Abstract
Cocaine covalently modifies proteins through a reaction in which the methyl ester of cocaine acylates the ɛ-amino group of lysine residues. This reaction is highly specific in vitro, because no other amino acid reacts with cocaine, and only cocaine's methyl ester reacts with the lysine side chain. Covalently modified proteins were present in the plasma of rats and human subjects chronically exposed to cocaine. Modified endogenous proteins are immunogenic, and specific antibodies were elicited in mouse and detected in the plasma of human subjects. Covalent modification of proteins could explain cocaine's autoimmune effects and provide a new biochemical approach to cocaine's long-term actions.
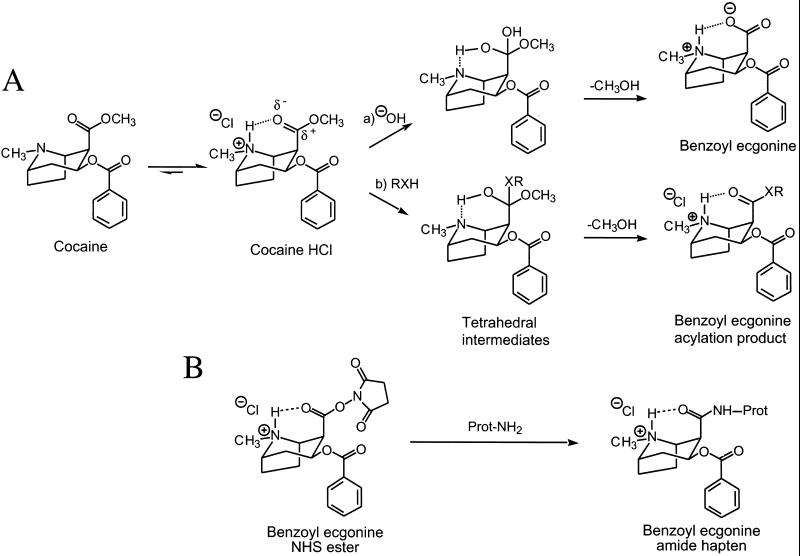
Although cocaine binds only transiently to its receptors and is cleared rapidly from plasma (1, 2), some of its effects persist after weeks or even months of abstinence (3–5). Persistence of effects could be caused by cellular mechanisms (6, 7) but also by direct chemical modification of proteins. For example, an unspecified reactive metabolite of cocaine is conjectured to contribute to cocaine-induced alterations in immunity (8) and could explain associated conditions such as thrombocytopenia (9) and vasculitis (10, 11). No such reactivity has been hypothesized for cocaine itself, but our recent finding (12) that the methyl ester of cocaine is unusually labile because of intramolecular catalysis of hydrolysis led us to reexamine the biochemistry of cocaine.
Cocaine is cleared from plasma in humans through hydrolysis of its ester groups (2). Benzoyl ester hydrolysis is mediated by various enzymes, but significant hydrolysis of the methyl ester occurs spontaneously under physiologic conditions. We recently found that the unusual lability of the methyl ester was caused by intramolecular acid catalysis mediated by the adjacent tropane nitrogen (ref. 12; Fig. 1A, path a). The tropane (pKa 8.6) is protonated predominately at physiologic pH, and transfer of the proton to the incipient alkoxide stabilizes the transition state to the rate-determined tetrahedral intermediate. This mechanism led us to consider the possibility of reaction with other nucleophiles such as those presented by proteins, of interest because covalent modification of proteins can irreversibly alter their function (13) or antigenicity (ref. 14; Fig. 1A, path b).
Figure 1.
Intramolecular acid-catalyzed reactions of cocaine at pH 7.4. (A) alkaline hydrolysis to yield benzoyl ecgonine and nucleophile (RXH) transfer to yield benzoyl ecgonine acylation product. (B) Controlled synthesis of benzoyl ecgonine amide hapten:benzoyl ecgonine N-hydroxy succinimide (NHS) ester reacts with lysine ɛ-amino groups of protein to yield a benzoyl ecgonine amide hapten.
Materials and Methods
Covalent Modification of Protein by 125I-Labeled Cocaine.
