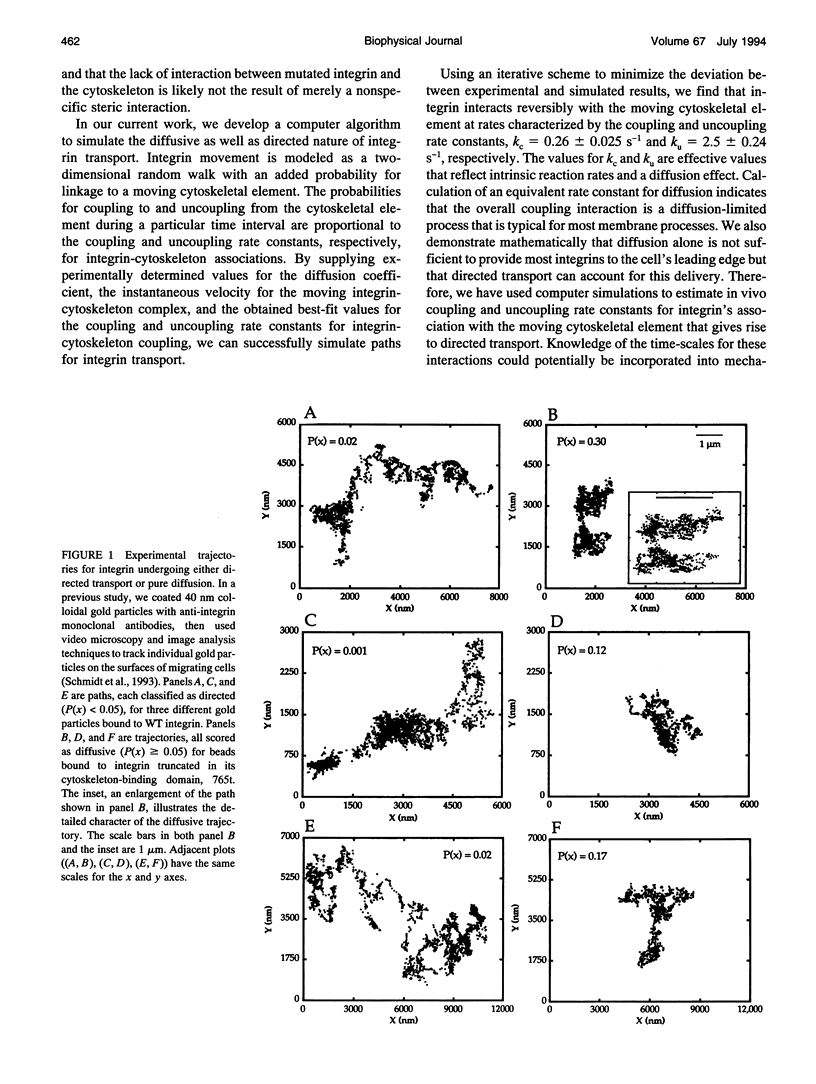
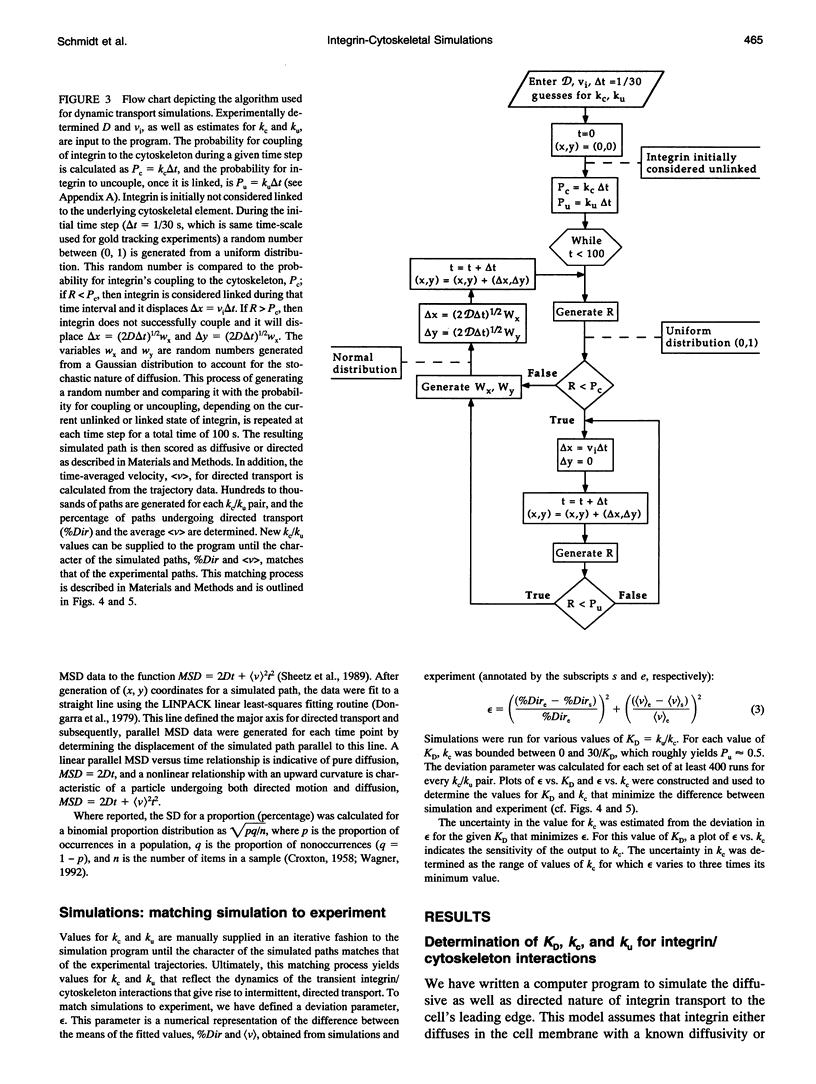
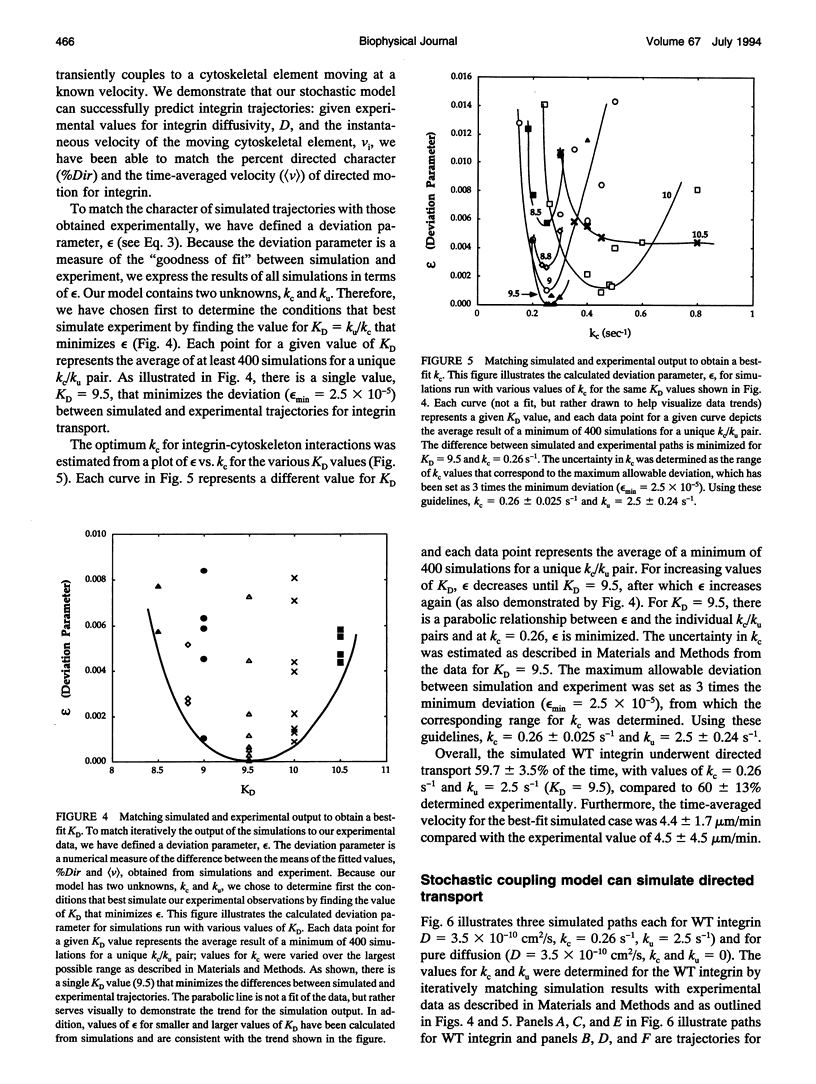
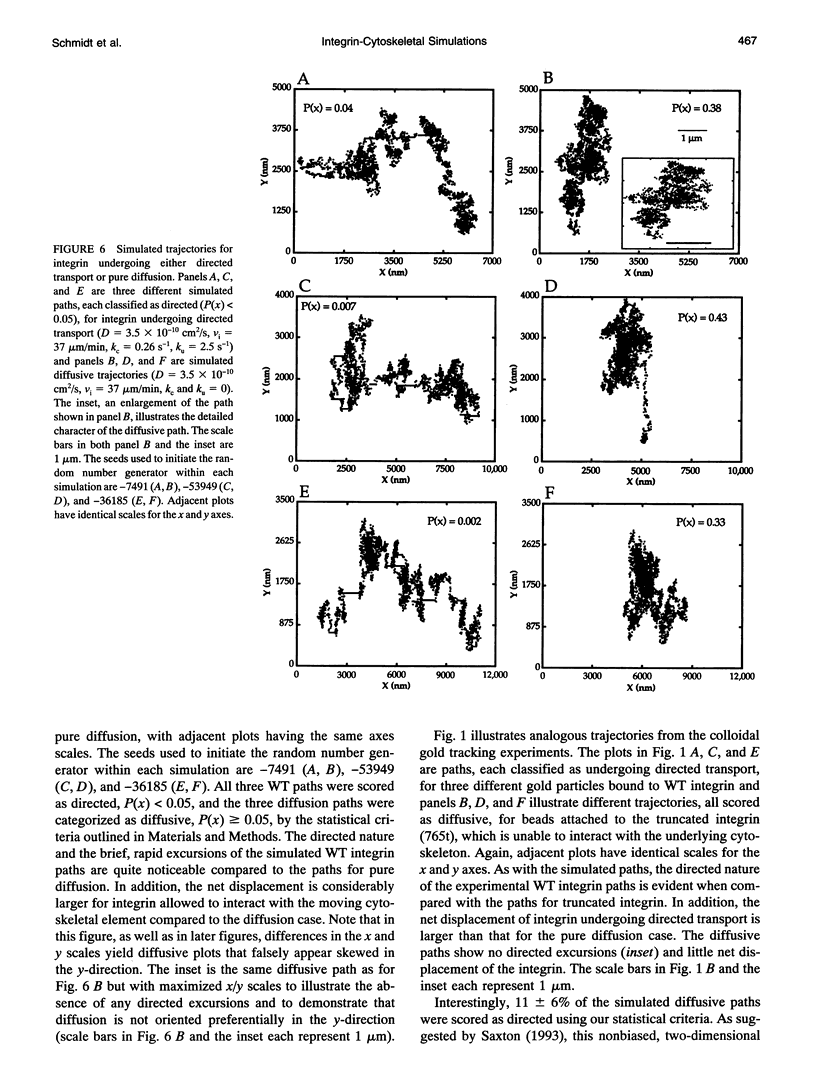
Abstract
Cell migration is a dynamic phenomenon requiring a physical interaction between the internal cell motile machinery and the external substratum in which adhesion receptors, such as integrins, serve as the transmembrane link. To analyze quantitatively this interaction, we apply a modified Brownian dynamics algorithm to simulate cytoskeleton-mediated transport of integrin on the dorsal surfaces of migrating fibroblasts. Previously, we experimentally demonstrated that integrin is transported in an intermittent fashion, with directed excursions interspersed by diffusive periods, preferentially toward the cell edge where the integrin is likely used in the formation of nascent adhesions. Integrins containing mutations in the cytoskeleton-binding region of the cytoplasmic domain display statistically different degrees of directed transport, indicating that this phenomenon is dependent on cytoskeletal associations. In the present work, we develop a computer algorithm generating simulated integrin transport trajectories, given estimates for the rate constants defining coupling (kc) and uncoupling (ku) of integrin with cytoskeletal components. Other parameters supplied to the program, the diffusion coefficient (D) for integrin in the membrane and the instantaneous velocity (vi) of the integrin/cytoskeleton complex, have been measured independently in our experimental system. By comparing the simulated trajectories with those obtained experimentally, we are able to estimate the coupling and uncoupling rate constants for the interaction of integrin with cytoskeletal elements in vivo. We find that integrin couples with