Abstract
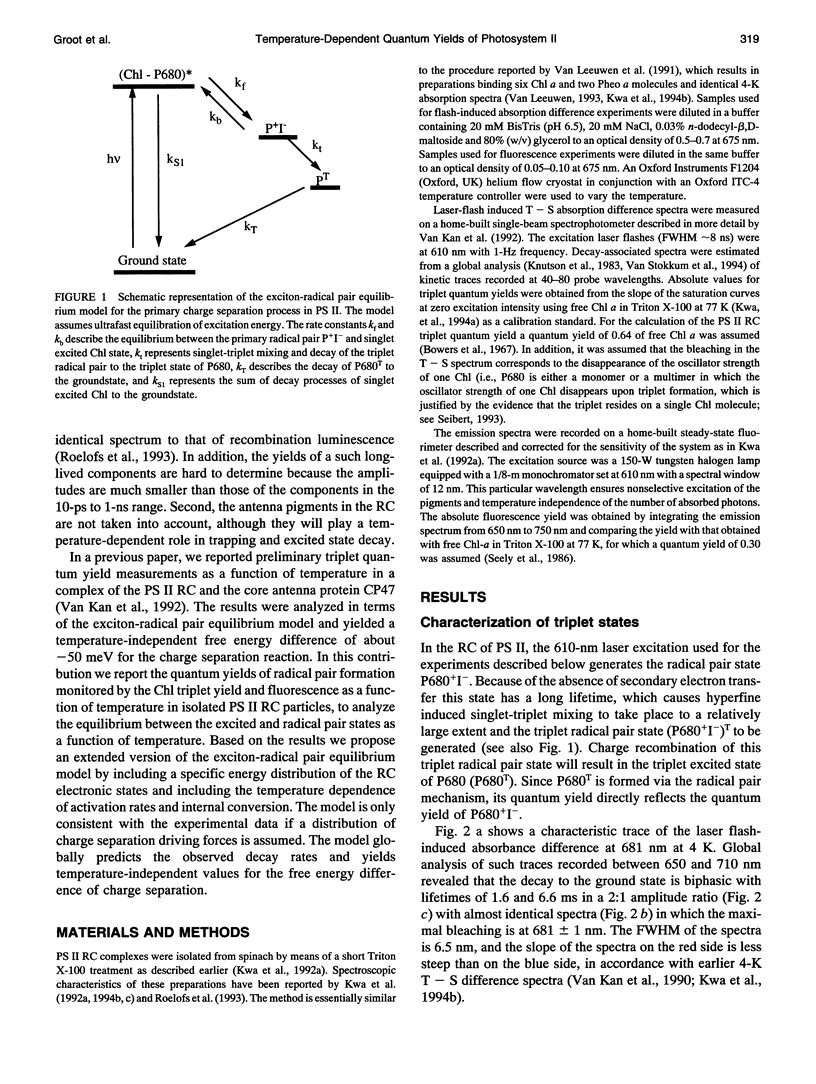
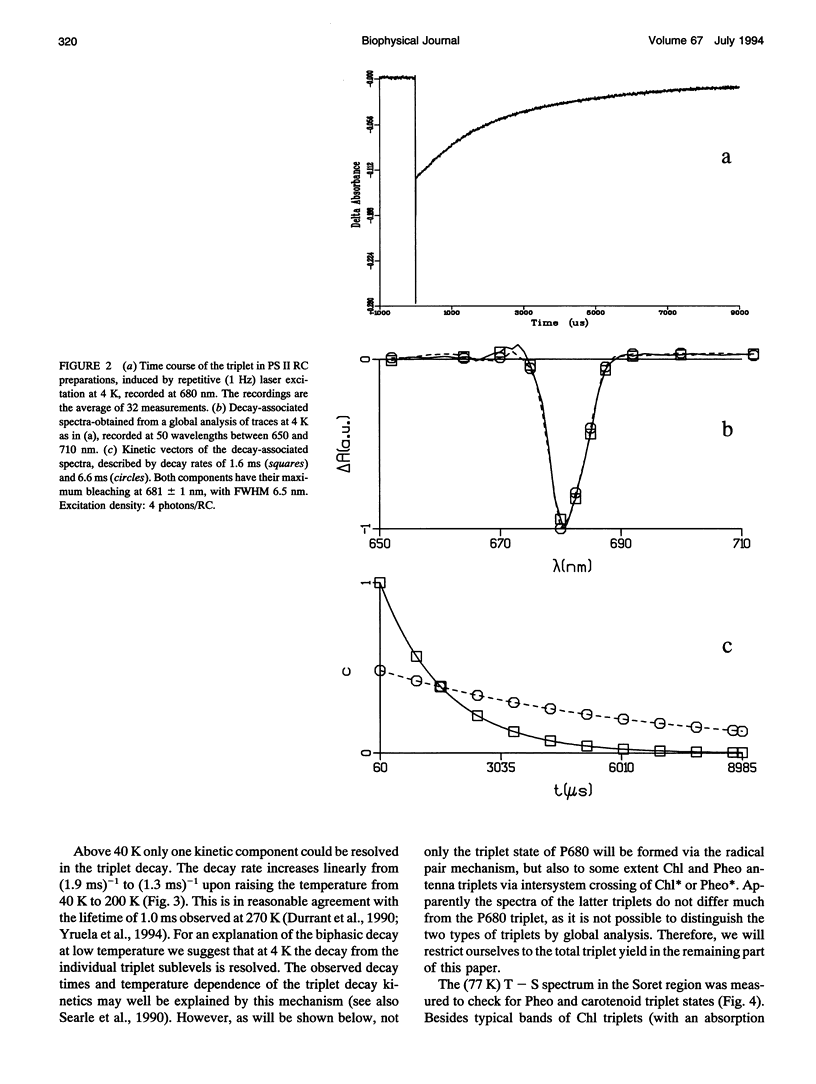
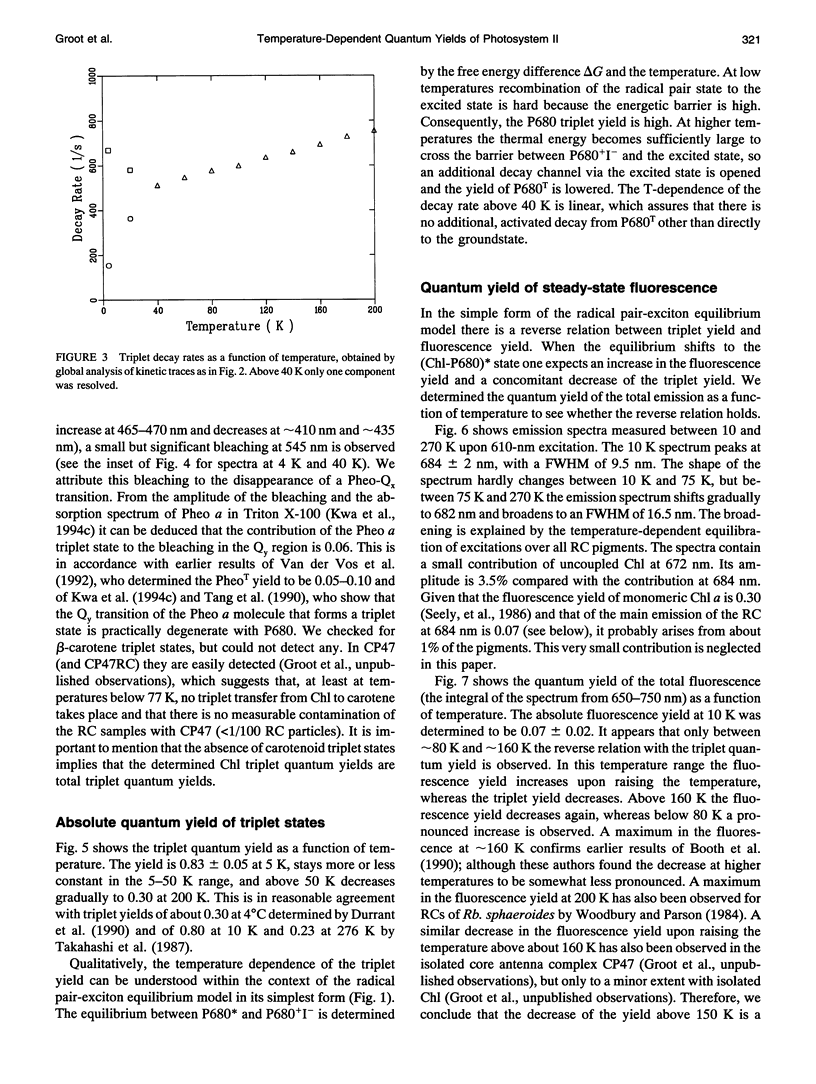
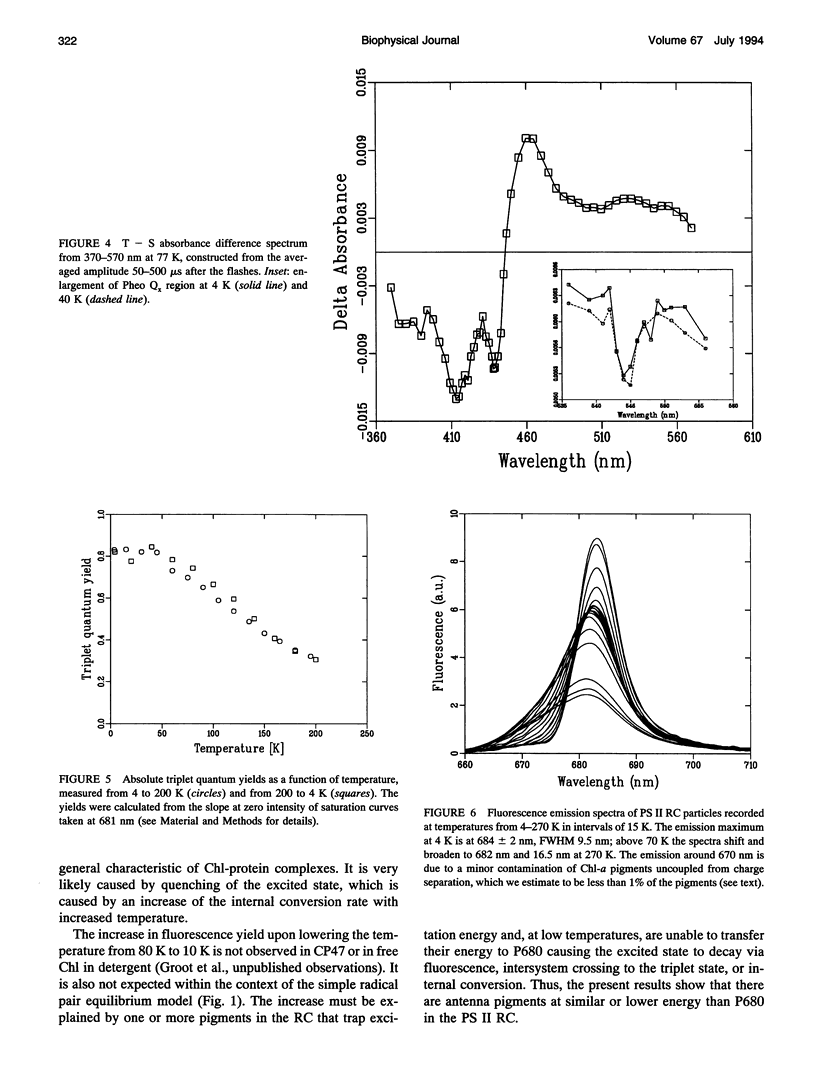
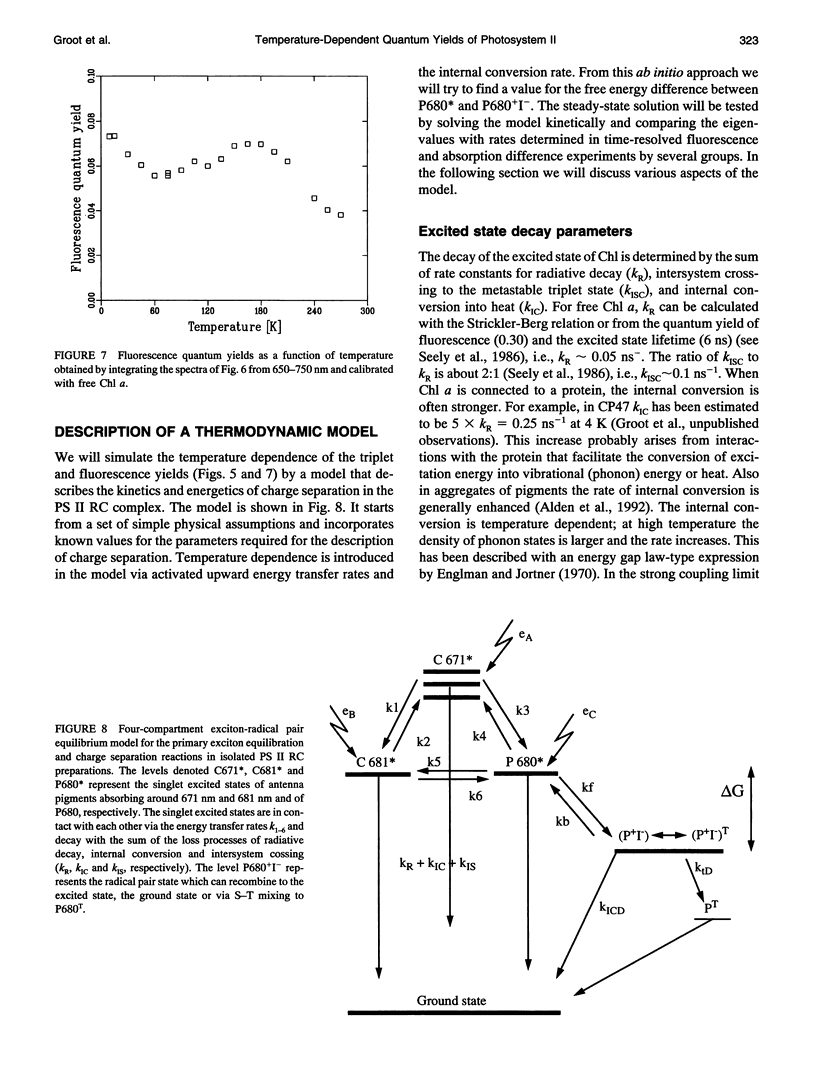
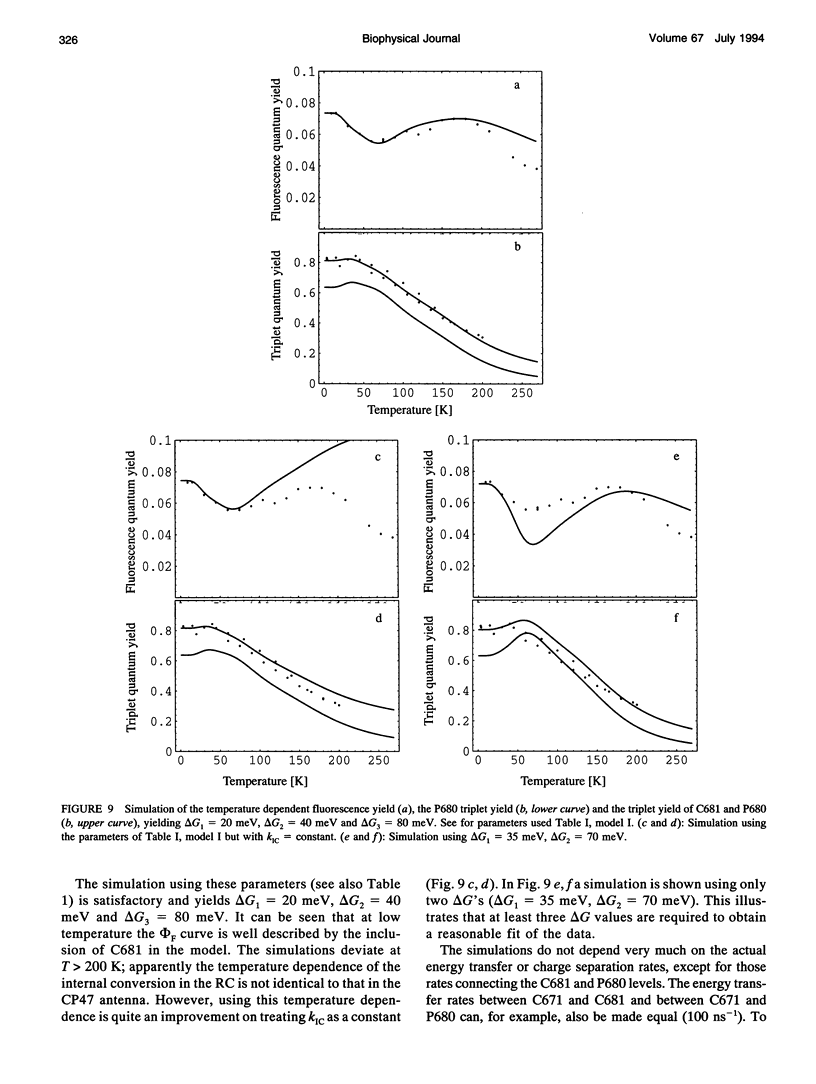
A key step in the photosynthetic reactions in photosystem II of green plants is the transfer of an electron from the singlet-excited chlorophyll molecule called P680 to a nearby pheophytin molecule. The free energy difference of this primary charge separation reaction is determined in isolated photosystem II reaction center complexes as a function of temperature by measuring the absolute quantum yield of P680 triplet formation and the time-integrated fluorescence emission yield. The total triplet yield is found to be 0.83 +/- 0.05 at 4 K, and it decreases upon raising the temperature to 0.30 at 200 K. It is suggested that the observed triplet states predominantly arise from P680 but to a minor extent also from antenna chlorophyll present in the photosystem