Abstract
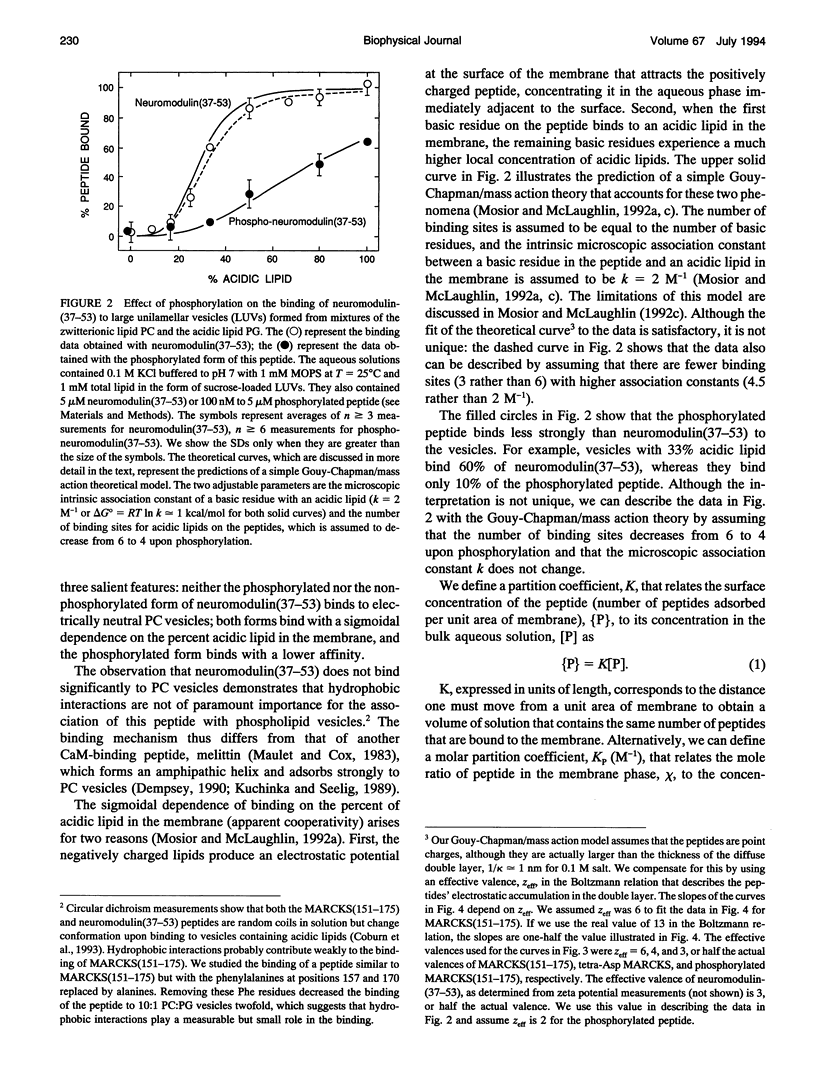
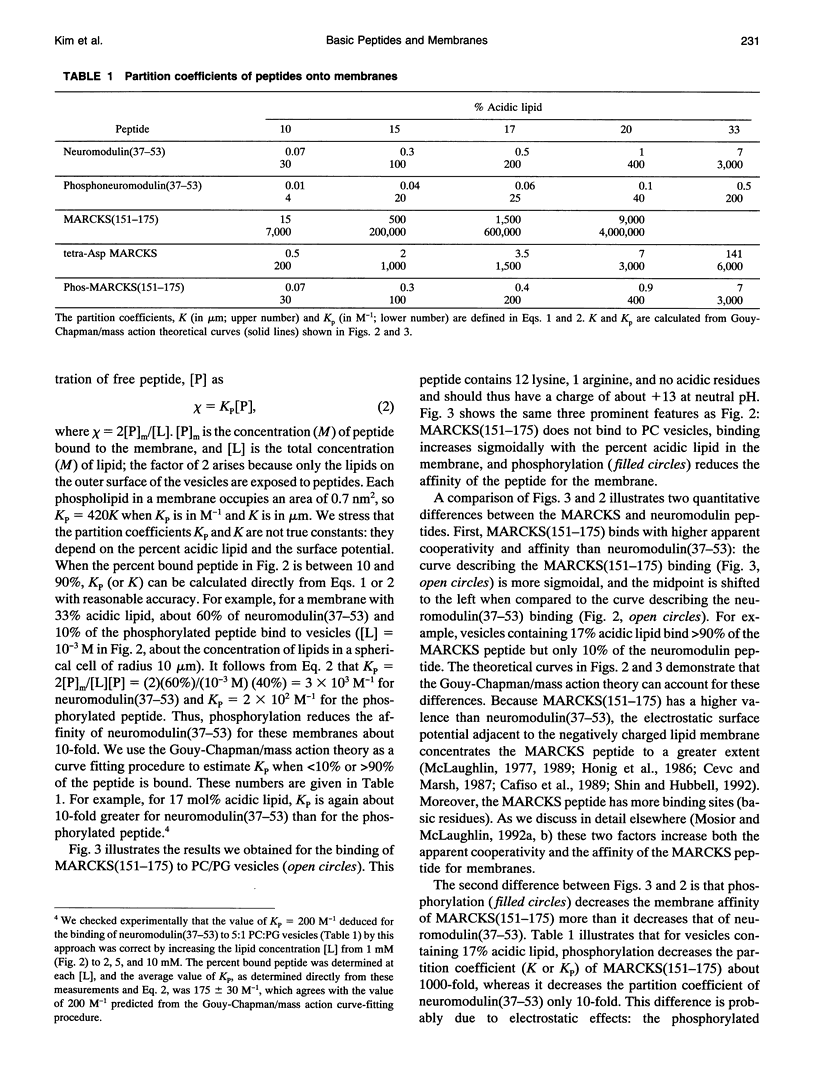
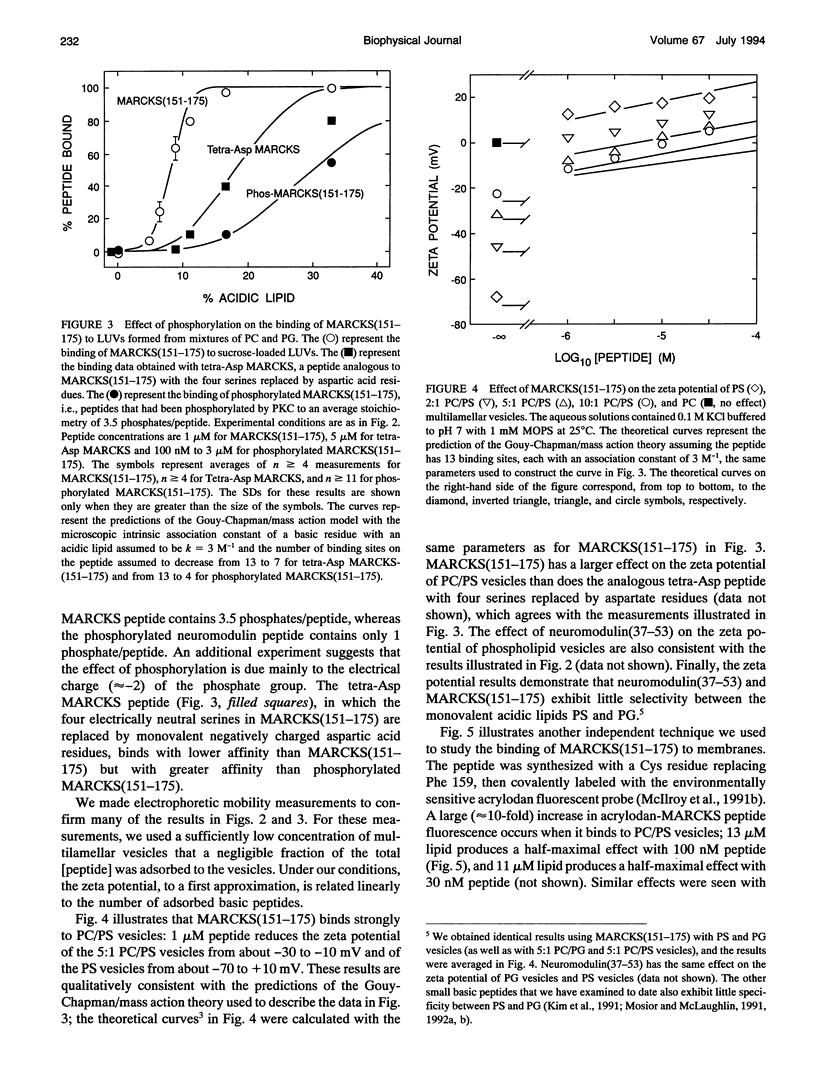
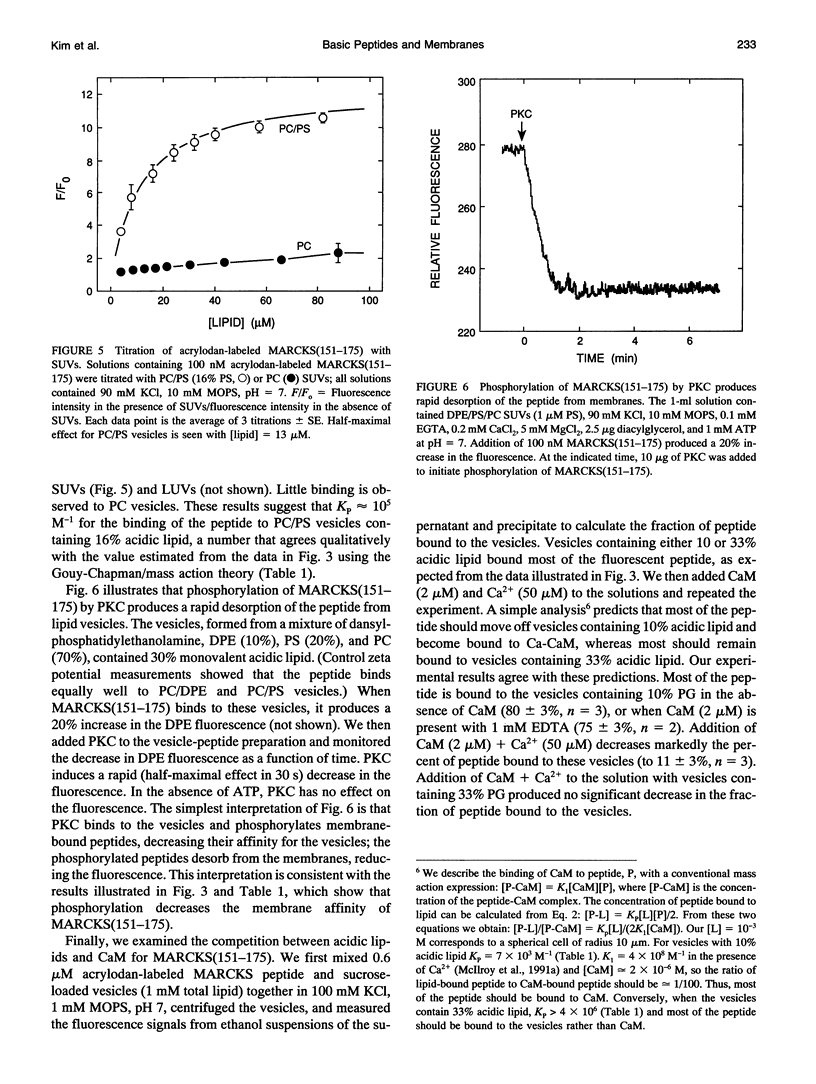
Several groups have observed that phosphorylation causes the MARCKS (Myristoylated Alanine-Rich C Kinase Substrate) protein to move off cell membranes and phospholipid vesicles. Our working hypothesis is that significant membrane binding of MARCKS requires both hydrophobic insertion of the N-terminal myristate into the bilayer and electrostatic association of the single cluster of basic residues in the protein with acidic lipids and that phosphorylation reverses this electrostatic association. Membrane binding measurements with myristoylated peptides and phospholipid vesicles show this hydrophobic moiety could, at best, barely attach proteins to plasma membranes. We report here membrane binding measurements with basic peptides that correspond to the phosphorylation domains of MARCKS and neuromodulin. Binding of these peptides increases sigmoidally with the percent acidic lipid in the phospholipid vesicle and can be described by a Gouy-Chapman/mass action theory that explains how electrostatics and reduction of dimensionality produce apparent cooperativity. The electrostatic affinity of the MARCKS peptide for membranes containing 10% acidic phospholipids (10(4) M-1 = chi/[P], where chi is the mole ratio of peptide bound to the outer monolayer of the vesicles and [P] is the concentration of peptide in the aqueous phase) is the same as the hydrophobic affinity of the myristate moiety for bilayer membranes. Phosphorylation decreases the affinity of the MARCKS peptide for membranes containing 15% acidic lipid about 1000-fold and produces a rapid (t1/2 < 30 s) dissociation of the peptide from phospholipid vesicles.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aderem A. The MARCKS brothers: a family of protein kinase C substrates. Cell. 1992 Nov 27;71(5):713–716. doi: 10.1016/0092-8674(92)90546-o. [DOI] [PubMed] [Google Scholar]
