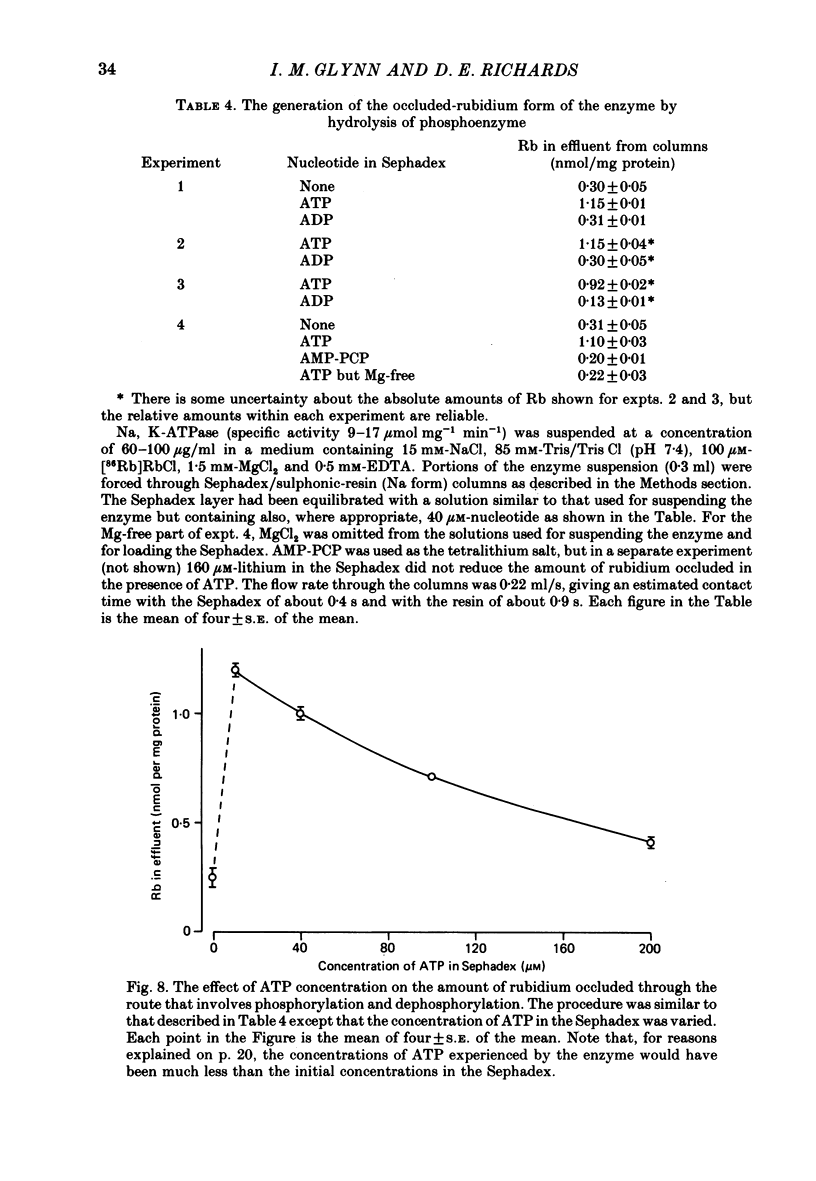
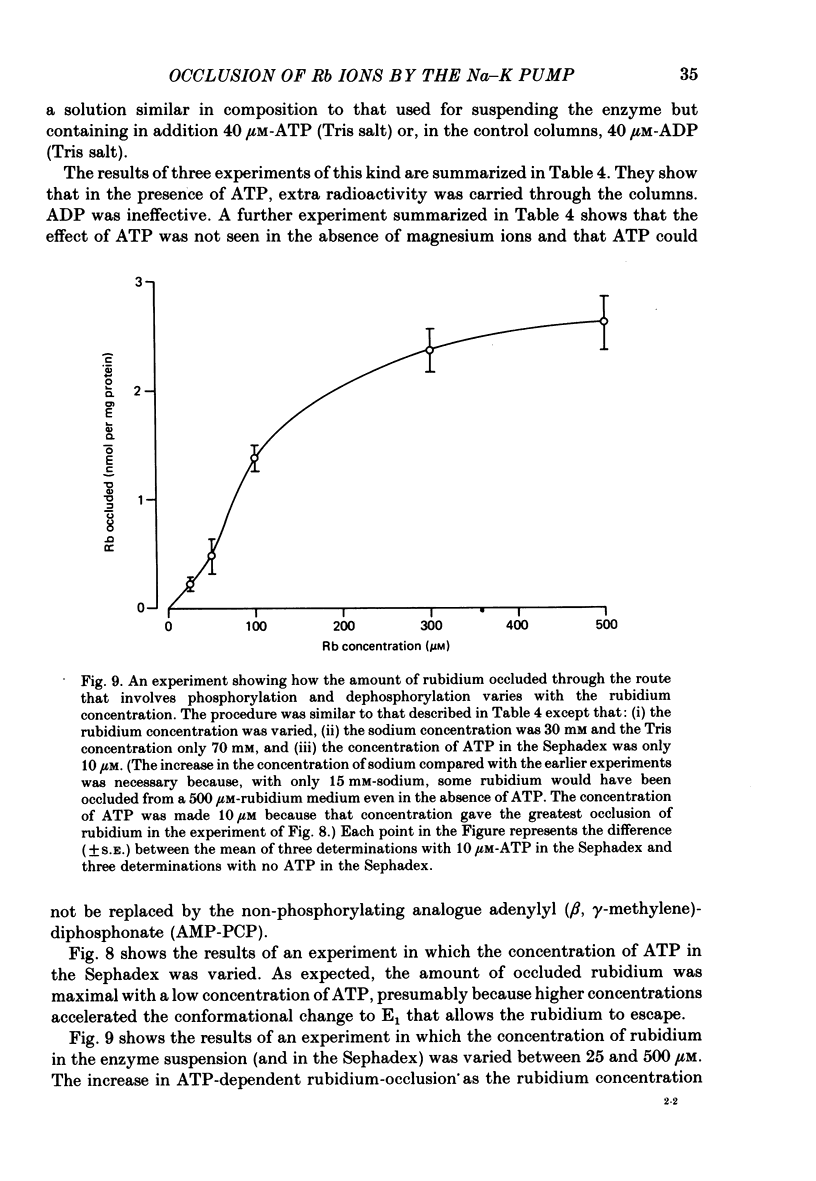
9. We have found that the occluded-rubidium form of the enzyme can also be formed by allowing rubidium ions to catalyse the hydrolysis of phosphoenzyme generated by the addition of ATP to enzyme suspended in a high-sodium medium.
10. The properties of the occluded-rubidium form of the enzyme, and of the two routes that can lead to its formation, suggest that an analagous occluded-potassium form plays a central role in the transport of potassium ions through the sodium—potassium pump. This hypothesis is supported by a detailed consideration of the probable magnitudes of the rate constants of the individual reactions making up the two routes.
Full text
PDF


















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albers R. W., Krishnan N. Application of the miniature ultracentrifuge in receptor-binding assays. Anal Biochem. 1979 Jul 15;96(2):395–402. doi: 10.1016/0003-2697(79)90598-0. [DOI] [PubMed] [Google Scholar]
- Beaugé L. A., Cavieres J. J., Glynn, Grantham J. J. The effects of vanadate on the fluxes of sodium and potassium ions through the sodium pump. J Physiol. 1980 Apr;301:7–23. doi: 10.1113/jphysiol.1980.sp013184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaugé L. A., Glynn I. M. Occlusion of K ions in the unphosphorylated sodium pump. Nature. 1979 Aug 9;280(5722):510–512. doi: 10.1038/280510a0. [DOI] [PubMed] [Google Scholar]
- Beaugé L. A., Glynn I. M. Sodium ions, acting at high-affinity extracellular sites, inhibit sodium-ATPase activity of the sodium pump by slowing dephosphorylation. J Physiol. 1979 Apr;289:17–31. doi: 10.1113/jphysiol.1979.sp012722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaugé L. A., Glynn I. M. The equilibrium between different conformations of the unphosphorylated sodium pump: effects of ATP and of potassium ions, and their relevance to potassium transport. J Physiol. 1980 Feb;299:367–383. doi: 10.1113/jphysiol.1980.sp013130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blostein R., Chu L. Sidedness of (sodium, potassium)-adenosine triphosphate of inside-out red cell membrane vesicles. Interactions with potassium. J Biol Chem. 1977 May 10;252(9):3035–3043. [PubMed] [Google Scholar]
- Cantley L. C., Jr, Cantley L. G., Josephson L. A characterization of vanadate interactions with the (Na,K)-ATPase. Mechanistic and regulatory implications. J Biol Chem. 1978 Oct 25;253(20):7361–7368. [PubMed] [Google Scholar]
