Abstract
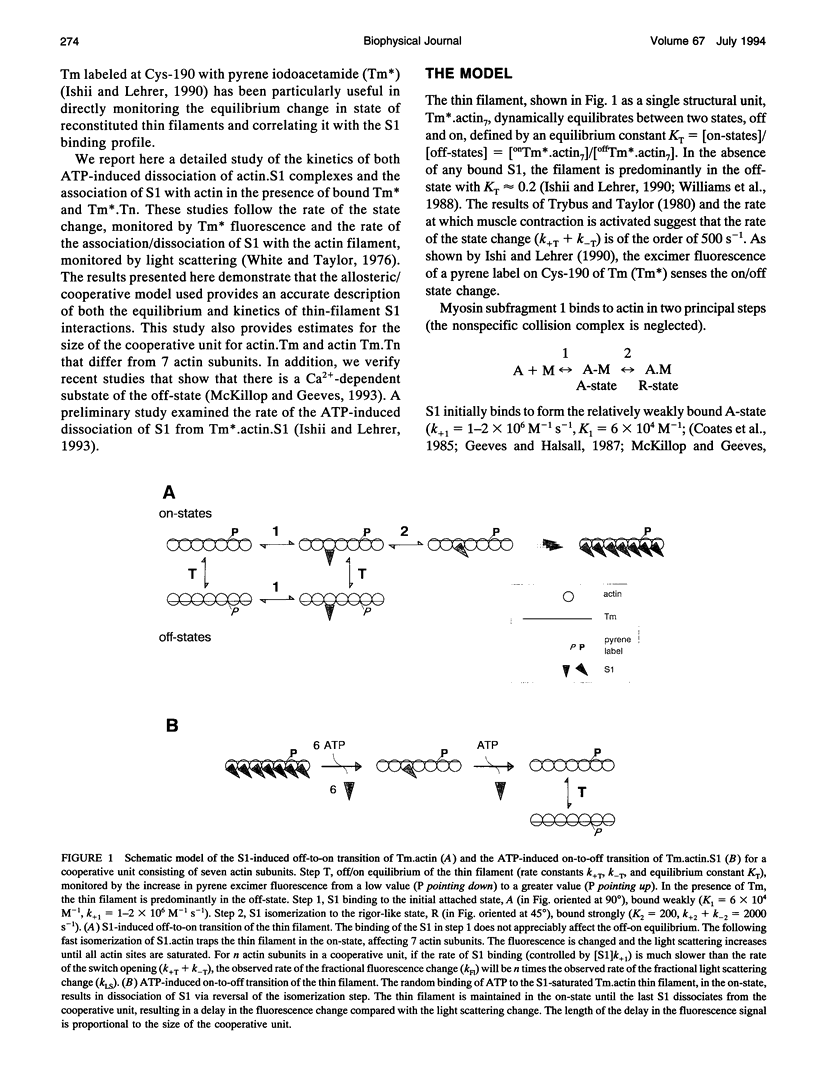
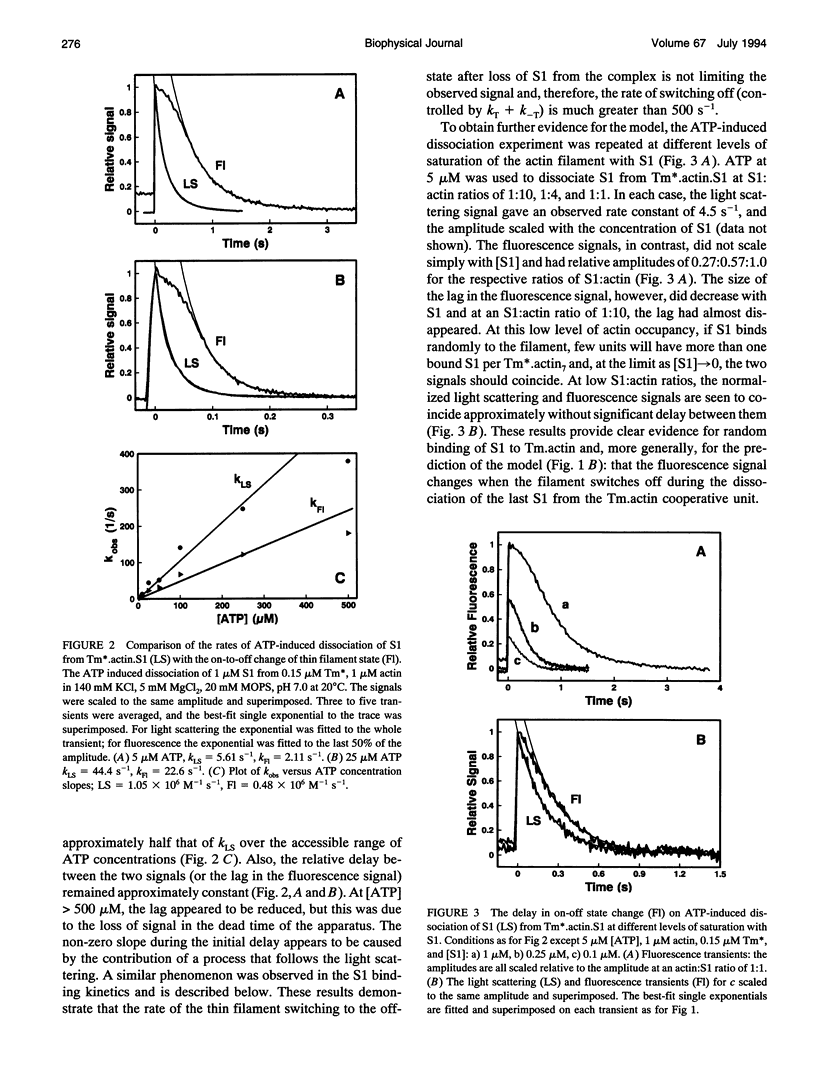
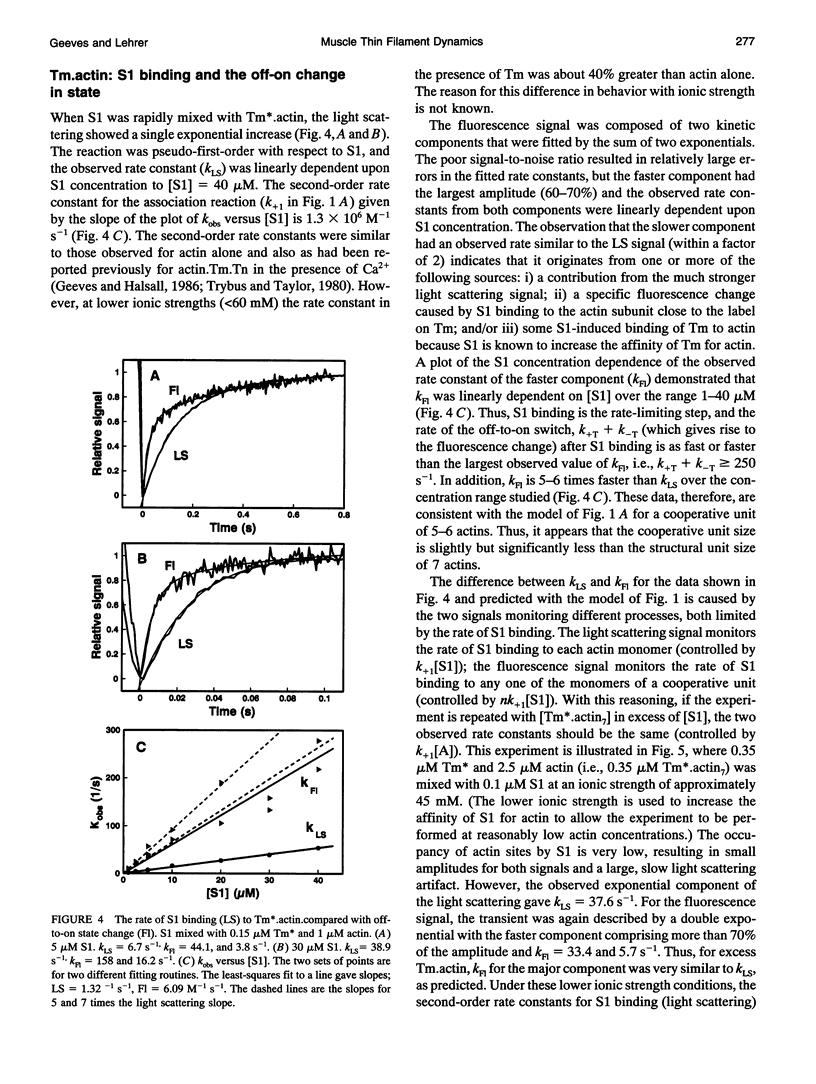
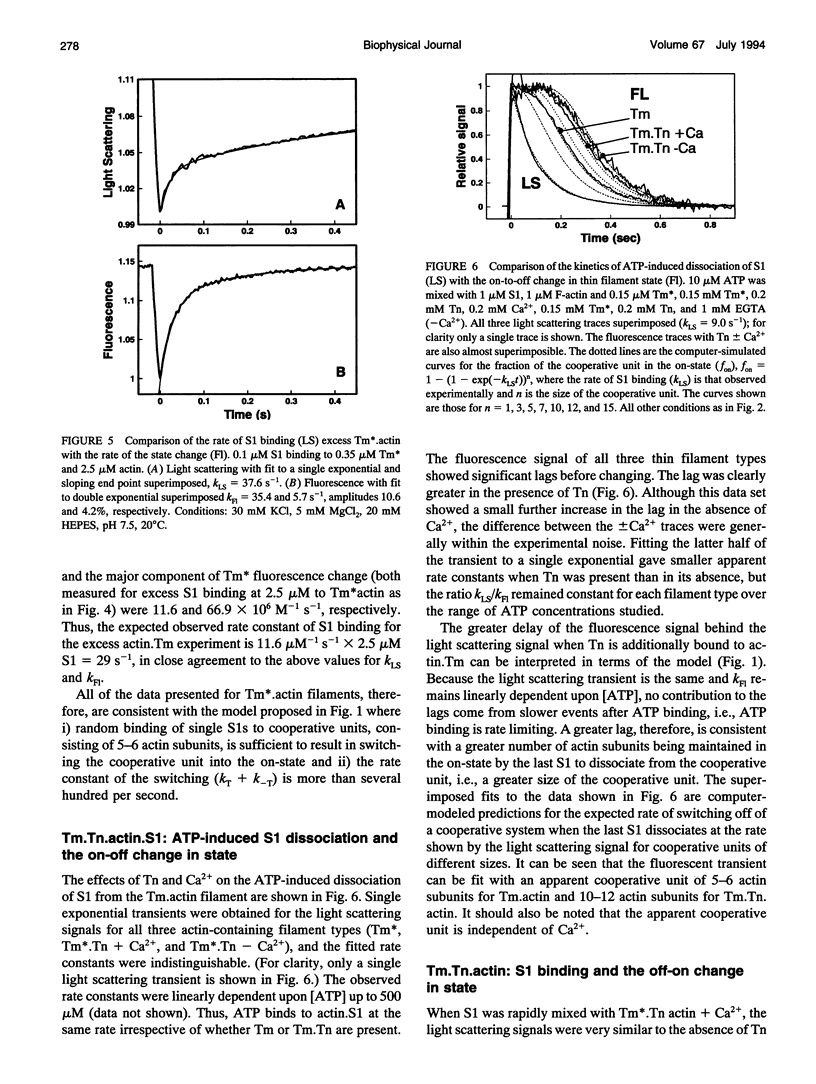
Actin thin filaments containing bound tropomyosin (Tm) or tropomyosin troponin (Tm.Tn) exist in two states ("off" and "on") with different affinities for myosin heads (S1), which results in the cooperative binding of S1. The rate of S1 binding to, and dissociating from, actin, Tm.actin, and Tm.Tn.actin, monitored by light scattering (LS), was compared with the rate of change in state, monitored by the excimer fluorescence (Fl) of a pyrene label attached to Tm. The ATP-induced S1 dissociation showed similar exponential decreases in LS for actin.S1, Tm.actin.S1, and Tm.Tn.actin.S1 +/- Ca2+. The Fl change, however, showed a delay that was greater for Tm.Tn.actin than Tm.actin, independent of Ca2+. The S1 binding kinetics gave observed rate constants for the S1-induced change in state that were 5-6 times the observed rate constants of S1 binding to Tm.actin, which were increased to 10-12 for Tm.Tn.actin, independent of Ca2+. The rate of the Fl signals showed that the on/off states were in rapid equilibrium. These data indicate that the apparent cooperative unit for Tm.actin is 5-6 actin subunits rather than the minimum structural unit size of 7, and is increased to 10-12 subunits for Tm.Tn.actin, independent of the presence of Ca2+. Thus, Tm appears semi-flexible, and Tn increases communication between neighboring structural units. A general model for the dynamic transitions involved in muscle regulation is presented.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benesch R., Benesch R. E. The effect of organic phosphates from the human erythrocyte on the allosteric properties of hemoglobin. Biochem Biophys Res Commun. 1967 Jan 23;26(2):162–167. doi: 10.1016/0006-291x(67)90228-8. [DOI] [PubMed] [Google Scholar]
- Brandt P. W., Diamond M. S., Rutchik J. S., Schachat F. H. Co-operative interactions between troponin-tropomyosin units extend the length of the thin filament in skeletal muscle. J Mol Biol. 1987 Jun 20;195(4):885–896. doi: 10.1016/0022-2836(87)90492-x. [DOI] [PubMed] [Google Scholar]