- Albert K. A., Nairn A. C., Greengard P. The 87-kDa protein, a major specific substrate for protein kinase C: purification from bovine brain and characterization. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7046–7050. doi: 10.1073/pnas.84.20.7046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander K. A., Cimler B. M., Meier K. E., Storm D. R. Regulation of calmodulin binding to P-57. A neurospecific calmodulin binding protein. J Biol Chem. 1987 May 5;262(13):6108–6113. [PubMed] [Google Scholar]
- Alexander K. A., Wakim B. T., Doyle G. S., Walsh K. A., Storm D. R. Identification and characterization of the calmodulin-binding domain of neuromodulin, a neurospecific calmodulin-binding protein. J Biol Chem. 1988 Jun 5;263(16):7544–7549. [PubMed] [Google Scholar]
- Apel E. D., Byford M. F., Au D., Walsh K. A., Storm D. R. Identification of the protein kinase C phosphorylation site in neuromodulin. Biochemistry. 1990 Mar 6;29(9):2330–2335. doi: 10.1021/bi00461a017. [DOI] [PubMed] [Google Scholar]
- Baudier J., Bronner C., Kligman D., Cole R. D. Protein kinase C substrates from bovine brain. Purification and characterization of neuromodulin, a neuron-specific calmodulin-binding protein. J Biol Chem. 1989 Jan 25;264(3):1824–1828. [PubMed] [Google Scholar]
- Bell R. M., Burns D. J. Lipid activation of protein kinase C. J Biol Chem. 1991 Mar 15;266(8):4661–4664. [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and calcium signalling. Nature. 1993 Jan 28;361(6410):315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- Blackshear P. J. The MARCKS family of cellular protein kinase C substrates. J Biol Chem. 1993 Jan 25;268(3):1501–1504. [PubMed] [Google Scholar]
- Cafiso D., McLaughlin A., McLaughlin S., Winiski A. Measuring electrostatic potentials adjacent to membranes. Methods Enzymol. 1989;171:342–364. doi: 10.1016/s0076-6879(89)71019-3. [DOI] [PubMed] [Google Scholar]
- Chakravarthy B. R., Bussey A., Whitfield J. F., Sikorska M., Williams R. E., Durkin J. P. The direct measurement of protein kinase C (PKC) activity in isolated membranes using a selective peptide substrate. Anal Biochem. 1991 Jul;196(1):144–150. doi: 10.1016/0003-2697(91)90130-l. [DOI] [PubMed] [Google Scholar]
