Abstract
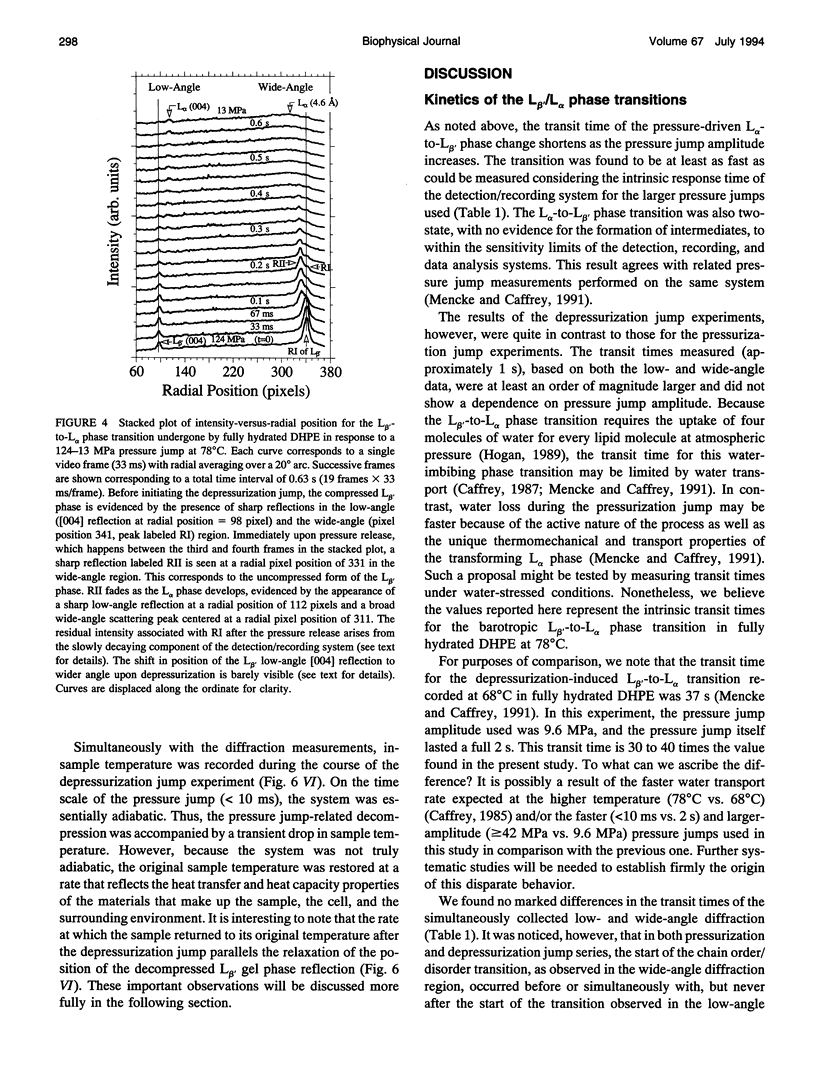
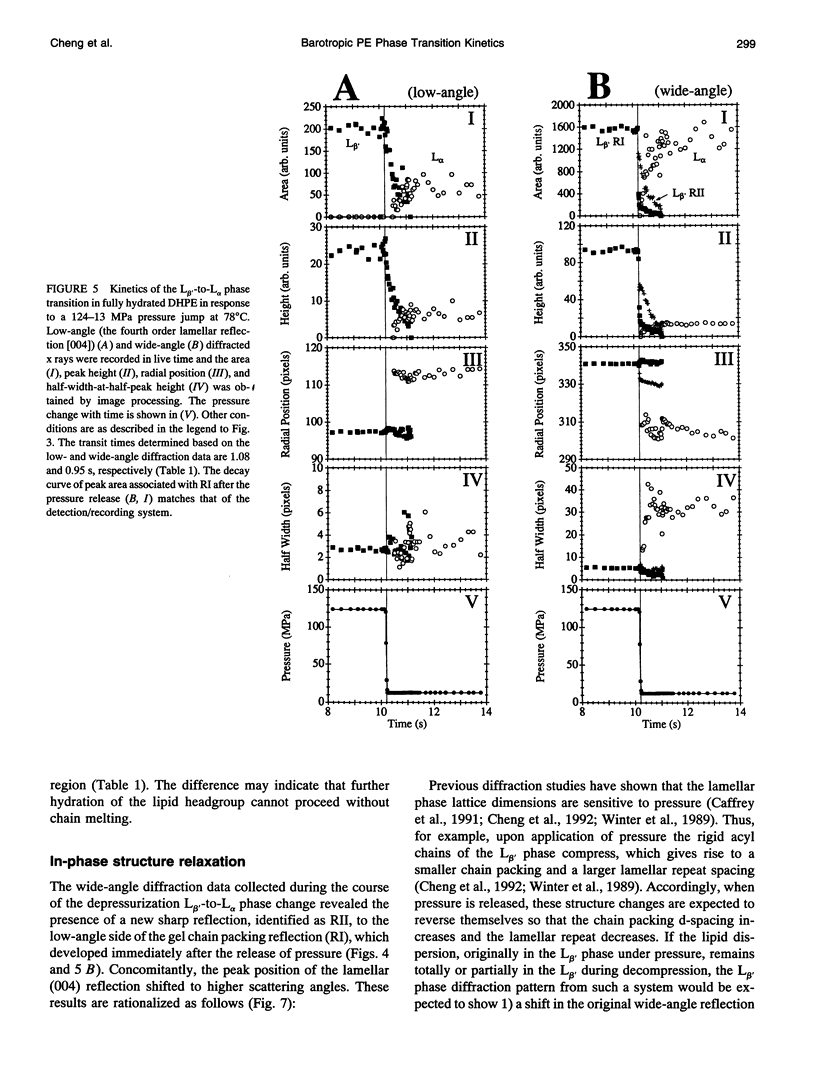
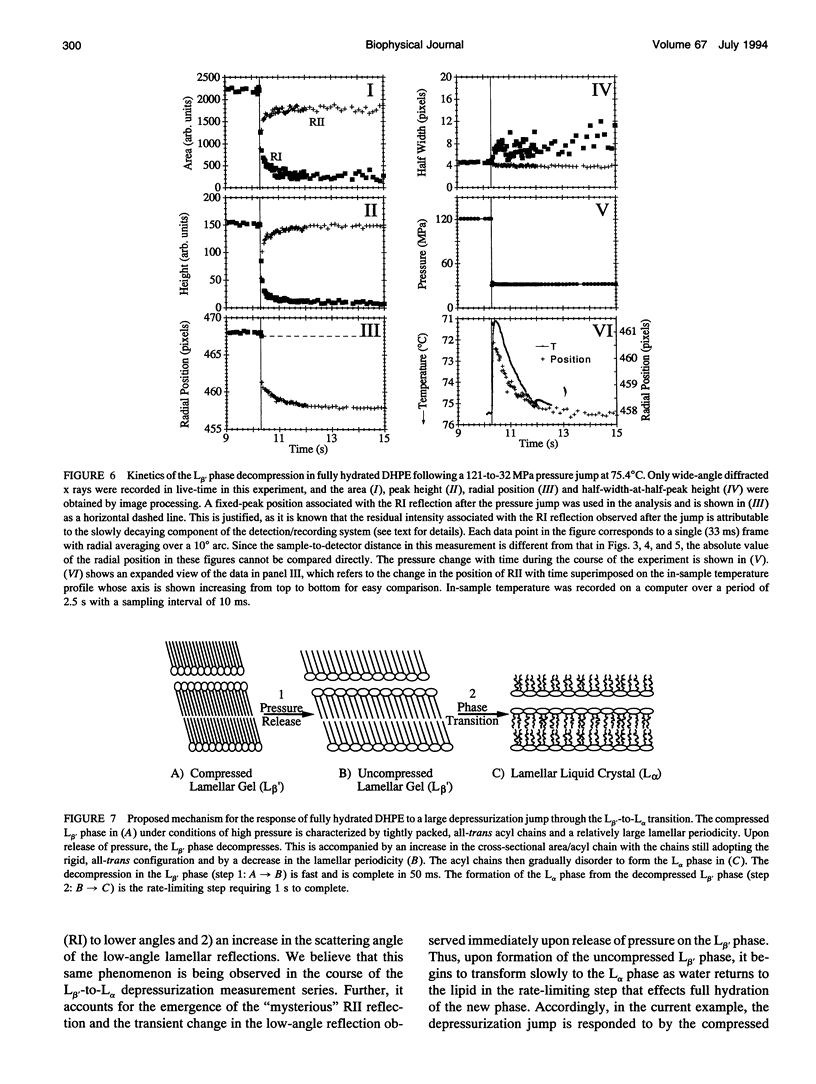
The kinetics and mechanism of the barotropic lamellar gel (L beta')/lamellar liquid crystal (L alpha) phase transition in fully hydrated 1,2-dihexadecyl-sn-glycero-3-phosphoethanolamine (DHPE) has been studied using time-resolved x-ray diffraction (TRXRD). The phase transition was induced by pressure jumps of varying amplitudes in both the pressurization and depressurization directions at controlled temperature (78 degrees C). Both low- and wide-angle diffracted x rays were recorded simultaneously in live time using an x-ray-sensitive image intensifier coupled to a CCD camera and Super-VHS videotape recorder. Such an arrangement allowed for the direct and quantitative characterization of the long- (lamellar repeat spacing) and short-range order (chain packing) during a kinetic experiment. The image-processed live-time x-ray diffraction data were fitted using a nonlinear least-squares model, and the parameters of the fits were monitored continuously throughout the transition. The pressure-induced transitions from the L alpha to the L beta' phase and from the L beta' to the L alpha phase was two-state (no formation of intermediates apparent during the transition) to within the sensitivity limits of the method. The corresponding transit time (the time during which both phases coexist) associated with the long- and short-range order of the pressurization-induced L alpha-to-L beta' phase transition decreased to a limiting value of approximately 50 ms with increasing pressure jump amplitude. This limiting value was close to the response time of the detector/recording system. Thus, the intrinsic transit time of this transition in fully hydrated DHPE at 78 degrees C was