- Brisson J. R., Golosinska K., Smillie L. B., Sykes B. D. Interaction of tropomyosin and troponin T: a proton nuclear magnetic resonance study. Biochemistry. 1986 Aug 12;25(16):4548–4555. doi: 10.1021/bi00364a014. [DOI] [PubMed] [Google Scholar]
- Chalovich J. M. Actin mediated regulation of muscle contraction. Pharmacol Ther. 1992;55(2):95–148. doi: 10.1016/0163-7258(92)90013-p. [DOI] [PubMed] [Google Scholar]
- Coates J. H., Criddle A. H., Geeves M. A. Pressure-relaxation studies of pyrene-labelled actin and myosin subfragment 1 from rabbit skeletal muscle. Evidence for two states of acto-subfragment 1. Biochem J. 1985 Dec 1;232(2):351–356. doi: 10.1042/bj2320351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flicker P. F., Phillips G. N., Jr, Cohen C. Troponin and its interactions with tropomyosin. An electron microscope study. J Mol Biol. 1982 Dec 5;162(2):495–501. doi: 10.1016/0022-2836(82)90540-x. [DOI] [PubMed] [Google Scholar]
- Geeves M. A. Dynamic interaction between actin and myosin subfragment 1 in the presence of ADP. Biochemistry. 1989 Jul 11;28(14):5864–5871. doi: 10.1021/bi00440a024. [DOI] [PubMed] [Google Scholar]
- Geeves M. A., Halsall D. J. The dynamics of the interaction between myosin subfragment 1 and pyrene-labelled thin filaments, from rabbit skeletal muscle. Proc R Soc Lond B Biol Sci. 1986 Oct 22;229(1254):85–95. doi: 10.1098/rspb.1986.0076. [DOI] [PubMed] [Google Scholar]
- Geeves M. A., Halsall D. J. Two-step ligand binding and cooperativity. A model to describe the cooperative binding of myosin subfragment 1 to regulated actin. Biophys J. 1987 Aug;52(2):215–220. doi: 10.1016/S0006-3495(87)83208-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geeves M. A., Jeffries T. E., Millar N. C. ATP-induced dissociation of rabbit skeletal actomyosin subfragment 1. Characterization of an isomerization of the ternary acto-S1-ATP complex. Biochemistry. 1986 Dec 30;25(26):8454–8458. doi: 10.1021/bi00374a020. [DOI] [PubMed] [Google Scholar]