II reaction center. No carotenoid triplet states could be detected, demonstrating that the contamination of the preparation with CP47 complexes is less than 1/100 reaction centers. The fluorescence yield is 0.07 +/- 0.02 at 10 K, and it decreases upon raising the temperature to reach a value of 0.05-0.06 at 60-70 K, increases upon raising the temperature to 0.07 at approximately 165 K and decreases again upon further raising the temperature. The complex dependence of fluorescence quantum yield on temperature is explained by assuming the presence of one or more pigments in the photosystem II reaction center that are energetically degenerate with the primary electron donor P680 and below 60-70 K trap part of the excitation energy, and by temperature-dependent excited state decay above 165 K. A four-compartment model is presented that describes the observed triplet and fluorescence quantum yields at all temperatures and includes pigments that are degenerate with P680, temperature-dependent excited state decay and activated upward energy transfer rates. The eigenvalues of the model are in accordance with the lifetimes observed in fluorescence and absorption difference measurements by several workers. The model suggests that the free energy difference between singlet-excited P680 and the radical pair state P680+l- is temperature independent, and that a distribution of free energy differences represented by at least three values of about 20, 40, and 80 meV, is needed to get an appropriate fit of the data.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Booth P. J., Crystall B., Ahmad I., Barber J., Porter G., Klug D. R. Observation of multiple radical pair states in photosystem 2 reaction centers. Biochemistry. 1991 Jul 30;30(30):7573–7586. doi: 10.1021/bi00244a029. [DOI] [PubMed] [Google Scholar]
- Durrant J. R., Hastings G., Joseph D. M., Barber J., Porter G., Klug D. R. Rate of oxidation of P680 in isolated photosystem 2 reaction centers monitored by loss of chlorophyll stimulated emission. Biochemistry. 1993 Aug 17;32(32):8259–8267. doi: 10.1021/bi00083a029. [DOI] [PubMed] [Google Scholar]