cytoskeletal elements at a rate approximately 10 times slower than its rate of uncoupling (kc = 0.3 s-1, ku = 3 s-1). Comparison of these rate constants with an equivalent rate constant for diffusion, k+ = 0.4 s-1, indicates that the coupling interaction is likely a diffusion-limited process, as is typically expected for membrane processes. We further show by calculation that directed transport is necessary for integrin to traverse the length of an extending lamellipod to its leading edge; diffusion alone is not sufficiently fast to supply adhesion receptors to points of new cell/substratum contact.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams R. J., Pollard T. D. Binding of myosin I to membrane lipids. Nature. 1989 Aug 17;340(6234):565–568. doi: 10.1038/340565a0. [DOI] [PubMed] [Google Scholar]
- Akiyama S. K., Yamada K. M. The interaction of plasma fibronectin with fibroblastic cells in suspension. J Biol Chem. 1985 Apr 10;260(7):4492–4500. [PubMed] [Google Scholar]
- Berg H. C., Purcell E. M. Physics of chemoreception. Biophys J. 1977 Nov;20(2):193–219. doi: 10.1016/S0006-3495(77)85544-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher M. S. Endocytosis and recycling of the fibronectin receptor in CHO cells. EMBO J. 1989 May;8(5):1341–1348. doi: 10.1002/j.1460-2075.1989.tb03514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burridge K., Fath K., Kelly T., Nuckolls G., Turner C. Focal adhesions: transmembrane junctions between the extracellular matrix and the cytoskeleton. Annu Rev Cell Biol. 1988;4:487–525. doi: 10.1146/annurev.cb.04.110188.002415. [DOI] [PubMed] [Google Scholar]
- Cima L. G., Vacanti J. P., Vacanti C., Ingber D., Mooney D., Langer R. Tissue engineering by cell transplantation using degradable polymer substrates. J Biomech Eng. 1991 May;113(2):143–151. doi: 10.1115/1.2891228. [DOI] [PubMed] [Google Scholar]