- Chapman E. R., Au D., Alexander K. A., Nicolson T. A., Storm D. R. Characterization of the calmodulin binding domain of neuromodulin. Functional significance of serine 41 and phenylalanine 42. J Biol Chem. 1991 Jan 5;266(1):207–213. [PubMed] [Google Scholar]
- Cimler B. M., Andreasen T. J., Andreasen K. I., Storm D. R. P-57 is a neural specific calmodulin-binding protein. J Biol Chem. 1985 Sep 5;260(19):10784–10788. [PubMed] [Google Scholar]
- Coggins P. J., Zwiers H. B-50 (GAP-43): biochemistry and functional neurochemistry of a neuron-specific phosphoprotein. J Neurochem. 1991 Apr;56(4):1095–1106. doi: 10.1111/j.1471-4159.1991.tb11398.x. [DOI] [PubMed] [Google Scholar]
- Crothers D. M., Metzger H. The influence of polyvalency on the binding properties of antibodies. Immunochemistry. 1972 Mar;9(3):341–357. doi: 10.1016/0019-2791(72)90097-3. [DOI] [PubMed] [Google Scholar]
- Dempsey C. E. The actions of melittin on membranes. Biochim Biophys Acta. 1990 May 7;1031(2):143–161. doi: 10.1016/0304-4157(90)90006-x. [DOI] [PubMed] [Google Scholar]
- Epand R. M., Stafford A. R., Bottega R., Ball E. H. Studies on the mechanism of action of a bilayer stabilizing inhibitor of protein kinase C: cholesterylphosphoryldimethylethanolamine. Biosci Rep. 1989 Jun;9(3):315–328. doi: 10.1007/BF01114684. [DOI] [PubMed] [Google Scholar]
- Estep R. P., Alexander K. A., Storm D. R. Regulation of free calmodulin levels in neurons by neuromodulin: relationship to neuronal growth and regeneration. Curr Top Cell Regul. 1990;31:161–180. doi: 10.1016/b978-0-12-152831-7.50006-8. [DOI] [PubMed] [Google Scholar]
- Gawrisch K., Han K. H., Yang J. S., Bergelson L. D., Ferretti J. A. Interaction of peptide fragment 828-848 of the envelope glycoprotein of human immunodeficiency virus type I with lipid bilayers. Biochemistry. 1993 Mar 30;32(12):3112–3118. doi: 10.1021/bi00063a024. [DOI] [PubMed] [Google Scholar]
- George D. J., Blackshear P. J. Membrane association of the myristoylated alanine-rich C kinase substrate (MARCKS) protein appears to involve myristate-dependent binding in the absence of a myristoyl protein receptor. J Biol Chem. 1992 Dec 5;267(34):24879–24885. [PubMed] [Google Scholar]