- Geeves M. A. The dynamics of actin and myosin association and the crossbridge model of muscle contraction. Biochem J. 1991 Feb 15;274(Pt 1):1–14. doi: 10.1042/bj2740001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graceffa P., Lehrer S. S. The excimer fluorescence of pyrene-labeled tropomyosin. A probe of conformational dynamics. J Biol Chem. 1980 Dec 10;255(23):11296–11300. [PubMed] [Google Scholar]
- Greene L. E., Eisenberg E. Cooperative binding of myosin subfragment-1 to the actin-troponin-tropomyosin complex. Proc Natl Acad Sci U S A. 1980 May;77(5):2616–2620. doi: 10.1073/pnas.77.5.2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammes G. G., Wu C. W. Regulation of enzyme activity. The activity of enzymes can be controlled by a multiplicity of conformational equilibria. Science. 1971 Jun 18;172(3989):1205–1211. doi: 10.1126/science.172.3989.1205. [DOI] [PubMed] [Google Scholar]
- Heeley D. H., Golosinska K., Smillie L. B. The effects of troponin T fragments T1 and T2 on the binding of nonpolymerizable tropomyosin to F-actin in the presence and absence of troponin I and troponin C. J Biol Chem. 1987 Jul 25;262(21):9971–9978. [PubMed] [Google Scholar]
- Hill L. E., Mehegan J. P., Butters C. A., Tobacman L. S. Analysis of troponin-tropomyosin binding to actin. Troponin does not promote interactions between tropomyosin molecules. J Biol Chem. 1992 Aug 15;267(23):16106–16113. [PubMed] [Google Scholar]
- Hill T. L., Eisenberg E., Greene L. Theoretical model for the cooperative equilibrium binding of myosin subfragment 1 to the actin-troponin-tropomyosin complex. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3186–3190. doi: 10.1073/pnas.77.6.3186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii Y., Lehrer S. S. Excimer fluorescence of pyrenyliodoacetamide-labeled tropomyosin: a probe of the state of tropomyosin in reconstituted muscle thin filaments. Biochemistry. 1990 Feb 6;29(5):1160–1166. doi: 10.1021/bi00457a010. [DOI] [PubMed] [Google Scholar]
