Abstract
The vitamin A metabolite, all-trans retinoic acid (atRA), plays an important role in neuronal development, including neurite outgrowth. However, the genes that lie downstream of atRA and its receptors in neuronal cells are largely unknown. By using the human neuroblastoma cell line, SH-SY5Y, we have identified an atRA-responsive gene (RAINB1: retinoic acid inducible in neuroblastoma cells) that is induced within 4 h after exposure of SH-SY5Y cells to atRA. RAINB1 mRNA is highly expressed in the nervous system (10.5- to 11-kb transcript) in both developing embryos and adults. Its expression is perturbed in developing rat embryos exposed to excess or insufficient atRA. RAINB1 is present on chromosome 11 and is spread over 38 exons, resulting in a putative ORF of 2,429 amino acids. The RAINB1 protein shows high similarity to a gene in Caenorhabditis elegans, unc-53, that is required for axonal elongation of mechanosensory neurons, suggesting that these proteins are orthologs. Thus, RAINB1 may represent a critical downstream gene in atRA-mediated neurite outgrowth.
The vitamin A metabolite, all-trans retinoic acid (atRA), plays an important role in vertebrate development. In the embryo, atRA is essential in patterning the developing nervous system and in the support of neuronal survival, migration, and differentiation (1, 2). atRA acts by binding to nuclear retinoic acid receptors (RARs), which alter gene transcription (3). However, the genes that participate in atRA-mediated changes in neuronal differentiation and adhesion/migration and the pathway of events leading to these changes are not yet known.
Human neuroblastoma is a malignant pediatric tumor of neural crest origin (4). Clonal cell lines exhibiting a neuronal phenotype (N-type), such as SH-SY5Y (5), have been used as a model system to study atRA effects. N-type cells express all three subtypes of RAR (α, β, and γ) mRNA as well as functional RAR protein (6). Treatment of SH-SY5Y cells with atRA results in the disaggregation of cells, the inhibition of cell growth, and the elongation of multiple neuritic processes on cells. Treatment of N-type cells also results in the modulation of several well-characterized atRA-responsive genes including RARβ and cellular retinoic acid-binding protein II (CRABPII; refs. 7 and 8). However, atRA regulates these genes in many cell types, which suggests that induction of these mRNAs alone cannot account for the specific atRA-induced changes in morphology that occur in SH-SY5Y cells. To identify novel genes that could be involved in neurite outgrowth, we used a cloning strategy based on subtractive hybridization. Here we report the identification of an atRA-responsive cDNA clone (RAINB1: retinoic acid inducible in neuroblastoma cells) that shares sequence similarity to a gene in Caenorhabditis elegans (unc-53) involved in axonal elongation. RAINB1 mRNA is regulated by atRA in SH-SY5Y cells, and its distribution is altered in the nervous system of rat embryos receiving either too little atRA or exposed to a teratogenic dose of atRA, indicating a role for RAINB1 in neuronal development.
Materials and Methods
Subtractive Library Construction and Screening.
Subtractive libraries were constructed as described (9) with minor modifications. atRA was analyzed for purity by HPLC (>96%; ref. 10). The clonal human neuroblastoma cell line, SH-SY5Y, was cultured as described (6). Cells were treated with 1 × 10−7 M atRA or vehicle (0.25% ethanol) for 4, 8, 16, or 24 h, and total RNA was harvested (11). CRABPII mRNA was analyzed by Northern blotting, and morphology was examined in cell cultures exposed to atRA for 4 days to confirm responsiveness to atRA (12). Poly(A)+ RNA was generated by using oligo(dT)-cellulose chromatography as described (13), except that SDS was omitted from the wash solutions. An equivalent amount of poly(A)+ RNA from each time point (0.125 μg) was pooled for the generation of double-stranded cDNA. Five rounds of amplification and subtraction were performed to search for atRA-induced genes as described (9), except that the biotinylated driver cDNA was prepared by PCR amplification in the presence of 0.15 mM biotinylated Bio-11-dUTP (Enzo Diagnostics) and 0.3 mM dTTP. Because the detection of CRABPII (an abundant mRNA) and RARβ (a low-abundance mRNA) cDNAs was maximized in the atRA-minus-vehicle sample after three subtractions, this cDNA was used for library construction. Colonies (XL1 Blue cells) containing inserts were amplified by PCR and immobilized on a positively charged nylon membrane. cDNAs that hybridized more intensely by using a 32P-labeled probe generated from the 3× or 4× atRA-minus-vehicle groups compared with membranes probed with the 3× or 4× vehicle-minus-vehicle samples were selected as putative positives. atRA responsiveness was confirmed by Northern blotting (8).
Generation of Rat Embryos.