- Graff J. M., Gordon J. I., Blackshear P. J. Myristoylated and nonmyristoylated forms of a protein are phosphorylated by protein kinase C. Science. 1989 Oct 27;246(4929):503–506. doi: 10.1126/science.2814478. [DOI] [PubMed] [Google Scholar]
- Graff J. M., Rajan R. R., Randall R. R., Nairn A. C., Blackshear P. J. Protein kinase C substrate and inhibitor characteristics of peptides derived from the myristoylated alanine-rich C kinase substrate (MARCKS) protein phosphorylation site domain. J Biol Chem. 1991 Aug 5;266(22):14390–14398. [PubMed] [Google Scholar]
- Graff J. M., Stumpo D. J., Blackshear P. J. Characterization of the phosphorylation sites in the chicken and bovine myristoylated alanine-rich C kinase substrate protein, a prominent cellular substrate for protein kinase C. J Biol Chem. 1989 Jul 15;264(20):11912–11919. [PubMed] [Google Scholar]
- Graff J. M., Young T. N., Johnson J. D., Blackshear P. J. Phosphorylation-regulated calmodulin binding to a prominent cellular substrate for protein kinase C. J Biol Chem. 1989 Dec 25;264(36):21818–21823. [PubMed] [Google Scholar]
- Hartwig J. H., Thelen M., Rosen A., Janmey P. A., Nairn A. C., Aderem A. MARCKS is an actin filament crosslinking protein regulated by protein kinase C and calcium-calmodulin. Nature. 1992 Apr 16;356(6370):618–622. doi: 10.1038/356618a0. [DOI] [PubMed] [Google Scholar]
- Honig B. H., Hubbell W. L., Flewelling R. F. Electrostatic interactions in membranes and proteins. Annu Rev Biophys Biophys Chem. 1986;15:163–193. doi: 10.1146/annurev.bb.15.060186.001115. [DOI] [PubMed] [Google Scholar]
- Houbre D., Duportail G., Deloulme J. C., Baudier J. The interactions of the brain-specific calmodulin-binding protein kinase C substrate, neuromodulin (GAP 43), with membrane phospholipids. J Biol Chem. 1991 Apr 15;266(11):7121–7131. [PubMed] [Google Scholar]
- James G., Olson E. N. Myristoylation, phosphorylation, and subcellular distribution of the 80-kDa protein kinase C substrate in BC3H1 myocytes. J Biol Chem. 1989 Dec 15;264(35):20928–20933. [PubMed] [Google Scholar]