- Ishii Y., Lehrer S. S. Fluorescence studies of the conformation of pyrene-labeled tropomyosin: effects of F-actin and myosin subfragment 1. Biochemistry. 1985 Nov 5;24(23):6631–6638. doi: 10.1021/bi00344a050. [DOI] [PubMed] [Google Scholar]
- Ishii Y., Lehrer S. S. Kinetics of the "on-off" change in regulatory state of the muscle thin filament. Arch Biochem Biophys. 1993 Aug 15;305(1):193–196. doi: 10.1006/abbi.1993.1410. [DOI] [PubMed] [Google Scholar]
- Koshland D. E., Jr, Némethy G., Filmer D. Comparison of experimental binding data and theoretical models in proteins containing subunits. Biochemistry. 1966 Jan;5(1):365–385. doi: 10.1021/bi00865a047. [DOI] [PubMed] [Google Scholar]
- Leavis P. C., Gergely J. Thin filament proteins and thin filament-linked regulation of vertebrate muscle contraction. CRC Crit Rev Biochem. 1984;16(3):235–305. doi: 10.3109/10409238409108717. [DOI] [PubMed] [Google Scholar]
- Lehrer S. S., Ishii Y. Fluorescence properties of acrylodan-labeled tropomyosin and tropomyosin-actin: evidence for myosin subfragment 1 induced changes in geometry between tropomyosin and actin. Biochemistry. 1988 Aug 9;27(16):5899–5906. doi: 10.1021/bi00416a012. [DOI] [PubMed] [Google Scholar]
- Lehrer S. S., Kerwar G. Intrinsic fluorescence of actin. Biochemistry. 1972 Mar 28;11(7):1211–1217. doi: 10.1021/bi00757a015. [DOI] [PubMed] [Google Scholar]
- Lehrer S. S., Morris E. P. Dual effects of tropomyosin and troponin-tropomyosin on actomyosin subfragment 1 ATPase. J Biol Chem. 1982 Jul 25;257(14):8073–8080. [PubMed] [Google Scholar]
- Levine B. A., Moir A. J., Perry S. V. The interaction of troponin-I with the N-terminal region of actin. Eur J Biochem. 1988 Mar 1;172(2):389–397. doi: 10.1111/j.1432-1033.1988.tb13899.x. [DOI] [PubMed] [Google Scholar]
- MONOD J., WYMAN J., CHANGEUX J. P. ON THE NATURE OF ALLOSTERIC TRANSITIONS: A PLAUSIBLE MODEL. J Mol Biol. 1965 May;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- Mabuchi K. Melting of myosin and tropomyosin: electron microscopic observations. J Struct Biol. 1990 May;103(3):249–256. doi: 10.1016/1047-8477(90)90043-c. [DOI] [PubMed] [Google Scholar]