- Durrant J. R., Hastings G., Joseph D. M., Barber J., Porter G., Klug D. R. Subpicosecond equilibration of excitation energy in isolated photosystem II reaction centers. Proc Natl Acad Sci U S A. 1992 Dec 1;89(23):11632–11636. doi: 10.1073/pnas.89.23.11632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings G., Durrant J. R., Barber J., Porter G., Klug D. R. Observation of pheophytin reduction in photosystem two reaction centers using femtosecond transient absorption spectroscopy. Biochemistry. 1992 Aug 25;31(33):7638–7647. doi: 10.1021/bi00148a027. [DOI] [PubMed] [Google Scholar]
- Schatz G. H., Brock H., Holzwarth A. R. Kinetic and Energetic Model for the Primary Processes in Photosystem II. Biophys J. 1988 Sep;54(3):397–405. doi: 10.1016/S0006-3495(88)82973-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatz G. H., Brock H., Holzwarth A. R. Picosecond kinetics of fluorescence and absorbance changes in photosystem II particles excited at low photon density. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8414–8418. doi: 10.1073/pnas.84.23.8414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk M., Gilbert M., Rousseau G., Richter M., Ogrodnik A., Michel-Beyerle M. E. Similarity of primary radical pair recombination in photosystem II and bacterial reaction centers. FEBS Lett. 1993 Dec 27;336(2):357–362. doi: 10.1016/0014-5793(93)80837-k. [DOI] [PubMed] [Google Scholar]
- Woodbury N. W., Parson W. W. Nanosecond fluorescence from isolated photosynthetic reaction centers of Rhodopseudomonas sphaeroides. Biochim Biophys Acta. 1984 Nov 26;767(2):345–361. doi: 10.1016/0005-2728(84)90205-6. [DOI] [PubMed] [Google Scholar]
- Yruela I., van Kan P. J., Müller M. G., Holzwarth A. R. Characterization of a D1-D2-cyt b-559 complex containing 4 chlorophyll a/2 pheophytin a isolated with the use of MgSO4. FEBS Lett. 1994 Feb 14;339(1-2):25–30. doi: 10.1016/0014-5793(94)80377-3. [DOI] [PubMed] [Google Scholar]