- Dembo M., Harris A. K. Motion of particles adhering to the leading lamella of crawling cells. J Cell Biol. 1981 Nov;91(2 Pt 1):528–536. doi: 10.1083/jcb.91.2.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMilla P. A., Barbee K., Lauffenburger D. A. Mathematical model for the effects of adhesion and mechanics on cell migration speed. Biophys J. 1991 Jul;60(1):15–37. doi: 10.1016/S0006-3495(91)82027-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher G. W., Conrad P. A., DeBiasio R. L., Taylor D. L. Centripetal transport of cytoplasm, actin, and the cell surface in lamellipodia of fibroblasts. Cell Motil Cytoskeleton. 1988;11(4):235–247. doi: 10.1002/cm.970110403. [DOI] [PubMed] [Google Scholar]
- Fukui Y., Lynch T. J., Brzeska H., Korn E. D. Myosin I is located at the leading edges of locomoting Dictyostelium amoebae. Nature. 1989 Sep 28;341(6240):328–331. doi: 10.1038/341328a0. [DOI] [PubMed] [Google Scholar]
- Gelles J., Schnapp B. J., Sheetz M. P. Tracking kinesin-driven movements with nanometre-scale precision. Nature. 1988 Feb 4;331(6155):450–453. doi: 10.1038/331450a0. [DOI] [PubMed] [Google Scholar]
- Harris A., Dunn G. Centripetal transport of attached particles on both surfaces of moving fibroblasts. Exp Cell Res. 1972 Aug;73(2):519–523. doi: 10.1016/0014-4827(72)90084-5. [DOI] [PubMed] [Google Scholar]
- Heidemann S. R., Lamoureux P., Buxbaum R. E. Growth cone behavior and production of traction force. J Cell Biol. 1990 Nov;111(5 Pt 1):1949–1957. doi: 10.1083/jcb.111.5.1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz A., Duggan K., Buck C., Beckerle M. C., Burridge K. Interaction of plasma membrane fibronectin receptor with talin--a transmembrane linkage. Nature. 1986 Apr 10;320(6062):531–533. doi: 10.1038/320531a0. [DOI] [PubMed] [Google Scholar]