Sprague–Dawley rats were mated and embryos were collected as described (14). One group of pregnant female rats (chow-fed) was given a teratogenic dose of atRA (50 mg/kg) or vehicle at ≈embryonic day (E) 9.4, and embryos were dissected 6 h later. Embryos were also obtained from vitamin A-deficient female rats maintained on either a 1.5 or 12 μg atRA/g diet, which enables implantation but produces embryos with varying degrees of vitamin A insufficiency (14). The control group was given a daily oral bolus dose of retinyl palmitate (500 units), which supports normal embryonic development. Embryos were fixed in 4% paraformaldehyde and used for whole-mount in situ hybridization or immunohistochemistry. In some cases, embryos were embedded in paraffin and sectioned (10–20 μm). A minimum of six embryos taken from at least three separate litters were examined for each stage/treatment.
Northern Blotting, and Whole-Mount in Situ Hybridization and Immunohistochemistry.
Poly(A)+ RNA was isolated from adult rat tissues as described above. The Quickprep micro mRNA Purification Kit (Amersham Biosciences) was used to isolate mRNA from E10.5–11.5 rat embryos for Northern blotting. For in situ hybridization studies, paraformaldehyde-fixed embryos were hybridized with digoxigenin-labeled riboprobe (15, 16) with modifications as described (14). A rat RAINB1 partial cDNA was obtained by PCR amplification from rat embryo (E12.5) RT-mRNA with primers designed from a mouse expressed sequence tag clone (GenBank accession no. AW553832; 5′-TCC CGC TGC TGC TTC TCC TTC TCT-′3 and 5′-TTC CGT CCC TGT TAA CTG CTC CTC-3′), subcloned into pGEMTeasy (Promega) and sequenced. Hoxb-1 protein was detected by using an antibody (Babco, Richmond, CA; 1:100) as described (14) in hemisected embryos fixed in 4% paraformaldehyde.
Results and Discussion
Isolation and Characterization of RAINB1 in Neuroblastoma Cells.
A partial cDNA clone (657 bp), RAINB1, which is inducible by atRA, was identified in the human neuroblastoma cell line SH-SY5Y. RAINB1 was identified by screening cDNA clones (a total of 10,000) on a Southern blot with 32P-labeled subtracted cDNA. Putative positive clones (387 total) were identified, and 80 were sequenced and compared with DNA databases. One of these putative positives was the RAINB1 clone. Sixteen additional clones represented partial cDNAs for genes identified as atRA-responsive in neuroblastoma cells including CRABPII (7, 8), RARβ (6), and cRET (17).
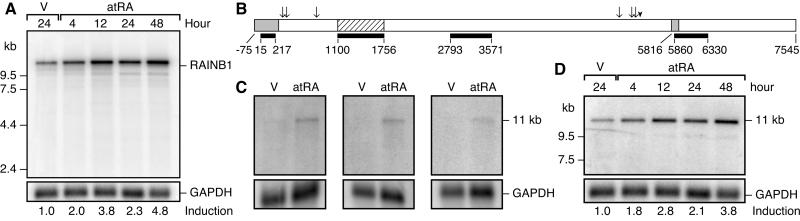
Regulation of RAINB1 by atRA in SH-SY5Y cells was confirmed by radiolabeling the 657-bp clone and probing a Northern blot. A 2-fold increase in the RAINB1 transcript was observed as early as 4 h after exposing cells to atRA, and steady-state levels of RAINB1 mRNA in the atRA-treated cells were increased 4.8-fold compared with vehicle-treated cells at 48 h (Fig. 1A). Thus, RAINB1 is induced very early after the exposure of SH-SY5Y cells to atRA and remains elevated for at least 2 days.
Figure 1.
Induction of RAINB1 by atRA. (A) Northern blot analysis of RAINB1 mRNA from SH-SY5Y cells treated with atRA (10−7 M) or 24 h with vehicle. (B) A schematic representation of the cDNA amplified from reverse transcribed mRNA and the cDNA probes used for the Northern blot analysis. The A of the ATG start site of the predicted ORF is designated nucleotide +1, and the sequence is numbered accordingly. The striped box represents the original 657-bp RAINB1 cDNA clone, and the black bars shown beneath represent the location of the cDNA probes used in Northern blotting studies. The gray boxes (the most 5′ prime region and site of 101-bp deletion starting at nucleotide 5816) and the arrowhead (site of 9-bp deletion) indicate the regions that can vary between the 12 related sequences from the patent database (AX034574 and AX009316–26). The arrows indicate the six nucleotide positions where the RAINB1 sequence differs from the AX009316 sequence. (C) mRNA from SH-SY5Y cells treated for 16 h with atRA or vehicle was probed with the original RAINB1 cDNA (nucleotides 1100–1756) (Left), the probe ranging from nucleotides 2793 to 3571 (Center), and that from nucleotides 5860 to 6330 (Right). (D) Northern blot analysis of the membrane shown in A with the probe comprising nucleotides 15–217. Hybridization with a radiolabeled glyceraldehyde-3-phosphate dehydrogenase (GAPDH) cDNA probe (nucleotides 11–1053 within the ORF of the human cDNA) was used to correct for any differences in mRNA loaded when calculating fold-induction.