less than or equal to 50 ms. In contrast, the depressurization-induced L beta'-to-L alpha phase transition was slower, taking approximately 1 s to complete, and occurred with no obvious dependence of the transit time on pressure jump amplitude. In the depressurization jump experiment, the lipid responded rapidly to the pressure jump in the L beta' phase up to the rate-determining L beta'-to-L alpha transition. Such behavior was examined carefully, as it could complicate the interpretation of phase transition kinetic measurements.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Caffrey M., Hogan J., Mencke A. Kinetics of the barotropic ripple (P beta')/lamellar liquid crystal (L alpha) phase transition in fully hydrated dimyristoylphosphatidylcholine (DMPC) monitored by time-resolved x-ray diffraction. Biophys J. 1991 Aug;60(2):456–466. doi: 10.1016/S0006-3495(91)82072-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caffrey M. Kinetics and mechanism of the lamellar gel/lamellar liquid-crystal and lamellar/inverted hexagonal phase transition in phosphatidylethanolamine: a real-time X-ray diffraction study using synchrotron radiation. Biochemistry. 1985 Aug 27;24(18):4826–4844. doi: 10.1021/bi00339a017. [DOI] [PubMed] [Google Scholar]
- Caffrey M. The study of lipid phase transition kinetics by time-resolved X-ray diffraction. Annu Rev Biophys Biophys Chem. 1989;18:159–186. doi: 10.1146/annurev.bb.18.060189.001111. [DOI] [PubMed] [Google Scholar]
- Gruner S. M. Time-resolved x-ray diffraction of biological materials. Science. 1987 Oct 16;238(4825):305–312. doi: 10.1126/science.3310232. [DOI] [PubMed] [Google Scholar]
- Laggner P., Kriechbaum M. Phospholipid phase transitions: kinetics and structural mechanisms. Chem Phys Lipids. 1991 Mar;57(2-3):121–145. doi: 10.1016/0009-3084(91)90072-j. [DOI] [PubMed] [Google Scholar]