- Kucik D. F., Elson E. L., Sheetz M. P. Forward transport of glycoproteins on leading lamellipodia in locomoting cells. Nature. 1989 Jul 27;340(6231):315–317. doi: 10.1038/340315a0. [DOI] [PubMed] [Google Scholar]
- McCammon J. A. Computer-aided molecular design. Science. 1987 Oct 23;238(4826):486–491. doi: 10.1126/science.3310236. [DOI] [PubMed] [Google Scholar]
- Okabe S., Hirokawa N. Incorporation and turnover of biotin-labeled actin microinjected into fibroblastic cells: an immunoelectron microscopic study. J Cell Biol. 1989 Oct;109(4 Pt 1):1581–1595. doi: 10.1083/jcb.109.4.1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otey C. A., Pavalko F. M., Burridge K. An interaction between alpha-actinin and the beta 1 integrin subunit in vitro. J Cell Biol. 1990 Aug;111(2):721–729. doi: 10.1083/jcb.111.2.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regen C. M., Horwitz A. F. Dynamics of beta 1 integrin-mediated adhesive contacts in motile fibroblasts. J Cell Biol. 1992 Dec;119(5):1347–1359. doi: 10.1083/jcb.119.5.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxton M. J. Lateral diffusion in an archipelago. Single-particle diffusion. Biophys J. 1993 Jun;64(6):1766–1780. doi: 10.1016/S0006-3495(93)81548-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt C. E., Horwitz A. F., Lauffenburger D. A., Sheetz M. P. Integrin-cytoskeletal interactions in migrating fibroblasts are dynamic, asymmetric, and regulated. J Cell Biol. 1993 Nov;123(4):977–991. doi: 10.1083/jcb.123.4.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sczekan M. M., Juliano R. L. Internalization of the fibronectin receptor is a constitutive process. J Cell Physiol. 1990 Mar;142(3):574–580. doi: 10.1002/jcp.1041420317. [DOI] [PubMed] [Google Scholar]