In parallel, 4′-[125I]iodococaine [1 mM, 200 μCi/mmol (1 Ci = 37 GBq)], 4′-[125I]iodobenzoyl ecgonine (1 mM, 200 μCi/mmol), and 4-[125I]iodobenzoic acid were incubated for 15 min or 3 days with human serum albumin (HSA, 0.5 ml, 1 mg/ml) in 100 mM PBS, pH 7.4, at 37°C. After the removal of free 4′-[125I]iodococaine by dialysis against PBS [3 exchanges, 1:1,000 (vol/vol)], identical volumes of samples were separated by SDS/PAGE and assayed by autoradiography. This protocol was repeated for HSA permethylated via excess formaldehyde and sodium borohydride (15), for HSA blocked at the N-terminal amino group by transamination with glyoxalic acid (16), and for HSA blocked at serine and tyrosine hydroxyl groups by reaction with diisopropyl fluorophosphate (17). 4′-[125I]Iodococaine (1.0 mCi/mmol), 4-[125I]iodobenzoyl ecgonine (1.0 mCi/mmol), and 4-[125I]iodobenzoic acid were prepared by adapting the methods described (18).
Acylation of Amino Acid by [14C]Cocaine.
To Nα-acetyllysine N-methyl amide (lysine L, 20 mg, 0.02 mmol) in PBS (0.1 ml, 100 mM, pH 7.4) was added [N-methyl-14C]cocaine (50 μCi/mmol: 94 μCi at t = 0 and 64 μCi at t = 48 h). At 72 h a sample was assayed by TLC on an analytic silica gel plate along with authentic [N-methyl-14C]-Nɛ-benzoyl ecgonine Nα-acetyllysine N-methyl amide as a standard. [N-methyl-14C]Cocaine (1.0 mCi/mmol) and [benzoylcarbonyl-14C]cocaine (1.0 mCi/mmol) and the corresponding benzoyl ecgonines were prepared by adapting methods described previously (19). [benzoylcarbonyl-14C]-Nɛ-Benzoyl Nα-acetyllysine N-methyl amide was prepared from Nα-acetyllysine N-methyl amide and [benzoylcarbonyl-14C]benzoic acid (NEN) via coupling with dicyclohexylcarbodiimide; by the same method, [14CH3-N]-Nɛ-benzoyl ecgonine Nα-acetyllysine N-methyl amide was prepared from [14CH3-N]-benzoyl ecgonine and lysine L. New compounds were characterized by 1H and 13C NMR spectroscopy and mass spectrometry.
Western Blot Detection of Cocaine-Modified Proteins.
mAb 46H1, purified by protein G affinity chromatography, did not react significantly with HSA or plasma proteins at 1:2,000 dilution. Tosyl-activated magnetic beads (4 × 107) were coated with mAb 46H1, crosslinked with dimethyl pimelimidate dihydrochloride, and washed free of noncovalently bound antibody with detergent. Blood was obtained from subjects after informed consent was given in accordance with a protocol approved by the Institutional Review Boards at Columbia-Presbyterian Medical Center and the New York Psychiatric Institute. Cocaine-exposed albumin or plasma samples were dialyzed against PBS [1:1,000 (vol/vol), 3 exchanges] and immunoprecipitated over 4 h at 4°C. Beads were washed four times with PBS, suspended in sample buffer, and boiled for 5 min. SDS/PAGE and electroblot transfer to poly(vinylidene difluoride) membranes was followed by Western blot with mAb 46H1 (1:2,000 dilution).
Biacore Assay of Antibodies to Cocaine-Modified Proteins.
The N-hydroxysuccinimide ester of benzoyl ecgonine (100 μg in 100 μl of PBS, pH 7.0) was administered to mice by tail-vein injection biweekly for 3 doses. Anticocaine antibodies were identified in serum samples by Biacore surface plasmon resonance measurement against cocaine attached at the carbomethoxyl group by a 10-atom tether constructed by methods described previously (20). Response units were measured as the difference between binding in a tethered cocaine cell and a reference cell. Species-specific goat anti-Ig (heavy and light chain) were obtained from Southern Biotechnology Associates.