- Castro J., Farley R. A. Proteolytic fragmentation of the catalytic subunit of the sodium and potassium adenosine triphosphatase. Alignment of tryptic and chymotryptic fragments and location of sites labeled with ATP and iodoacetate. J Biol Chem. 1979 Apr 10;254(7):2221–2228. [PubMed] [Google Scholar]
- Garrahan P. J., Glynn I. M. The incorporation of inorganic phosphate into adenosine triphosphate by reversal of the sodium pump. J Physiol. 1967 Sep;192(1):237–256. doi: 10.1113/jphysiol.1967.sp008298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn I. M., Lew V. L., Lüthi U. Reversal of the potassium entry mechanism in red cells, with and without reversal of the entire pump cycle. J Physiol. 1970 Apr;207(2):371–391. doi: 10.1113/jphysiol.1970.sp009067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn I. M., Lew V. L. Synthesis of adenosine triphosphate at the expense of downhill cation movements in intact human red cells. J Physiol. 1970 Apr;207(2):393–402. doi: 10.1113/jphysiol.1970.sp009068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings D., Skou J. C. Potassium binding to the (Na+ + K+)-ATPase. Biochim Biophys Acta. 1980 Sep 18;601(2):380–385. doi: 10.1016/0005-2736(80)90542-8. [DOI] [PubMed] [Google Scholar]
- Hegyvary C., Jorgensen P. L. Conformational changes of renal sodium plus potassium ion-transport adenosine triphosphatase labeled with fluorescein. J Biol Chem. 1981 Jun 25;256(12):6296–6303. [PubMed] [Google Scholar]
- Hegyvary C., Post R. L. Binding of adenosine triphosphate to sodium and potassium ion-stimulated adenosine triphosphatase. J Biol Chem. 1971 Sep 10;246(17):5234–5240. [PubMed] [Google Scholar]
- Jorgensen P. L. Purification and characterization of (Na+ plus K+ )-ATPase. 3. Purification from the outer medulla of mammalian kidney after selective removal of membrane components by sodium dodecylsulphate. Biochim Biophys Acta. 1974 Jul 12;356(1):36–52. doi: 10.1016/0005-2736(74)90292-2. [DOI] [PubMed] [Google Scholar]
- Jorgensen P. L. Purification and characterization of (Na+, K+)-ATPase. V. Conformational changes in the enzyme Transitions between the Na-form and the K-form studied with tryptic digestion as a tool. Biochim Biophys Acta. 1975 Sep 2;401(3):399–415. doi: 10.1016/0005-2736(75)90239-4. [DOI] [PubMed] [Google Scholar]