- Sheetz M. P., Baumrind N. L., Wayne D. B., Pearlman A. L. Concentration of membrane antigens by forward transport and trapping in neuronal growth cones. Cell. 1990 Apr 20;61(2):231–241. doi: 10.1016/0092-8674(90)90804-n. [DOI] [PubMed] [Google Scholar]
- Sheetz M. P. Glycoprotein motility and dynamic domains in fluid plasma membranes. Annu Rev Biophys Biomol Struct. 1993;22:417–431. doi: 10.1146/annurev.bb.22.060193.002221. [DOI] [PubMed] [Google Scholar]
- Sheetz M. P., Turney S., Qian H., Elson E. L. Nanometre-level analysis demonstrates that lipid flow does not drive membrane glycoprotein movements. Nature. 1989 Jul 27;340(6231):284–288. doi: 10.1038/340284a0. [DOI] [PubMed] [Google Scholar]
- Singer S. J., Kupfer A. The directed migration of eukaryotic cells. Annu Rev Cell Biol. 1986;2:337–365. doi: 10.1146/annurev.cb.02.110186.002005. [DOI] [PubMed] [Google Scholar]
- Symons M. H., Mitchison T. J. Control of actin polymerization in live and permeabilized fibroblasts. J Cell Biol. 1991 Aug;114(3):503–513. doi: 10.1083/jcb.114.3.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N., Butler J. P., Ingber D. E. Mechanotransduction across the cell surface and through the cytoskeleton. Science. 1993 May 21;260(5111):1124–1127. doi: 10.1126/science.7684161. [DOI] [PubMed] [Google Scholar]
- Wang Y. L. Exchange of actin subunits at the leading edge of living fibroblasts: possible role of treadmilling. J Cell Biol. 1985 Aug;101(2):597–602. doi: 10.1083/jcb.101.2.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward M. D., Hammer D. A. A theoretical analysis for the effect of focal contact formation on cell-substrate attachment strength. Biophys J. 1993 Mar;64(3):936–959. doi: 10.1016/S0006-3495(93)81456-5. [DOI] [PMC free article] [PubMed] [Google Scholar]