- Kaibuchi K., Takai Y., Nishizuka Y. Cooperative roles of various membrane phospholipids in the activation of calcium-activated, phospholipid-dependent protein kinase. J Biol Chem. 1981 Jul 25;256(14):7146–7149. [PubMed] [Google Scholar]
- Kim J., Mosior M., Chung L. A., Wu H., McLaughlin S. Binding of peptides with basic residues to membranes containing acidic phospholipids. Biophys J. 1991 Jul;60(1):135–148. doi: 10.1016/S0006-3495(91)82037-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klee C. B., Vanaman T. C. Calmodulin. Adv Protein Chem. 1982;35:213–321. doi: 10.1016/s0065-3233(08)60470-2. [DOI] [PubMed] [Google Scholar]
- Kuchinka E., Seelig J. Interaction of melittin with phosphatidylcholine membranes. Binding isotherm and lipid head-group conformation. Biochemistry. 1989 May 16;28(10):4216–4221. doi: 10.1021/bi00436a014. [DOI] [PubMed] [Google Scholar]
- LaBate M. E., Skene J. H. Selective conservation of GAP-43 structure in vertebrate evolution. Neuron. 1989 Sep;3(3):299–310. doi: 10.1016/0896-6273(89)90254-7. [DOI] [PubMed] [Google Scholar]
- Liu Y. C., Storm D. R. Dephosphorylation of neuromodulin by calcineurin. J Biol Chem. 1989 Aug 5;264(22):12800–12804. [PubMed] [Google Scholar]
- Liu Y. C., Storm D. R. Regulation of free calmodulin levels by neuromodulin: neuron growth and regeneration. Trends Pharmacol Sci. 1990 Mar;11(3):107–111. doi: 10.1016/0165-6147(90)90195-e. [DOI] [PubMed] [Google Scholar]
- MacNicol M., Schulman H. Cross-talk between protein kinase C and multifunctional Ca2+/calmodulin-dependent protein kinase. J Biol Chem. 1992 Jun 15;267(17):12197–12201. [PubMed] [Google Scholar]
- Mangels L. A., Gnegy M. E. Carbachol stimulates binding of a photoreactive calmodulin derivative to calmodulin-binding proteins in intact SK-N-SH human neuroblastoma cells. J Biol Chem. 1992 Mar 25;267(9):5847–5854. [PubMed] [Google Scholar]
- Maulet Y., Cox J. A. Structural changes in melittin and calmodulin upon complex formation and their modulation by calcium. Biochemistry. 1983 Nov 22;22(24):5680–5686. doi: 10.1021/bi00293a035. [DOI] [PubMed] [Google Scholar]