Results
125I-Labeled Cocaine Modifies Albumin in Vitro.
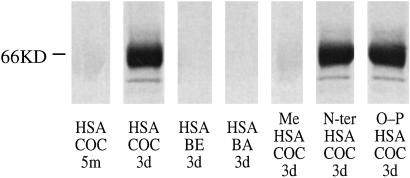
We exposed HSA to 4′-[125I]iodococaine under physiologic conditions: buffered saline at 37°C, pH 7.4. At intervals, aliquots were dialyzed to remove unbound radiolabel and subjected to polyacrylamide gel electrophoresis under denaturing conditions (SDS/PAGE) to remove radiolabel bound noncovalently. Autoradiography of a sample obtained at 3 days showed incorporation of radiolabel into the albumin band at 66 kDa (Fig. 2, lane 2, HSA+COC 3d). In contrast, a sample obtained after a brief exposure (5 min) that was expected to result in predominantly noncovalent interactions showed no incorporation (Fig. 2, lane 1, HSA+COC 5m). Benzoyl ecgonine, the product of methyl ester hydrolysis (see Fig. 1), is not susceptible to nucleophilic attack at the carboxylate group, and as expected, incubation of 4′-[125I]iodobenzoyl ecgonine with albumin for 3 days resulted in no incorporation (Fig. 2 lane 3, HSA+BE 3d). Although the methyl ester is more labile than the benzoyl ester, cocaine does hydrolyze slowly at the benzoyl group to release benzoic acid; 4-[125I]iodobenzoic acid incubated with albumin for 3 days, however, was not incorporated (Fig. 2, lane 4, HSA+BA 3d). These findings indicate that 4′-iodococaine can covalently modify protein spontaneously, and the likely mechanism is a nucleophilic attack by the protein at the cocaine methyl ester.
Figure 2.
Incorporation of radiolabeled cocaine into HSA. SDS/PAGE and autoradiogram of (i) HSA exposed to 4′-[125I]iodococaine for 15 min (lane 1, COC 15m) or 3 days (lane 2, COC 3d), 4′-[125I]iodobenzoyl ecgonine for 3 days (lane 3, BE 3d), or 4-[125I]iodobenzoic acid for 3 days (lane 4, BA 3d) or (ii) 4′-[125I]iodococaine incubated for 3 days with HSA methylated with CH2O/NaBH4 (lane 5, Me HSA+COC3d) or N-terminally blocked with glyoxalic acid (lane 6, N-ter HSA COC 3d) or blocked at serine and tyrosine hydroxyl groups with diisopropyl fluorophosphate (lane 7, O-P HSA COC 3d). n = 3; illustrative results are shown.
Specific Nucleophilic Attack by Lysine.
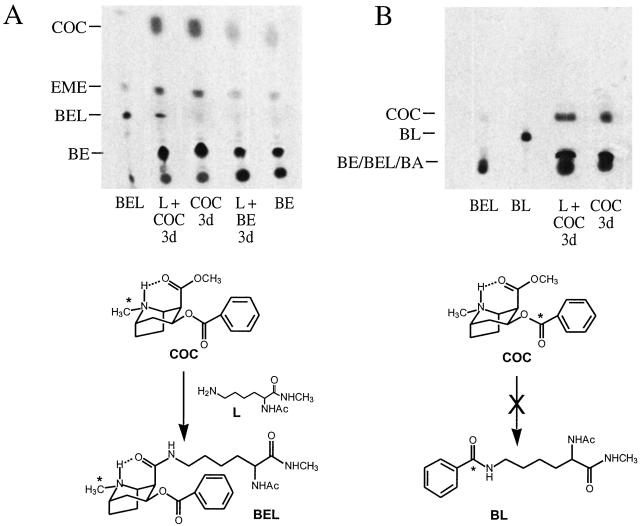
To identify potential attacking nucleophiles, we investigated the reaction of [N-methyl-14C]cocaine with individual amino acids likely to form stable products. We studied lysine, serine, tyrosine, and cysteine, each protected at the carboxylic acid group as an N-methyl amide and at the α-amino group as an acetamide, leaving only the side chain nucleophile as a potential reactant. In addition, glycine was studied as the N-methyl amide to model the α-amino group of an N-terminal amino acid. Each protected amino acid was exposed to [N-methyl-14C]cocaine for 3 days under physiologic conditions and then assayed by TLC under solvent conditions specifically defined to separate an authentic sample of the anticipated benzoyl ecgonine-amino acid adduct cleanly. As expected, the cocaine hydrolysis products ecgonine methyl ester (EME) and benzoyl ecgonine (BE) were observed in each reaction mixture. However, only the protected