Database searches with the RAINB1 cDNA revealed no known sequences with similarity in the nonredundant database (National Center for Biotechnology Information), but a 99% match was found to several related sequences in the National Center for Biotechnology Information patent database (AX034574 and AX009316–AX009326). These sequences are quite similar to one another with the exception of variability in the most 5′ and 3′ ends, and alternative forms containing 9- and/or 101-bp deletions. To determine how RAINB1 mRNA in SH-SY5Y cells might be related to these sequences, two sets of primers to the AX009316 sequence were designed (Fig. 1B). PCR analysis of atRA-treated SH-SY5Y cDNA (16 h) with these primers resulted in products with 100% identity to AX009316. Northern blot analysis of mRNA from SH-SY5Y cells (± atRA for 16 h) with these radiolabeled cDNAs as probes resulted in hybridization to a transcript identical in size (11 kb) with that recognized by the original RAINB1 cDNA (nucleotides 1,100–1,756), and all transcripts showed atRA inducibility (Fig. 1C). Analysis of the same Northern blot shown in Fig. 1A with a probe corresponding to the 5′ region of the AX009316 sequence (Fig. 1B) revealed an 11-kb transcript with induction by atRA similar to that observed with the original RAINB1 cDNA clone (compare Fig. 1 A and D).
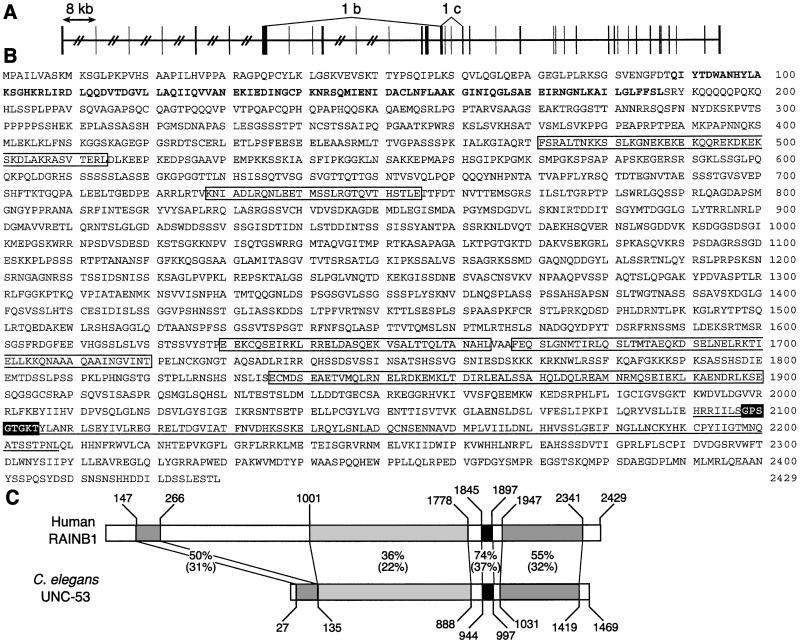
Additional primer sets were designed to the human patent database sequences and used to generate PCR products from SH-SY5Y cDNA for sequence analysis and comparison. Sequence information from at least three separate PCR products for all regions of the RAINB1 sequence was used to generate the final consensus sequence for the RAINB1 transcript in SH-SY5Y cells (GenBank accession no. AF466143). Analysis of this cDNA at the genomic level shows that it is present on chromosome 11 and is spread over 38 exons (Fig. 2A). Several putative retinoic acid response elements (DR5-type) are located 5′ to the first exon (data not shown). The PCR studies also revealed evidence for additional splice variants in SH-SY5Y, including a clone lacking nucleotides 1,291–4,376 (RAINB1b, deletion extending from midexon 8 to midexon 16), a variant lacking exons 17 and 18 (RAINB1c; Fig. 2A), and a clone containing an additional 9 bp inserted after nucleotide 5,325 (RAINB1d; GenBank accession no. AF466144). Additional studies will be needed to fully define the number of RAINB1 mRNA variants that exist in these cells.
Figure 2.
Genomic organization, protein sequence, and schematic comparison of RAINB1 to C. elegans UNC-53. (A) The genomic structure of the 38 exons of the RAINB1 mRNA. The vertical boxes represent the exons, the horizontal lines represent the introns, and the hatch marks indicate introns that are greater than 8 kb. The regions encompassed by 1b and 1c are deleted in alternatively spliced transcripts identified by PCR. (B) The amino acid sequence of the RAINB1 putative ORF. The bold sequence has similarity to the calponin domain, and the boxed sequences represent putative coiled–coil domains. The white lettering with black background is a sequence motif for a P-loop ATP/GTP binding site that is contained within the underlined AAA-domain ATPase. (C) A schematic alignment of the putative RAINB1 and C. elegans unc-53 gene products. The percent similarity and identity (in parentheses) between the amino acid sequences is shown for regions indicated by shaded boxes.