- McIlroy B. K., Walters J. D., Blackshear P. J., Johnson J. D. Phosphorylation-dependent binding of a synthetic MARCKS peptide to calmodulin. J Biol Chem. 1991 Mar 15;266(8):4959–4964. [PubMed] [Google Scholar]
- McIlroy B. K., Walters J. D., Johnson J. D. A continuous fluorescence assay for protein kinase C. Anal Biochem. 1991 May 15;195(1):148–152. doi: 10.1016/0003-2697(91)90310-p. [DOI] [PubMed] [Google Scholar]
- McLaughlin S. The electrostatic properties of membranes. Annu Rev Biophys Biophys Chem. 1989;18:113–136. doi: 10.1146/annurev.bb.18.060189.000553. [DOI] [PubMed] [Google Scholar]
- Montich G., Scarlata S., McLaughlin S., Lehrmann R., Seelig J. Thermodynamic characterization of the association of small basic peptides with membranes containing acidic lipids. Biochim Biophys Acta. 1993 Feb 23;1146(1):17–24. doi: 10.1016/0005-2736(93)90333-u. [DOI] [PubMed] [Google Scholar]
- Mosior M., Epand R. M. Mechanism of activation of protein kinase C: roles of diolein and phosphatidylserine. Biochemistry. 1993 Jan 12;32(1):66–75. doi: 10.1021/bi00052a010. [DOI] [PubMed] [Google Scholar]
- Mosior M., McLaughlin S. Binding of basic peptides to acidic lipids in membranes: effects of inserting alanine(s) between the basic residues. Biochemistry. 1992 Feb 18;31(6):1767–1773. doi: 10.1021/bi00121a026. [DOI] [PubMed] [Google Scholar]
- Mosior M., McLaughlin S. Electrostatics and reduction of dimensionality produce apparent cooperativity when basic peptides bind to acidic lipids in membranes. Biochim Biophys Acta. 1992 Mar 23;1105(1):185–187. doi: 10.1016/0005-2736(92)90178-o. [DOI] [PubMed] [Google Scholar]
- Mosior M., McLaughlin S. Peptides that mimic the pseudosubstrate region of protein kinase C bind to acidic lipids in membranes. Biophys J. 1991 Jul;60(1):149–159. doi: 10.1016/S0006-3495(91)82038-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton A. C. Interaction of proteins with lipid headgroups: lessons from protein kinase C. Annu Rev Biophys Biomol Struct. 1993;22:1–25. doi: 10.1146/annurev.bb.22.060193.000245. [DOI] [PubMed] [Google Scholar]