lysine L yielded a new radiolabeled product (Fig. 3A, lane 2, L+COC 3d; other amino acids are not shown) that was not observed with the incubation of cocaine alone (lane 3, COC 3d). The mobility of the product matched an authentic sample of Nɛ-benzoyl ecgonine adduct of the protected lysine L (lane 1, BEL), and mass spectrometric analysis showed a parent ion consistent with this structure. Quantitative mass balance showed that at >50% cocaine consumption, the acylation accounted for ≈0.05% of the input of cocaine with the remainder consumed by the ester hydrolyses. No covalent modification was observed with [N-methyl-14C]benzoyl ecgonine (Fig. 3A, lane 4, L+BE 3d), which lacks the carboxymethyl electrophile.
Figure 3.
Acylation of lysine by the methyl ester of 14C-cocaine (COC). n = 4; illustrative results are shown. (A) Analytical TLC (silica gel) (CHCl3/CH3OH/NH4OH 15:1:0.05) and autoradiogram of lysine L and [N-methyl-14C]cocaine after 3 days (lane 2, COC+L 3d); authentic acylation product BEL (lane 1, BEL); [N-methyl-14C]cocaine alone after 3 days (lane 3, COC 3d); [N-methyl-14C]benzoyl ecgonine and lysine L after 3 days (lane 4, BE+L 3d); and hydrolysis product [N-methyl-14C]benzoyl ecgonine (lane 5, BE). (B) TLC (CHCl3/MeOH 50:1) and autoradiogram of lysine L and [benzoylcarbonyl-14C]cocaine incubated for 3 days (lane 3, COC+L 3d); authentic acylation product BL (lane 2, BL); authentic BEL (lane 1, BEL); and [benzoylcarbonyl-14C]cocaine after 3 days (lane 4, COC 3d). Authentic benzoyl ecgonine (not shown) and benzoic acid (BA, not shown) remain near the origin.
These results indicating a unique reaction between lysine and cocaine were supported further by the effect of blocking of side chain functional groups on incorporation. Thus, permethylation of the amino groups of albumin with formaldehyde and sodium borohydride blocked reaction with 4′-[125I]iodococaine in vitro (Fig. 2, lane 5, MeHSA+COC 3d). This permethylation method is highly selective for lysine ɛ-amino groups (15), and the resulting dimethyl amino groups would be incapable of forming a stable acylation product with cocaine. In contrast, selective blocking of N-terminal amino groups (16) or serine and tyrosine hydroxyl groups (17) failed to block incorporation of label (Fig. 2, lanes 6 and 7).
The reaction of cocaine with the ɛ-amino group of lysine was selective for the methyl ester of cocaine. Thus, no reaction was observed at the benzoyl ester when the radiolabel was repositioned to the benzoylcarbonyl group (e.g., [benzoylcarbonyl-14C]cocaine) and chromatographic conditions were readjusted to report nucleophilic attack at this site (Fig. 3B, lane 3, L+COC 3d). Also, no reaction was observed when the carbomethoxyl group of [N-methyl-14C]cocaine was epimerized from the α configuration to the β to preclude the formation of a hydrogen bond with the tropane NH (data not shown). These results are consistent with the specific acylation of lysine ɛ-amino groups by the methyl ester of cocaine through intramolecular catalysis.
Cocaine Modifies Plasma Proteins in Vivo.
