Abstract
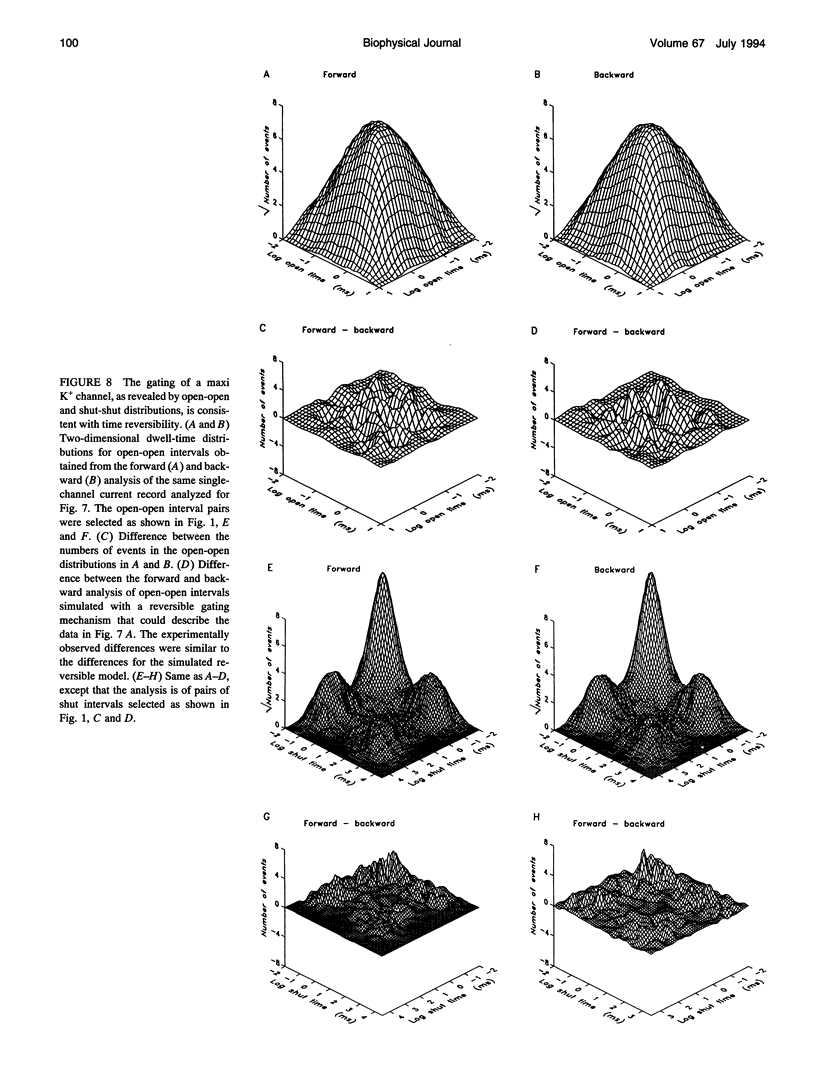
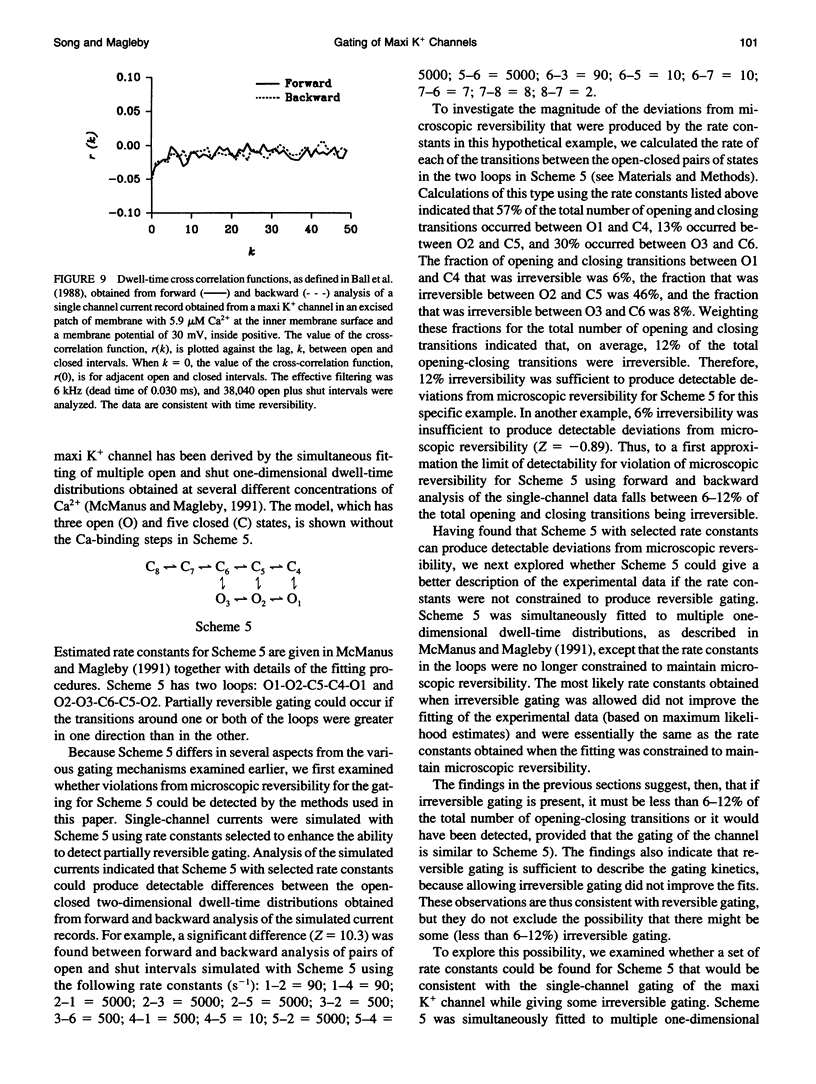
An assumption usually made when developing kinetic models for the gating of ion channels is that the transitions among the various states involved in the gating obey microscopic reversibility. If this assumption is incorrect, then the models and estimated rate constants made with the assumption would be in error. This paper examines whether the gating of a large conductance Ca-activated K+ channel in skeletal muscle is consistent with microscopic reversibility. If microscopic reversibility is obeyed, then the number of forward and backward transitions per unit time for each individual reaction step will, on average, be identical and, consequently, the gating must show time reversibility. To look for time reversibility, two-dimensional dwell-time distributions of the durations of open and closed intervals were obtained from single-channel current records analyzed in the forward and in the backward directions. Two-dimensional dwell-time distributions of pairs of open intervals and of pairs of closed intervals were also analyzed to extend the resolution of the method to special circumstances in which intervals from different closed (or open) states might have similar durations. No significant differences were observed between the forward and backward analysis of the two-dimensional dwell-time distributions, suggesting time reversibility. Thus, we find no evidence to indicate that the gating of the maxi K+ channel violates microscopic reversibility.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ball F. G., Kerry C. J., Ramsey R. L., Sansom M. S., Usherwood P. N. The use of dwell time cross-correlation functions to study single-ion channel gating kinetics. Biophys J. 1988 Aug;54(2):309–320. doi: 10.1016/S0006-3495(88)82961-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett J. N., Magleby K. L., Pallotta B. S. Properties of single calcium-activated potassium channels in cultured rat muscle. J Physiol. 1982 Oct;331:211–230. doi: 10.1113/jphysiol.1982.sp014370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatz A. L., Magleby K. L. Quantitative description of three modes of activity of fast chloride channels from rat skeletal muscle. J Physiol. 1986 Sep;378:141–174. doi: 10.1113/jphysiol.1986.sp016212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D., Hawkes A. G. A note on correlations in single ion channel records. Proc R Soc Lond B Biol Sci. 1987 Feb 23;230(1258):15–52. doi: 10.1098/rspb.1987.0008. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Hawkes A. G. On the stochastic properties of bursts of single ion channel openings and of clusters of bursts. Philos Trans R Soc Lond B Biol Sci. 1982 Dec 24;300(1098):1–59. doi: 10.1098/rstb.1982.0156. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Hawkes A. G. On the stochastic properties of single ion channels. Proc R Soc Lond B Biol Sci. 1981 Mar 6;211(1183):205–235. doi: 10.1098/rspb.1981.0003. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Hawkes A. G. Relaxation and fluctuations of membrane currents that flow through drug-operated channels. Proc R Soc Lond B Biol Sci. 1977 Nov 14;199(1135):231–262. doi: 10.1098/rspb.1977.0137. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Sakmann B. Fast events in single-channel currents activated by acetylcholine and its analogues at the frog muscle end-plate. J Physiol. 1985 Dec;369:501–557. doi: 10.1113/jphysiol.1985.sp015912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson W. B., McManus O. B., Magleby K. L. Opening and closing transitions for BK channels often occur in two steps via sojourns through a brief lifetime subconductance state. Biophys J. 1993 Aug;65(2):702–714. doi: 10.1016/S0006-3495(93)81097-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Sakmann B. Multiple conductance states of single acetylcholine receptor channels in embryonic muscle cells. Nature. 1981 Dec 3;294(5840):462–464. doi: 10.1038/294462a0. [DOI] [PubMed] [Google Scholar]
- Kerry C. J., Ramsey R. L., Sansom M. S., Usherwood P. N. Glutamate receptor channel kinetics: the effect of glutamate concentration. Biophys J. 1988 Jan;53(1):39–52. doi: 10.1016/S0006-3495(88)83064-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kijima S., Kijima H. Statistical analysis of channel current from a membrane patch. I. Some stochastic properties of ion channels or molecular systems in equilibrium. J Theor Biol. 1987 Oct 21;128(4):423–434. doi: 10.1016/s0022-5193(87)80188-1. [DOI] [PubMed] [Google Scholar]
- Kirber M. T., Singer J. J., Walsh J. V., Jr, Fuller M. S., Peura R. A. Possible forms for dwell-time histograms from single-channel current records. J Theor Biol. 1985 Sep 7;116(1):111–126. doi: 10.1016/s0022-5193(85)80133-8. [DOI] [PubMed] [Google Scholar]
- Latorre R., Oberhauser A., Labarca P., Alvarez O. Varieties of calcium-activated potassium channels. Annu Rev Physiol. 1989;51:385–399. doi: 10.1146/annurev.ph.51.030189.002125. [DOI] [PubMed] [Google Scholar]
- Magleby K. L., Weiss D. S. Estimating kinetic parameters for single channels with simulation. A general method that resolves the missed event problem and accounts for noise. Biophys J. 1990 Dec;58(6):1411–1426. doi: 10.1016/S0006-3495(90)82487-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magleby K. L., Weiss D. S. Identifying kinetic gating mechanisms for ion channels by using two-dimensional distributions of simulated dwell times. Proc Biol Sci. 1990 Sep 22;241(1302):220–228. doi: 10.1098/rspb.1990.0089. [DOI] [PubMed] [Google Scholar]
- Marty A. Ca-dependent K channels with large unitary conductance in chromaffin cell membranes. Nature. 1981 Jun 11;291(5815):497–500. doi: 10.1038/291497a0. [DOI] [PubMed] [Google Scholar]
- McManus O. B., Blatz A. L., Magleby K. L. Inverse relationship of the durations of adjacent open and shut intervals for C1 and K channels. Nature. 1985 Oct 17;317(6038):625–627. doi: 10.1038/317625a0. [DOI] [PubMed] [Google Scholar]