The largest predicted ORF for the RAINB1 cDNA encodes for a protein of 261 kDa (Fig. 2B). Database analysis (National Center for Biotechnology Information-Conserved Domain search) of the coding region revealed a region of 99 aa at the N-terminal end of RAINB1 with high similarity to a calponin homology or actin-binding domain. A second 118-aa region in the C-terminal end of the protein showed similarity to a domain found in proteins with cellular ATPase activity (AAA-domain), and a P-loop representing an ATP/GTP-binding site motif was found embedded in this domain (scanProsite). Regions within the protein also showed high similarity to translated expressed sequence tags from rat, zebrafish, and Xenopus, and a genomic clone of Drosophila melanogaster (data not shown) in addition to the C. elegans gene unc-53 (Fig. 2C). Particularly striking was the similarity between the C-terminal end of RAINB1 and UNC-53. In C. elegans the unc-53 gene plays an essential role in the longitudinal growth of neurons involved in mechanosensation, and several mutant alleles with varying severity have been examined (18). Whole neuronal process bundles are misguided, suggesting that pioneering neurons are affected by this mutation. The fact that atRA induces RAINB1 mRNA and causes neurite elongation in SH-SY5Y cells suggests that RAINB1 is an ortholog of UNC-53. The regulation of RAINB1 in humans must, however, differ from that of unc-53 in C. elegans, because the latter do not express RARs. That humans and nematodes could express a gene product with similar function yet regulate that gene in different ways is not surprising. For example, both D. melanogaster and humans express Hox genes (referred to as the HOM-C complex in D. melanogaster), but only in vertebrates are several of these genes regulated by atRA and its receptors (19, 20).
RAINB1 Tissue Expression.
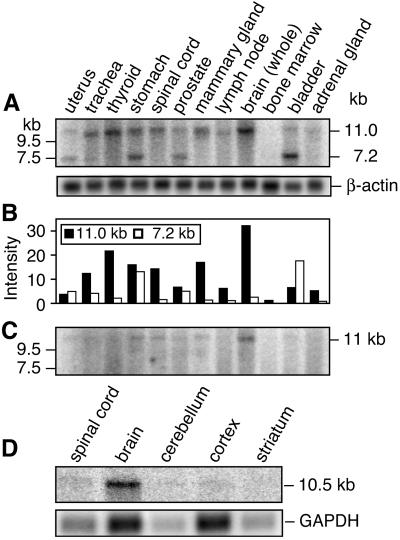
A multiple tissue expression array (CLONTECH) containing 76 different human mRNAs was analyzed by using the original 657-bp RAINB1 cDNA clone. Human fetal brain and heart, and adult cerebellum, bladder, aorta, jejunum, and interventricular septum showed the highest level of RAINB1 mRNA (data not shown). A human multiple-tissue Northern blot showed the highest expression of a single 11-kb RAINB1 transcript in brain (Fig. 3A), followed by thyroid, mammary gland, and spinal cord. In uterus, stomach, prostate, and bladder both 11-kb and 7.2-kb transcripts were observed. When the same Northern blot was hybridized with a probe specific to the 5′ region (nucleotides 15–217; see Fig. 1B) of RAINB1, only the 11-kb transcript was detected (Fig. 3C).
Figure 3.
RAINB1 expression in normal adult human and rat tissues. (A) Northern blot analysis of RAINB1 mRNA in adult human tissues. The original 657-bp RAINB1 cDNA clone was radiolabeled and used as a probe. (B) The relative amounts of the 11-kb (filled bars) and the 7.2-kb (empty bars) transcripts in adult human tissues from A, as determined by analysis with a PhosphorImaging system (Molecular Dynamics). (C) Hybridization of the 5′ end of the RAINB1 cDNA (nucleotides 15–17) to the human multiple tissue blot. (D) Expression of RAINB1 in rat neural tissue with a rat partial cDNA as a probe. The cDNA clone (474 bp; GenBank accession no. AF466145) was obtained by PCR amplification of reverse-transcribed RNA from E10.5 rat embryos with primers to regions that are similar between the human cDNA and a partial mouse sequence available in the expressed sequence tag database (AW553832). The rat cDNA clone shows 83% (320/385 bp) sequence identity when compared with the human RAINB1 and 93% (400/430) when compared with the mouse expressed sequence tag in this region (data not shown).
mRNA was isolated from adult rat tissues and examined by Northern blotting (Fig. 3D). A single 10.5-kb transcript was expressed in whole brain, and fainter signals were detected in the cerebellum, cortex, and spinal cord samples. Transcript was not detectable above background in other tissues examined (liver, kidney, heart, lung, duodenum, and olfactory bulb; data not shown). Thus, in both the rat and human, RAINB1 mRNA is expressed prominently in the brain with lesser amounts in the spinal cord. In addition, only the larger transcript of 10.5–11 kb is detected in the nervous system of both species.
Expression of RAINB1 in Rat Embryos.