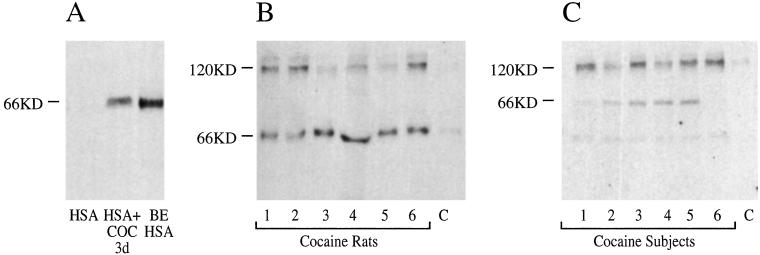
To determine whether modification of proteins occurs in vivo, we developed an immunoassay for the benzoyl ecgonine acylation product. mAb 46H1 was elicited to benzoyl ecgonine attached to a carrier protein by an amide bond (ref. 20; Fig. 1C). On Western blot, mAb 46H1 bound authentic benzoyl ecgonine-HSA adduct but not HSA (Fig. 4A). Exposure of HSA to cocaine for 3 days followed by immunoprecipitation and Western blot with mAb 46H1 showed anti-benzoyl ecgonine amide immunoreactivity (Fig. 4A, HSA+COC 3d). To test the effect of in vivo exposure, we treated rats (n = 6) with cocaine (32 mg/kg) i.p. twice daily for 7 days. Plasma samples harvested after an abstinence of 1 day, during which free cocaine cleared from the plasma, showed two dominant immunoreactive bands (66 and 120 kDa) not found in pretreatment control plasma (Fig. 4B). We performed a similar analysis on the plasma of people who used 1 g or more of cocaine per week. Plasma samples obtained after an observed abstinence of 1–3 days showed anti-benzoyl ecgonine amide immunoreactivity not found in controls, with dominant bands at 66 and 120 kDa and a minor band at 39 kDa (Fig. 4C). No immunoreactivity was found in controls. The major immunoprecipitated bands were identified by mass spectrometric analysis of tryptic digest fragments (21) as serum albumin and α2-macroglobulin, respectively.
Figure 4.
Incorporation of anti-benzoyl ecgonine immunoreactivity into proteins. SDS/PAGE chromatography and Western blot with mAb 46H1 of samples are shown. n = 3; illustrative results are shown. (A) HSA (lane 1); HSA incubated with cocaine and immunoprecipitated with mAb 46H1 (lane 2, HSA+COC); and HSA acylated as per Fig. 1C with N-hydroxyphthalimide-activated benzoyl ecgonine (lane 3, BE HSA). (B) Plasma from rats exposed to cocaine twice daily for 7 days (lanes 1–6) and plasma from an unexposed control (lane 7) immunoprecipitated with mAb 46H1. (C) Plasma from human cocaine users (lanes 1–6) and an unexposed control subject (lane 7) immunoprecipitated with mAb 46H1. Each lane represents material from an individual.
Antibodies to Modified Proteins in Mouse and Man.
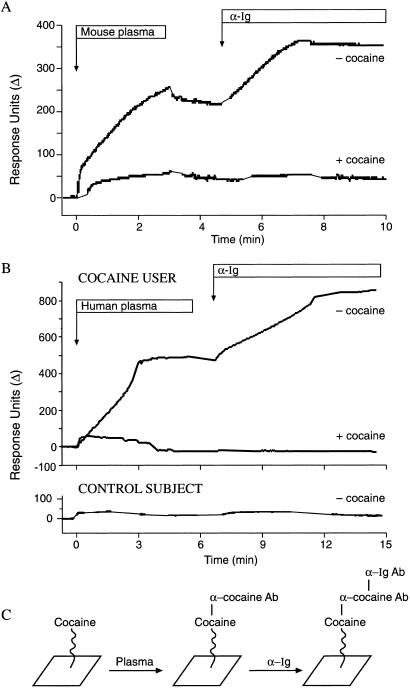
Covalent modification of proteins by small molecules can alter their antigenicity significantly (14). To evaluate the immunogenicity of endogenous proteins modified by cocaine, the activated analog of cocaine, benzoyl ecgonine N-hydroxysuccinimide ester, was administered directly to mice at biweekly intervals. This ester could react with plasma proteins (Fig. 1B) in situ and elicit antibodies that would crossreact with cocaine. Cocaine was attached through a 10-atom tether to a gold surface, and binding to it was measured by Biacore surface plasmon resonance. Plasma from injected mice (n = 3) demonstrated a new binding activity against tethered cocaine. The presence of an antibody in the binding complex was confirmed by recognition with goat anti-mouse Ig antibody. The binding of the murine antibody could be blocked by pretreating the plasma with free cocaine, thereby confirming its anti-cocaine specificity (Fig. 5 A and C). We concluded that in vivo modification of endogenous proteins by the benzoyl ecgonine moiety of cocaine could yield an effective immunogen that would elicit anti-cocaine antibodies.