- McKillop D. F., Geeves M. A. Regulation of the acto.myosin subfragment 1 interaction by troponin/tropomyosin. Evidence for control of a specific isomerization between two acto.myosin subfragment 1 states. Biochem J. 1991 Nov 1;279(Pt 3):711–718. doi: 10.1042/bj2790711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKillop D. F., Geeves M. A. Regulation of the interaction between actin and myosin subfragment 1: evidence for three states of the thin filament. Biophys J. 1993 Aug;65(2):693–701. doi: 10.1016/S0006-3495(93)81110-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki M., Iio T. Kinetics of structural changes of reconstituted skeletal muscle thin filaments observed by fluorescence resonance energy transfer. J Biol Chem. 1993 Apr 5;268(10):7101–7106. [PubMed] [Google Scholar]
- Millar N. C., Geeves M. A. The limiting rate of the ATP-mediated dissociation of actin from rabbit skeletal muscle myosin subfragment 1. FEBS Lett. 1983 Aug 22;160(1-2):141–148. doi: 10.1016/0014-5793(83)80954-5. [DOI] [PubMed] [Google Scholar]
- Moss R. L., Allen J. D., Greaser M. L. Effects of partial extraction of troponin complex upon the tension-pCa relation in rabbit skeletal muscle. Further evidence that tension development involves cooperative effects within the thin filament. J Gen Physiol. 1986 May;87(5):761–774. doi: 10.1085/jgp.87.5.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagashima H., Asakura S. Studies on co-operative properties of tropomyosin-actin and tropomyosin-troponin-actin complexes by the use of N-ethylmaleimide-treated and untreated species of myosin subfragment 1. J Mol Biol. 1982 Mar 15;155(4):409–428. doi: 10.1016/0022-2836(82)90479-x. [DOI] [PubMed] [Google Scholar]
- Parry D. A., Squire J. M. Structural role of tropomyosin in muscle regulation: analysis of the x-ray diffraction patterns from relaxed and contracting muscles. J Mol Biol. 1973 Mar 25;75(1):33–55. doi: 10.1016/0022-2836(73)90527-5. [DOI] [PubMed] [Google Scholar]