- McManus O. B., Blatz A. L., Magleby K. L. Sampling, log binning, fitting, and plotting durations of open and shut intervals from single channels and the effects of noise. Pflugers Arch. 1987 Nov;410(4-5):530–553. doi: 10.1007/BF00586537. [DOI] [PubMed] [Google Scholar]
- McManus O. B., Magleby K. L. Accounting for the Ca(2+)-dependent kinetics of single large-conductance Ca(2+)-activated K+ channels in rat skeletal muscle. J Physiol. 1991 Nov;443:739–777. doi: 10.1113/jphysiol.1991.sp018861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McManus O. B., Magleby K. L. Kinetic states and modes of single large-conductance calcium-activated potassium channels in cultured rat skeletal muscle. J Physiol. 1988 Aug;402:79–120. doi: 10.1113/jphysiol.1988.sp017195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McManus O. B., Magleby K. L. Kinetic time constants independent of previous single-channel activity suggest Markov gating for a large conductance Ca-activated K channel. J Gen Physiol. 1989 Dec;94(6):1037–1070. doi: 10.1085/jgp.94.6.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E., Stevens C. F. Conductance fluctuations and ionic pores in membranes. Annu Rev Biophys Bioeng. 1977;6:345–381. doi: 10.1146/annurev.bb.06.060177.002021. [DOI] [PubMed] [Google Scholar]
- Pallotta B. S., Magleby K. L., Barrett J. N. Single channel recordings of Ca2+-activated K+ currents in rat muscle cell culture. Nature. 1981 Oct 8;293(5832):471–474. doi: 10.1038/293471a0. [DOI] [PubMed] [Google Scholar]
- Patlak J. B. Sodium channel subconductance levels measured with a new variance-mean analysis. J Gen Physiol. 1988 Oct;92(4):413–430. doi: 10.1085/jgp.92.4.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rae J. L., Dewey J., Rae J. S., Cooper K. A maxi calcium-activated potassium channel from chick lens epithelium. Curr Eye Res. 1990 Sep;9(9):847–861. doi: 10.3109/02713689008999557. [DOI] [PubMed] [Google Scholar]
- Richard E. A., Miller C. Steady-state coupling of ion-channel conformations to a transmembrane ion gradient. Science. 1990 Mar 9;247(4947):1208–1210. doi: 10.1126/science.2156338. [DOI] [PubMed] [Google Scholar]
- Sigworth F. J., Sine S. M. Data transformations for improved display and fitting of single-channel dwell time histograms. Biophys J. 1987 Dec;52(6):1047–1054. doi: 10.1016/S0006-3495(87)83298-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg I. Z. Frequencies of paired open-closed durations of ion channels. Method of evaluation from single-channel recordings. Biophys J. 1987 Jul;52(1):47–55. doi: 10.1016/S0006-3495(87)83187-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg I. Z. Relationship between statistical properties of single ionic channel recordings and the thermodynamic state of the channels. J Theor Biol. 1987 Jan 7;124(1):71–87. doi: 10.1016/s0022-5193(87)80253-9. [DOI] [PubMed] [Google Scholar]
- Tyerman S. D., Terry B. R., Findlay G. P. Multiple conductances in the large K+ channel from Chara corallina shown by a transient analysis method. Biophys J. 1992 Mar;61(3):736–749. doi: 10.1016/S0006-3495(92)81878-7. [DOI] [PMC free article] [PubMed] [Google Scholar]