- Newton A. C., Koshland D. E., Jr High cooperativity, specificity, and multiplicity in the protein kinase C-lipid interaction. J Biol Chem. 1989 Sep 5;264(25):14909–14915. [PubMed] [Google Scholar]
- Nielander H. B., Schrama L. H., van Rozen A. J., Kasperaitis M., Oestreicher A. B., Gispen W. H., Schotman P. Mutation of serine 41 in the neuron-specific protein B-50 (GAP-43) prohibits phosphorylation by protein kinase C. J Neurochem. 1990 Oct;55(4):1442–1445. doi: 10.1111/j.1471-4159.1990.tb03159.x. [DOI] [PubMed] [Google Scholar]
- Op den Kamp J. A. Lipid asymmetry in membranes. Annu Rev Biochem. 1979;48:47–71. doi: 10.1146/annurev.bi.48.070179.000403. [DOI] [PubMed] [Google Scholar]
- Peitzsch R. M., McLaughlin S. Binding of acylated peptides and fatty acids to phospholipid vesicles: pertinence to myristoylated proteins. Biochemistry. 1993 Oct 5;32(39):10436–10443. doi: 10.1021/bi00090a020. [DOI] [PubMed] [Google Scholar]
- Rebecchi M., Peterson A., McLaughlin S. Phosphoinositide-specific phospholipase C-delta 1 binds with high affinity to phospholipid vesicles containing phosphatidylinositol 4,5-bisphosphate. Biochemistry. 1992 Dec 29;31(51):12742–12747. doi: 10.1021/bi00166a005. [DOI] [PubMed] [Google Scholar]
- Sawai T., Negishi M., Nishigaki N., Ohno T., Ichikawa A. Enhancement by protein kinase C of prostacyclin receptor-mediated activation of adenylate cyclase through a calmodulin/myristoylated alanine-rich C kinase substrate (MARCKS) system in IC2 mast cells. J Biol Chem. 1993 Jan 25;268(3):1995–2000. [PubMed] [Google Scholar]
- Shin Y. K., Hubbell W. L. Determination of electrostatic potentials at biological interfaces using electron-electron double resonance. Biophys J. 1992 Jun;61(6):1443–1453. doi: 10.1016/S0006-3495(92)81950-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skene J. H. Axonal growth-associated proteins. Annu Rev Neurosci. 1989;12:127–156. doi: 10.1146/annurev.ne.12.030189.001015. [DOI] [PubMed] [Google Scholar]