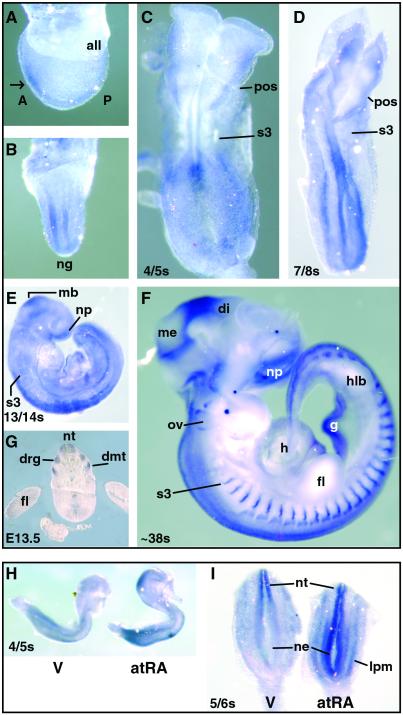
The normal distribution of RAINB1 mRNA was studied in developing rat embryos both by Northern blotting and whole-mount in situ hybridization. By Northern analysis, a single 10.5-kb transcript was detected in mRNA (2 μg) isolated from normal, vitamin A-sufficient embryos at E10.5 and E11.5 (data not shown). Whole-mount in situ hybridization studies conducted on embryos ranging from E9.25 (late gastrula stage) through E13.5 revealed a dynamic RAINB1 expression pattern (Fig. 4 Upper). RAINB1 mRNA was first detected in the developing nervous system at E9.25 (presomite; Fig. 4 A and B). By the 4/5-somite stage, RAINB1 expression was localized to a small region of neuroectoderm with an anterior limit of approximately the third somite and extending caudally to the location of the primitive node (Fig. 4C). By E9.75 (7/8 somite stage), expression in the neuroectoderm had expanded caudally (Fig. 4D), and RAINB1 was also prominently expressed in the lateral plate mesoderm (data from sections not shown). Embryo sections revealed that, adjacent and caudal to somite 3, transcript was expressed throughout the closed neural tube and open neuroepithelium (data not shown).
Figure 4.
Whole-mount in situ hybridization of RAINB1 in normal rat embryos (E9.25–13.5; Upper) and alteration of RAINB1 expression in atRA-treated embryos (Lower). (Upper) Lateral (A) and frontal (B) views of a normal vitamin A-sufficient E9.25 (presomite) embryo. RAINB1 staining is observed most prominently in the anterior region of the embryo (white arrow) concentrated in the neural folds lateral to the neural groove. Dorsal view of a 4/5 somite embryo (C). Expression is seen in the neural tube and the lateral plate mesoderm, with staining most prominent in regions associated with newly formed and forming somites. Dorsal view of a 7/8 somite embryo (D). Staining is most intense in the midbrain, lateral plate mesoderm, and the entire neural tube and neuroepithelium caudal to somite 3. Lateral view of 13/14 somite embryo (E). Expression is seen in the midbrain, nasal placode, neural tube caudal to somite 3, and gut. Lateral view of an E12.5 embryo (F) and transverse section of a E13.5 embryo (G). RAINB1 mRNA is found associated with the nasal placode, at the divisions between the mesencephalon/diencephalon, and midbrain/hindbrain, neural tube, dorsal root ganglia, dorsal aspect of the otic vesicle, gut, and dermamyotome. (Lower) Lateral (H) and (I) dorsal views of early embryos exposed to excess atRA compared with vehicle-exposed embryos. Both more intense and ectopic expression of RAINB1 mRNA occurred in the embryos exposed to a teratogenic dose of atRA. Abbreviations: A, anterior; all, allantois; di, diencephalon; dmt, dermamyotome; drg, dorsal root ganglia; fl, forelimb; g, gut; h, heart; hlb, hindlimb bud; lpm, lateral plate mesoderm; mb, midbrain; me, mesencephalon; ne, neuroepithelium; ng, neural groove; np, nasal placode; nt, neural tube; ov, otic vesicle; P, posterior; pos, preotic sulcus; s3, somite 3; V, vehicle.
Expression of RAINB1 mRNA in the midbrain region was evident both at the 7/8 and 13/14 somite stages (Fig. 4 D and E). Specific RAINB1 staining was also observed in what seemed to be neural crest cells migrating from the midbrain neural fold/prorhombomere A region and in the nasal process at E10.5 (Fig. 4E). RAINB1 mRNA continued to be expressed in the dorsal neural epithelium, but by the 20/21 somite stage (E11) expression in the ventral neural tube could no longer be detected. At this time RAINB1 expression was also seen in the dorsal somitic tissue extending caudally from approximately somite 3 and became more punctate at the midline of the somite as development proceeded (data not shown).