Figure 5.
Detection of anti-cocaine antibodies by Biacore. Continuous flow of plasma assayed against cocaine immobilized on a tether followed by a continuous flow of species-specific goat anti-Ig antibody (α-Ig) is shown. (A) Plasma from a mouse subjected to in situ haptenization with benzoyl ecgonine N-hydroxysuccinimide ester. n = 3; illustrative results are shown. A binding signal with plasma is observed, and enhancement of the signal after the addition of goat α-Ig confirms the presence of murine anti-cocaine antibodies. (B) Plasma from a human cocaine user (Upper) and a control subject (Lower). The signal is observed only from the cocaine user, and enhancement after the addition of goat α-Ig confirms the presence of human anti-cocaine antibodies. Pretreatment of plasma with 100 μM free cocaine (+cocaine) inhibits binding to the tethered cocaine, confirming the anti-cocaine specificity of the antibodies detected in plasma. (C) Schematic of putative binding events. Cocaine tethered to the gold surface is bound by anti-cocaine antibodies (α cocaine Ab) from experimental plasma, which in turn are bound by anti-Ig antibodies (α-Ig Ab).
Based on these results, we screened plasma samples from long-term (14 ± 3 yr) cocaine users and nonuser control subjects by using our Biacore assay for anti-cocaine antibodies. Two of seven cocaine users (but none of 10 controls) showed significant plasma protein binding to tethered cocaine (Fig. 5 B and C). The presence of Ig in the binding complex was confirmed by recognition with goat anti-human Ig antibody. The anti-cocaine specificity of the human antibodies was confirmed by inhibition of binding in plasma samples pretreated with free cocaine (Fig. 5B, Lower). Covalent modification by cocaine of IgG (22) did not contribute measurably to the immune response, because antibody binding to free cocaine was reversible (data not shown).
Discussion
The results presented herein show that cocaine covalently modifies proteins. A spontaneous chemical reaction was observed in vitro and found to be restricted to the benzoyl ester group of cocaine and the ɛ-amino group of lysine. Modified proteins were demonstrated in vivo in an animal model and in 6 of 6 human users tested. Spontaneous reaction of lysine ɛ-amino groups with cocaine could be responsible for the in vivo modification, but the efficiency in vitro is low, and we cannot exclude a parallel modification of lysine or other amino acids through an enzymatic reaction. Because repetitive self-administration of cocaine is the hallmark of addiction, haptenization at even a low level of efficiency could have clinical consequences, which we observed in 2 of 7 users tested.
As an apparent consequence of covalent modification, we detected antibodies to modified proteins in human long-term users of cocaine. By analogy to various drug-induced vasculitides (23) and penicillin-induced thrombocytopenia (14), the vascular (10, 11) and blood cell abnormalities (9) noted idiosyncratically with chronic cocaine abuse may be a consequence of autoimmune phenomena initiated by covalent modification by cocaine.
Covalent modification of the constituents of blood could have diagnostic applications. Plasma or red blood cell levels of a long-lived cocaine adduct could provide a measure of past exposure and aid the management of abstinence. Such a reporter function would be analogous to the use of glycosylated hemoglobin (24) as a marker of past hyperglycemia and a guide to the management of diabetes. Alternatively, cocaine-modified blood proteins could serve as secondary markers for the modification of less accessible proteins. The use of modified plasma proteins as proxies would be similar to the use of advanced glycation end products-hemoglobin as a marker of widespread advanced glycosylation (25).