- Phillips G. N., Jr, Fillers J. P., Cohen C. Tropomyosin crystal structure and muscle regulation. J Mol Biol. 1986 Nov 5;192(1):111–131. doi: 10.1016/0022-2836(86)90468-7. [DOI] [PubMed] [Google Scholar]
- Syska H., Wilkinson J. M., Grand R. J., Perry S. V. The relationship between biological activity and primary structure of troponin I from white skeletal muscle of the rabbit. Biochem J. 1976 Feb 1;153(2):375–387. doi: 10.1042/bj1530375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao T., Gong B. J., Leavis P. C. Calcium-induced movement of troponin-I relative to actin in skeletal muscle thin filaments. Science. 1990 Mar 16;247(4948):1339–1341. doi: 10.1126/science.2138356. [DOI] [PubMed] [Google Scholar]
- Trybus K. M., Taylor E. W. Kinetic studies of the cooperative binding of subfragment 1 to regulated actin. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7209–7213. doi: 10.1073/pnas.77.12.7209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno H. Local structural changes in tropomyosin detected by a trypsin-probe method. Biochemistry. 1984 Sep 25;23(20):4791–4798. doi: 10.1021/bi00315a040. [DOI] [PubMed] [Google Scholar]
- Weeds A. G., Pope B. Studies on the chymotryptic digestion of myosin. Effects of divalent cations on proteolytic susceptibility. J Mol Biol. 1977 Apr;111(2):129–157. doi: 10.1016/s0022-2836(77)80119-8. [DOI] [PubMed] [Google Scholar]
- White H. D., Taylor E. W. Energetics and mechanism of actomyosin adenosine triphosphatase. Biochemistry. 1976 Dec 28;15(26):5818–5826. doi: 10.1021/bi00671a020. [DOI] [PubMed] [Google Scholar]
- Williams D. L., Jr, Greene L. E., Eisenberg E. Cooperative turning on of myosin subfragment 1 adenosinetriphosphatase activity by the troponin-tropomyosin-actin complex. Biochemistry. 1988 Sep 6;27(18):6987–6993. doi: 10.1021/bi00418a048. [DOI] [PubMed] [Google Scholar]
- Zot A. S., Potter J. D. Structural aspects of troponin-tropomyosin regulation of skeletal muscle contraction. Annu Rev Biophys Biophys Chem. 1987;16:535–559. doi: 10.1146/annurev.bb.16.060187.002535. [DOI] [PubMed] [Google Scholar]