- Jørgensen P. L., Karlish S. J. Defective conformational response in a selectively trypsinized (Na+ + K+)-ATPase studied with tryptophan fluorescence. Biochim Biophys Acta. 1980 Apr 10;597(2):305–317. doi: 10.1016/0005-2736(80)90108-x. [DOI] [PubMed] [Google Scholar]
- Karlish S. J., Beaugé L. A., Glynn I. M. Vanadate inhibits (Na+ + K+)ATPase by blocking a conformational change of the unphosphorylated form. Nature. 1979 Nov 15;282(5736):333–335. doi: 10.1038/282333a0. [DOI] [PubMed] [Google Scholar]
- Karlish S. J. Characterization of conformational changes in (Na,K) ATPase labeled with fluorescein at the active site. J Bioenerg Biomembr. 1980 Aug;12(3-4):111–136. doi: 10.1007/BF00744678. [DOI] [PubMed] [Google Scholar]
- Karlish S. J., Pick U. Sidedness of the effects of sodium and potassium ions on the conformational state of the sodium-potassium pump. J Physiol. 1981 Mar;312:505–529. doi: 10.1113/jphysiol.1981.sp013641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlish S. J., Stein W. D. Passive rubidium fluxes mediated by Na-K-ATPase reconstituted into phospholipid vesicles when ATP- and phosphate-free. J Physiol. 1982 Jul;328:295–316. doi: 10.1113/jphysiol.1982.sp014265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlish S. J., Yates D. W., Glynn I. M. Conformational transitions between Na+-bound and K+-bound forms of (Na+ + K+)-ATPase, studied with formycin nucleotides. Biochim Biophys Acta. 1978 Jul 7;525(1):252–264. doi: 10.1016/0005-2744(78)90219-x. [DOI] [PubMed] [Google Scholar]
- Karlish S. J., Yates D. W. Tryptophan fluorescence of (Na+ + K+)-ATPase as a tool for study of the enzyme mechanism. Biochim Biophys Acta. 1978 Nov 10;527(1):115–130. doi: 10.1016/0005-2744(78)90261-9. [DOI] [PubMed] [Google Scholar]
- Matsui H., Hayashi Y., Homareda H., Kimimura M. Ouabain-sensitive 42K binding to Na+, K+-ATPase purified from canine kidney outer medulla. Biochem Biophys Res Commun. 1977 Mar 21;75(2):373–380. doi: 10.1016/0006-291x(77)91052-x. [DOI] [PubMed] [Google Scholar]
- Mårdh S. Bovine brain Na+,K+-stimulated ATP phosphohydrolase studied by a rapid-mixing technique. K+-stimulated liberation of [32P] orthophosphate from [32P] phosphoenzyme and resolution of the dephosphorylation into two phases. Biochim Biophys Acta. 1975 Jun 24;391(2):448–463. doi: 10.1016/0005-2744(75)90269-7. [DOI] [PubMed] [Google Scholar]