By E12.5 and E13.5, a broad band of RAINB1 staining was present at the mesencephalon/diencephalon border, and prominent staining was noted at the metencephalon/mesencephalon (midbrain/hindbrain) border (Fig. 4F). In adult rat brain tissues analyzed by Northern blotting, the cerebellum, which has its embryonic origin in the metencephalon, showed RAINB1 expression (approximately one-half the amount present in whole brain when corrected for loading differences). At this stage of development, RAINB1 staining was also present in the developing hindbrain, dorsal root ganglia (DRG), dorsal aspect of the otic vesicle, and dorsal neural tube with expression extending caudally to the tailbud (Fig. 4 F and G). In addition, prominent expression was observed in the developing gut and as sharp bands in the dermamyotome beginning with the third somite and all those more posterior.
An important question that needs to be addressed is how much of the embryonic expression of RAINB1 is under the regulation of atRA. Retinoic acid synthesis is initiated in the mid- to late-gastrula stage (21). Tissue adjacent to the node (22) and that associated with undifferentiated somites and mesenchyme surrounding the neural tube are sites of early atRA synthesis by the action of retinaldehyde dehydrogenase type 2 (RALDH2) (23, 24). Like RALDH2, RAINB1 mRNA is found in regions associated with the most recently formed somites in early embryos. A second atRA biosynthetic enzyme (RALDH1) has been detected in the ventral mesencephalon of the early mouse embryo, corresponding in time of appearance with RAINB1 expression in the midbrain (24). RALDH (type 3) has been reported in the ventral facial region and olfactory pit in mouse embryos at the same time that RAINB1 mRNA is observed in the rat embryo in the present studies (25, 26). In older embryos, RALDH2 is found in the developing gut (23), and RALDH1 and RALDH3 are present in the otic vesicle (24, 26), both sites of RAINB1 mRNA expression. Thus, atRA and its biosynthetic enzymes are present at times and in places where RAINB1 mRNA expression is observed, supporting the hypothesis that atRA could be involved in RAINB1 regulation.
Ectopic expression of mRNA in response to a teratogenic dose of atRA has been observed for genes that are under the direct regulation of atRA and its receptors (27, 28). For this reason, the expression of RAINB1 mRNA was examined in early-somite-stage rat embryos exposed to excess atRA. Embryos taken 6 h after the dams received a teratogenic dose of atRA showed an enhanced expression of RAINB1 in both the midbrain and tailbud when compared with the vehicle control embryos (Fig. 4H). Ectopic RAINB1 expression was observed throughout the entire caudal neural tube and expression was dramatically increased in the lateral plate mesoderm in embryos exposed to excess atRA (Fig. 4I). Thus, some early aspects of RAINB1 expression seem to be under the regulation of atRA.
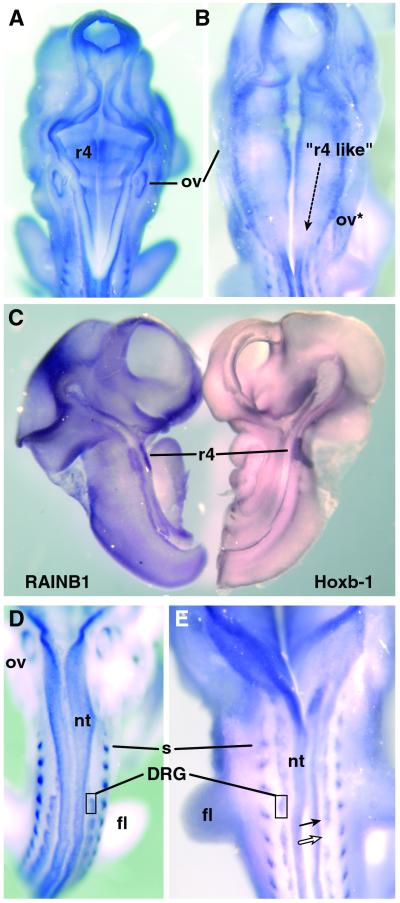
Because the development of the posterior hindbrain is particularly sensitive to a deficiency of vitamin A (14), expression of RAINB1 in this region of the embryo was examined more closely in both vitamin A-sufficient and atRA-insufficient embryos. The developing hindbrain of a normal rat embryo at E12.5 is segmented into seven morphologically distinct segments called rhombomeres. Rhombomeres develop as lineage-specific compartments giving rise to central nervous system neurons and neural crest cells, which form both neuronal and glial portions of the peripheral cranial ganglia, anterior ventral skull bones, and other connective tissues in the head and neck. In vitamin A deficiency, rhombomere 3 is enlarged, whereas the remainder of the posterior hindbrain is shortened, lacks segmentation (caudal to r4), and expresses mRNAs characteristic of more anterior rhombomeric compartments (14). Examination of the hindbrain of vitamin A-sufficient embryos at E12.5 showed normal segmentation with light but reproducible RAINB1 expression at the boundaries of rhombomeres 3/4, 4/5, 5/6, and 6/7. RAINB1 mRNA was also detected in the ventral/medial aspect of the hindbrain neuroepithelium, with particularly prominent expression in rhombomere 4 (Fig. 5 A and C). In contrast, embryos from mothers receiving insufficient atRA lacked morphological rhombomere borders and its associated RAINB1 staining caudal to rhombomere 3. Furthermore, the ventral/medial expression of RAINB1 mRNA in rhombomere 4 was expanded caudally (Fig. 5B). A caudal expansion of the atRA regulated Hoxb-1 protein has been reported in the hindbrain of atRA-insufficient embryos (14), whereas in normal embryos this protein is restricted to rhombomere 4 (Fig. 5C). Thus, RAINB1 could play a role in vitamin A-mediated segmentation of the posterior hindbrain or in maintaining cell compartmentalization within individual rhombomeres.