The modification of relatively few proteins in plasma was unexpected. Albumin and α2-macroglobulin are abundant, and the modification of a protein will be greater the greater its concentration or the number of accessible lysine residues and in vivo the signal will be greater the slower the turnover. Additionally, these proteins share function as pleiotropic-binding proteins (26, 27), and preconcentration of cocaine within a noncovalent binding pocket of these proteins (i.e., at a receptor) may have increased the efficiency of incorporation. Albumin is known to catalyze reactions through a lysine ɛ-amino group of atypically low pKa (28, 29), and such amino groups could function as particularly potent nucleophiles. More generally, functional groups of a protein could autocatalyze acylation by cocaine. Finally, we have noted that cocaine also labels only a few proteins in rat cerebral cortex membranes in vitro (N.B. and D.W.L., unpublished observation), and of particular interest is the possibility that enhanced covalent modification of one or more cocaine-binding neurotransmitter receptors or transporters (30–32) contributes to some of the long term neuropsychiatric effects of cocaine (3–5).
Acknowledgments
We thank J. H. Woods and R. J. Briscoe for providing plasma from cocaine-treated rats. This work was supported by the Office of National Drug Control Policy-Counterdrug Technology Assessment Center (to D.W.L.).
Abbreviation
- HSA
human serum albumin
References
- 1.Chow M J, Ambre J J, Ruo T I, Atkinson A J, Bowsher D J, Fisherman M W. Clin Pharmacol Ther. 1985;38:318–324. doi: 10.1038/clpt.1985.179. [DOI] [PubMed] [Google Scholar]
- 2.Isenschmid D S, Fischman M W, Foltin R W, Caplan Y H. J Anal Toxicol. 1992;16:311–314. doi: 10.1093/jat/16.5.311. [DOI] [PubMed] [Google Scholar]
- 3.Volkow N D, Hitzemann R, Wang G J, Fowler J S, Wolf A P, Dewey S L, Handlesman L. Synapse. 1992;11:184–190. doi: 10.1002/syn.890110303. [DOI] [PubMed] [Google Scholar]
- 4.Volkow N D, Wang G J, Fowler J S, Logan J, Gatley S J, Hitzemann R, Chen A D, Dewey S L, Pappas N. Nature (London) 1997;386:830–833. doi: 10.1038/386830a0. [DOI] [PubMed] [Google Scholar]
- 5.Karoum F, Suddath R I, Wyatt R J. Eur J Pharmacol. 1990;186:1–8. doi: 10.1016/0014-2999(90)94054-2. [DOI] [PubMed] [Google Scholar]
- 6.Kelz M B, Chen J, Carlezon W A, Whisler K, Gilden L, Beckmann A M, Steffen C, Zhang M J, Marotti L, Self D W, et al. Nature (London) 1999;401:272–276. doi: 10.1038/45790. [DOI] [PubMed] [Google Scholar]
- 7.Letchworth S R, Sexton T, Childers S R, Vrana K E, Vaughan R A, Davies H M, Porrino L J. J Neurochem. 1999;73:1982–1989. [PubMed] [Google Scholar]
- 8.Holsapple M P, Matulka R A, Stanulis E D, Jordan S D. Adv Exp Med Biol. 1993;335:121–126. doi: 10.1007/978-1-4615-2980-4_17. [DOI] [PubMed] [Google Scholar]
- 9.Burday M J, Martin S E. Am J Med. 1991;91:656–660. doi: 10.1016/0002-9343(91)90221-i. [DOI] [PubMed] [Google Scholar]
- 10.Krendel D A, Ditter S M, Frankel M R, Ross W K. Neurology. 1990;40:1092–1094. doi: 10.1212/wnl.40.7.1092. [DOI] [PubMed] [Google Scholar]