- Norby J. G., Jensen J. Binding of ATP to brain microsomal ATPase. Determination of the ATP-binding capacity and the dissociation constant of the enzyme-ATP complex as a function of K+ concentration. Biochim Biophys Acta. 1971 Mar 9;233(1):104–116. doi: 10.1016/0005-2736(71)90362-2. [DOI] [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Post R. L., Hegyvary C., Kume S. Activation by adenosine triphosphate in the phosphorylation kinetics of sodium and potassium ion transport adenosine triphosphatase. J Biol Chem. 1972 Oct 25;247(20):6530–6540. [PubMed] [Google Scholar]
- Post R. L., Toda G., Rogers F. N. Phosphorylation by inorganic phosphate of sodium plus potassium ion transport adenosine triphosphatase. Four reactive states. J Biol Chem. 1975 Jan 25;250(2):691–701. [PubMed] [Google Scholar]
- Richards D. E., Ellory J. C., Glynn I. M. Radiation inactivation of (Na+ + K+)-ATPase. A small target size for the K+-occluding mechanism. Biochim Biophys Acta. 1981 Nov 6;648(2):284–286. doi: 10.1016/0005-2736(81)90045-6. [DOI] [PubMed] [Google Scholar]
- Skou J. C., Esmann M. Effects of ATP and protons on the Na : K selectivity of the (Na+ + K+)-ATPase studied by ligand effects on intrinsic and extrinsic fluorescence. Biochim Biophys Acta. 1980 Sep 18;601(2):386–402. doi: 10.1016/0005-2736(80)90543-x. [DOI] [PubMed] [Google Scholar]
- Skou J. C., Esmann M. Eosin, a fluorescent probe of ATP binding to the (Na+ + K+)-ATPase. Biochim Biophys Acta. 1981 Oct 2;647(2):232–240. doi: 10.1016/0005-2736(81)90251-0. [DOI] [PubMed] [Google Scholar]
- Taniguchi K., Post R. L. Synthesis of adenosine triphosphate and exchange between inorganic phosphate and adenosine triphosphate in sodium and potassium ion transport adenosine triphosphatase. J Biol Chem. 1975 Apr 25;250(8):3010–3018. [PubMed] [Google Scholar]
- Yamaguchi M., Tonomura Y. Binding of monovalent cations to Na+,K+-dependent ATPase purified from porcine kidney. I. Simultaneous binding of three sodium and two potassium or rubidium ions to the enzyme. J Biochem. 1980 Nov;88(5):1365–1375. doi: 10.1093/oxfordjournals.jbchem.a133105. [DOI] [PubMed] [Google Scholar]
- Yamaguchi M., Tonomura Y. Simultaneous binding of three Na+ and two K+ ions to Na+,K+-dependent ATPase and changes in its affinities for the ions induced by the formation of a phosphorylated intermediate. J Biochem. 1979 Aug;86(2):509–523. doi: 10.1093/oxfordjournals.jbchem.a132551. [DOI] [PubMed] [Google Scholar]