- Skene J. H. GAP-43 as a 'calmodulin sponge' and some implications for calcium signalling in axon terminals. Neurosci Res Suppl. 1990;13:S112–S125. doi: 10.1016/0921-8696(90)90040-a. [DOI] [PubMed] [Google Scholar]
- Skene J. H., Virág I. Posttranslational membrane attachment and dynamic fatty acylation of a neuronal growth cone protein, GAP-43. J Cell Biol. 1989 Feb;108(2):613–624. doi: 10.1083/jcb.108.2.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumpo D. J., Graff J. M., Albert K. A., Greengard P., Blackshear P. J. Molecular cloning, characterization, and expression of a cDNA encoding the "80- to 87-kDa" myristoylated alanine-rich C kinase substrate: a major cellular substrate for protein kinase C. Proc Natl Acad Sci U S A. 1989 Jun;86(11):4012–4016. doi: 10.1073/pnas.86.11.4012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi H., Manenti S. Interaction of myristoylated alanine-rich protein kinase C substrate (MARCKS) with membrane phospholipids. J Biol Chem. 1993 May 15;268(14):9960–9963. [PubMed] [Google Scholar]
- Thelen M., Rosen A., Nairn A. C., Aderem A. Regulation by phosphorylation of reversible association of a myristoylated protein kinase C substrate with the plasma membrane. Nature. 1991 May 23;351(6324):320–322. doi: 10.1038/351320a0. [DOI] [PubMed] [Google Scholar]
- Wakim B. T., Alexander K. A., Masure H. R., Cimler B. M., Storm D. R., Walsh K. A. Amino acid sequence of P-57, a neurospecific calmodulin-binding protein. Biochemistry. 1987 Nov 17;26(23):7466–7470. doi: 10.1021/bi00397a040. [DOI] [PubMed] [Google Scholar]
- Wang J. K., Walaas S. I., Sihra T. S., Aderem A., Greengard P. Phosphorylation and associated translocation of the 87-kDa protein, a major protein kinase C substrate, in isolated nerve terminals. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2253–2256. doi: 10.1073/pnas.86.7.2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kruijff B., Rietveld A., Telders N., Vaandrager B. Molecular aspects of the bilayer stabilization induced by poly(L-lysines) of varying size in cardiolipin liposomes. Biochim Biophys Acta. 1985 Nov 7;820(2):295–304. doi: 10.1016/0005-2736(85)90124-5. [DOI] [PubMed] [Google Scholar]