- Marsh D. General features of phospholipid phase transitions. Chem Phys Lipids. 1991 Mar;57(2-3):109–120. doi: 10.1016/0009-3084(91)90071-i. [DOI] [PubMed] [Google Scholar]
- Mencke A. P., Caffrey M. Kinetics and mechanism of the pressure-induced lamellar order/disorder transition in phosphatidylethanolamine: a time-resolved X-ray diffraction study. Biochemistry. 1991 Mar 5;30(9):2453–2463. doi: 10.1021/bi00223a023. [DOI] [PubMed] [Google Scholar]
- Seddon J. M., Cevc G., Marsh D. Calorimetric studies of the gel-fluid (L beta-L alpha) and lamellar-inverted hexagonal (L alpha-HII) phase transitions in dialkyl- and diacylphosphatidylethanolamines. Biochemistry. 1983 Mar 1;22(5):1280–1289. doi: 10.1021/bi00274a045. [DOI] [PubMed] [Google Scholar]
- Tardieu A., Luzzati V., Reman F. C. Structure and polymorphism of the hydrocarbon chains of lipids: a study of lecithin-water phases. J Mol Biol. 1973 Apr 25;75(4):711–733. doi: 10.1016/0022-2836(73)90303-3. [DOI] [PubMed] [Google Scholar]
- Van Osdol W. W., Biltonen R. L., Johnson M. L. Measuring the kinetics of membrane phase transitions. J Biochem Biophys Methods. 1989;20(1):1–46. doi: 10.1016/0165-022x(89)90079-1. [DOI] [PubMed] [Google Scholar]
- Winter R., Xie C. L., Jonas J., Thiyagarajan P., Wong P. T. High-pressure small-angle neutron scattering (SANS) study of 1,2-dielaidoyl-sn-glycero-3-phosphocholine bilayers. Biochim Biophys Acta. 1989 Jun 26;982(1):85–88. doi: 10.1016/0005-2736(89)90177-6. [DOI] [PubMed] [Google Scholar]
- Yager P., Peticolas W. L. The kinetics of the main phase transition of aqueous dispersions of phospholipids induced by pressure jump and monitored by Raman spectroscopy. Biochim Biophys Acta. 1982 Jun 28;688(3):775–785. doi: 10.1016/0005-2736(82)90291-7. [DOI] [PubMed] [Google Scholar]