Figure 5.
Expression of RAINB1 in normal and atRA-insufficient embryos at E12.5. RAINB1 mRNA in the hindbrain of a normal vitamin A-sufficient embryo (A) compared with that in an atRA-insufficient embryo (B, maternal intake of 1.5 μg atRA/g diet). Vitamin A-deficient dams given 500 international units of retinyl palmitate daily by oral gavage yielded embryos exhibiting RAINB1 expression indistinguishable from that observed in embryos from chow-fed dams (data not shown). The location of RAINB1 staining in rhombomere 4 was confirmed by colocalization of Hoxb-1 in rhombomere 4 in a hemisected normal embryo (C). The expression of RAINB1 in the DRG, dermamyotome and neural tube of an E12.5 vitamin A-sufficient embryo (D) compared with one deficient in atRA (E, maternal intake of 1.5 μg atRA/g diet). The filled arrow indicates what appears to be fused DRG next to a region completely devoid of stain (open arrow). Abbreviations: fl, forelimb; nt, neural tube; ov, otic vesicle; ov*, ectopic otic vesicle; r4, rhombomere 4; s, somite.
In addition to the vitamin A deficiency-induced alteration in RAINB1 mRNA expression in the hindbrain, embryos from the group receiving the lowest amount of atRA (1.5 μg/g diet) exhibited fainter expression of RAINB1 in the DRG (Fig. 5E) than in vitamin A-sufficient embryos (Fig. 5D). The DRG neurons originate from the neural crest, which is also the precursor of SH-SY5Y cells from which RAINB1 was cloned. The cell bodies of these neurons develop to form ganglia adjacent to the neural tube in register with and under the influence of the somites. They are bipolar sensory neurons that extend their axons into the periphery as well as into the dorsal spinal cord, another site of RAINB1 expression (Fig. 4G). The ganglia in the 1.5 μg atRA/g diet group were irregular in pattern and in some cases seemed to be absent altogether (Fig. 5E), showing that vitamin A is essential for their proper formation. Retinoic acid has been implicated in the development of the DRG, because these cells do not differentiate properly in vitamin A-deficient quail and fail to extend neurites in culture (29). Whether RAINB1 plays an essential role in the genesis of the ganglia remains to be established. It is intriguing, however, that mutating the RAINB1 homolog in C. elegans (unc-53) results in a defect in mechanosensory neurons (18).
In conclusion, we have identified an atRA regulated mRNA (RAINB1) in the human neuroblastoma cell line SH-SY5Y. It is highly expressed in the nervous system in both the human and rat. In the rat, RAINB1 is developmentally regulated, its expression in early embryos is perturbed by excess atRA, and its expression in both the developing hindbrain and DRG is altered by retinoid insufficiency. RAINB1 is a homolog to the C. elegans gene unc-53 that is involved in axonal elongation. Thus, atRA may play a role in regulating neuronal development by regulating the expression of RAINB1.
Acknowledgments
This work was supported by grants from the U.S. Department of Agriculture CSREES (Project WISO4305) and National Research Initiative Competitive Grants Program (Project 9900802).