- 11.Merkel P A, Koroshetz W J, Irizarry M C, Cudkowicz M E. Semin Arthritis Rheum. 1995;25:172–183. doi: 10.1016/s0049-0172(95)80029-8. [DOI] [PubMed] [Google Scholar]
- 12.Li P, Zhao K, Deng S-X, Landry D W. Helv Chim Acta. 1999;82:85–89. [Google Scholar]
- 13.Vlassara H, Brownlee M, Manogue K R, Dinarello C A, Pasagian A. Science. 1988;240:1546–1548. doi: 10.1126/science.3259727. [DOI] [PubMed] [Google Scholar]
- 14.Murphy M F, Riordan J, Minchinton R M, Chapman J F, Amess J A, Shaw E J, Waters A J. Br J Haematol. 1983;55:155–160. doi: 10.1111/j.1365-2141.1983.tb01233.x. [DOI] [PubMed] [Google Scholar]
- 15.Means G E, Feeney R E. Biochemistry. 1968;7:2192–2201. doi: 10.1021/bi00846a023. [DOI] [PubMed] [Google Scholar]
- 16.Dixon H B F. Biochem J. 1964;92:661–666. doi: 10.1042/bj0920661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cohen J A, Osterbana R A, Berends F. Methods Enzymol. 1967;11:686–702. [Google Scholar]
- 18.Zea-Ponce Y, Baldwin R M, Laruelle M, Wang S. J Nucl Med. 1995;36:525–529. [PubMed] [Google Scholar]
- 19.Landry D W, Zhao K, Yang G X, Glickman M, Georgiadis T M. Science. 1993;259:1899–1901. doi: 10.1126/science.8456315. [DOI] [PubMed] [Google Scholar]
- 20.Henzel W J, Billeci T M, Stults J T, Wong S C, Grimley C, Wattanabe C. Proc Natl Acad Sci USA. 1993;90:5011–5015. doi: 10.1073/pnas.90.11.5011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang G, Chun J, Arakawa-Uramoto H, Wang X, Gawinowiccicz M A, Zhao K, Landry D W. J Am Chem Soc. 1996;118:5881–5890. [Google Scholar]
- 22.Wirshing P, Ashley J A, Lo C L, Janda K D, Lernaer R A. Science. 1995;270:775–782. doi: 10.1126/science.270.5243.1775. [DOI] [PubMed] [Google Scholar]
- 23.Merkel P A. Curr Opin Rheumatol. 1998;10:45–50. doi: 10.1097/00002281-199801000-00007. [DOI] [PubMed] [Google Scholar]
- 24.Bunn H F, Gabbay K H, Gallop P M. Science. 1978;200:21–27. doi: 10.1126/science.635569. [DOI] [PubMed] [Google Scholar]
- 25.Makita Z, Vlassara H, Rayfield E, Cartwright K, Friedman E, Rodby R. Science. 1992;258:651–653. doi: 10.1126/science.1411574. [DOI] [PubMed] [Google Scholar]
- 26.Weisiger R A. Proc Natl Acad Sci USA. 1985;82:1563–1567. doi: 10.1073/pnas.82.5.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Armstrong P B, Quigley J P. Dev Comp Immunol. 1999;23:375–390. doi: 10.1016/s0145-305x(99)00018-x. [DOI] [PubMed] [Google Scholar]
- 28.Hollfelder F, Kirby A J, Tawfik D S. Nature (London) 1996;383:60–62. doi: 10.1038/383060a0. [DOI] [PubMed] [Google Scholar]
- 29.Raylor R P, Vatz J B. J Am Chem Soc. 1973;95:5819–5820. doi: 10.1021/ja00798a093. [DOI] [PubMed] [Google Scholar]
- 30.Schechter M D, Meehan S M. Pharmacol Biochem Behav. 1995;51:521–523. doi: 10.1016/0091-3057(95)00046-y. [DOI] [PubMed] [Google Scholar]
- 31.Ritz M C, Lamb R J, Goldberg S R, Kuhar M J. Science. 1987;237:1219–1223. doi: 10.1126/science.2820058. [DOI] [PubMed] [Google Scholar]
- 32.Ritz M C, George F R. J Pharmacol Exp Ther. 1993;264:1333–1343. [PubMed] [Google Scholar]