Abbreviations
- atRA
all-trans retinoic acid
- RAR
retinoic acid receptor
- CRABPII
cellular retinoic acid-binding protein II
- En
embryonic day n
- DRG
dorsal root ganglia
Footnotes
References
- 1.Gavalas A, Krumlauf R. Curr Opin Genet Dev. 2000;10:380–386. doi: 10.1016/s0959-437x(00)00100-3. [DOI] [PubMed] [Google Scholar]
- 2.McCaffery P, Dräger U C. Cytokine Growth Factor Rev. 2000;11:233–249. doi: 10.1016/s1359-6101(00)00002-2. [DOI] [PubMed] [Google Scholar]
- 3.Chambon P. FASEB J. 1996;10:940–954. [PubMed] [Google Scholar]
- 4.Alexander F. Pediatr Urol Oncol. 2000;27:383–392. [Google Scholar]
- 5.Biedler J L, Roffler-Tarlov S, Schachner M, Freedman L S. Cancer Res. 1978;38:3751–3757. [PubMed] [Google Scholar]
- 6.Clagett-Dame M, Verhalen T J, Biedler J L, Repa J J. Arch Biochem Biophys. 1993;300:684–693. doi: 10.1006/abbi.1993.1095. [DOI] [PubMed] [Google Scholar]
- 7.Redfern C P F, Lovat P E, Malcolm A J, Pearson A D J. Biochem J. 1994;304:147–154. doi: 10.1042/bj3040147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Plum L A, Clagett-Dame M. Arch Biochem Biophys. 1995;319:457–463. doi: 10.1006/abbi.1995.1317. [DOI] [PubMed] [Google Scholar]
- 9.Bouillet P, Oulad-Abdelghani M, Vicaire S, Garnier J-M, Schuhbaur B, Dollé P, Chambon P. Dev Biol. 1995;170:420–433. doi: 10.1006/dbio.1995.1226. [DOI] [PubMed] [Google Scholar]
- 10.Motto M G, Facchine K I, Hamburg P F, Burinsky D J, Dunphy R, Oyler A R, Cotter M L. J Chromatogr. 1989;481:255–262. [Google Scholar]
- 11.Chomczynski P, Sacchi N. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 12.Påhlman S, Ruusala A-I, Abrahamsson L, Mattsson M E K, Esscher T. Cell Differ. 1984;14:135–144. doi: 10.1016/0045-6039(84)90038-1. [DOI] [PubMed] [Google Scholar]
- 13.Sambrook J, Fritsch E F, Maniatis T. Molecular Cloning: A Laboratory Manual. 2nd Ed. Plainview, NY: Cold Spring Harbor Lab. Press; 1989. pp. 7.26–7.29. [Google Scholar]
- 14.White J C, Highland M, Kaiser M, Clagett-Dame M. Dev Biol. 2000;220:263–284. doi: 10.1006/dbio.2000.9635. [DOI] [PubMed] [Google Scholar]
- 15.Xu Q, Wilkinson D G. In: In Situ Hybridization: A Practical Approach. Wilkinson D G, editor. London: Oxford Univ. Press; 1998. pp. 87–106. [Google Scholar]
- 16.Stern C D. In: Cellular and Molecular Procedures in Developmental Biology. de Pablo F, Ferrús A, Stern C D, editors. San Diego: Academic; 1998. pp. 223–243. [Google Scholar]
- 17.Tahira T, Ishizaka Y, Itoh F, Nakayasu M, Sugimura T, Nagao M. Oncogene. 1991;6:2333–2338. [PubMed] [Google Scholar]
- 18.Hekimi S, Kershaw D. J Neurosci. 1993;13:4254–4271. doi: 10.1523/JNEUROSCI.13-10-04254.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maconochie M, Nonchev S, Morrison A, Krumlauf R. Annu Rev Genet. 1996;30:529–556. doi: 10.1146/annurev.genet.30.1.529. [DOI] [PubMed] [Google Scholar]
- 20.Veraksa A, Del Campo M, McGinnis W. Mol Gen Metab. 2000;69:85–100. doi: 10.1006/mgme.2000.2963. [DOI] [PubMed] [Google Scholar]
- 21.Ulven S M, Gundersen T E, Weedon M S, Landaas V Ø, Sakhi A K, Sakhi A K, Fromm S H, Geronimo B A, Moskaug J O, Blomhoff R. Dev Biol. 2000;220:379–391. doi: 10.1006/dbio.2000.9634. [DOI] [PubMed] [Google Scholar]
- 22.Hogan B L M, Thaller C, Eichele G. Nature (London) 1992;359:237–241. doi: 10.1038/359237a0. [DOI] [PubMed] [Google Scholar]
- 23.Niederreither K, McCaffery P, Dräger U C, Chambon P, Dollé P. Mech Dev. 1997;62:67–78. doi: 10.1016/s0925-4773(96)00653-3. [DOI] [PubMed] [Google Scholar]
- 24.Haselbeck R J, Hoffmann I, Duester G. Dev Genet. 1999;25:353–364. doi: 10.1002/(SICI)1520-6408(1999)25:4<353::AID-DVG9>3.0.CO;2-G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suzuki R, Shintani T, Sakuta H, Kato A, Ohkawara T, Osumi N, Noda M. Mech Dev. 2000;98:37–50. doi: 10.1016/s0925-4773(00)00450-0. [DOI] [PubMed] [Google Scholar]
- 26.Mic F A, Molotkov A, Fan X, Cuenca A E, Duester G. Mech Dev. 2000;97:227–230. doi: 10.1016/s0925-4773(00)00434-2. [DOI] [PubMed] [Google Scholar]
- 27.Morriss-Kay G M, Murphy P, Hill R E, Davidson D R. EMBO J. 1991;10:2985–2995. doi: 10.1002/j.1460-2075.1991.tb07849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marshall H, Nonchev S, Sham M H, Muchamore I, Lumsden A, Krumlauf R. Nature (London) 1992;360:737–741. doi: 10.1038/360737a0. [DOI] [PubMed] [Google Scholar]
- 29.Maden M, Gale E, Kostetskii I, Zile M. Curr Biol. 1996;6:417–426. doi: 10.1016/s0960-9822(02)00509-2. [DOI] [PubMed] [Google Scholar]