Abstract
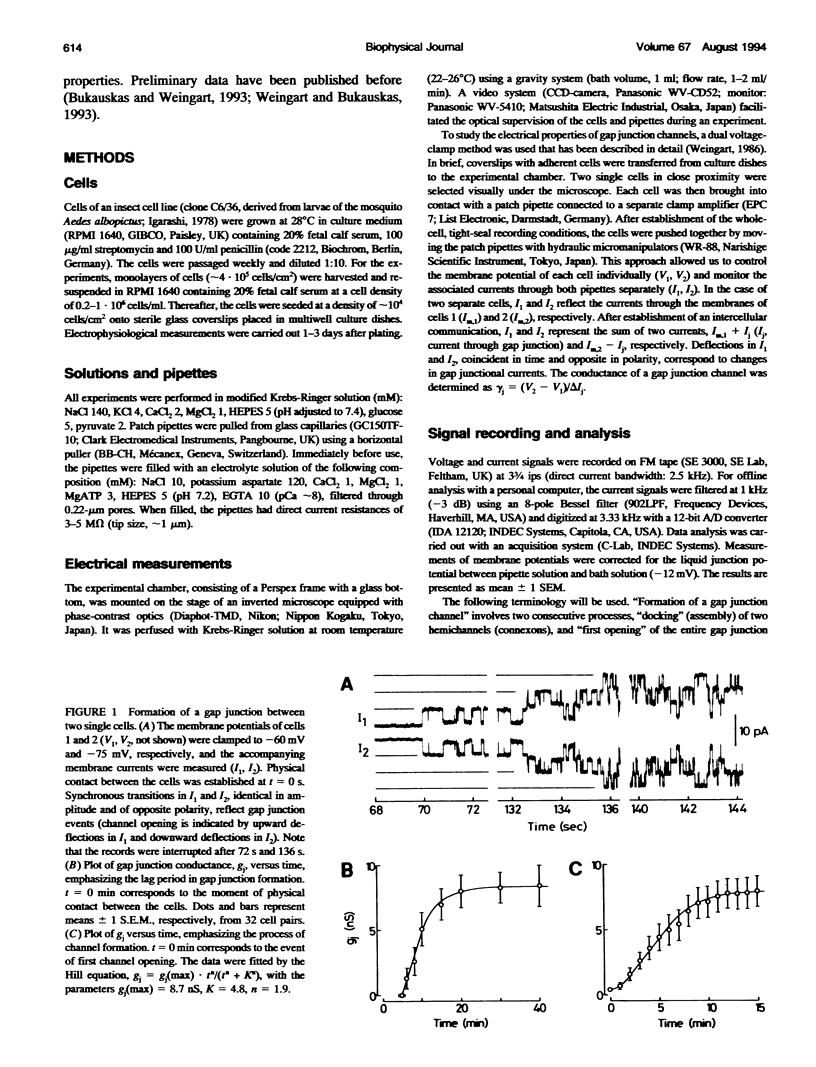
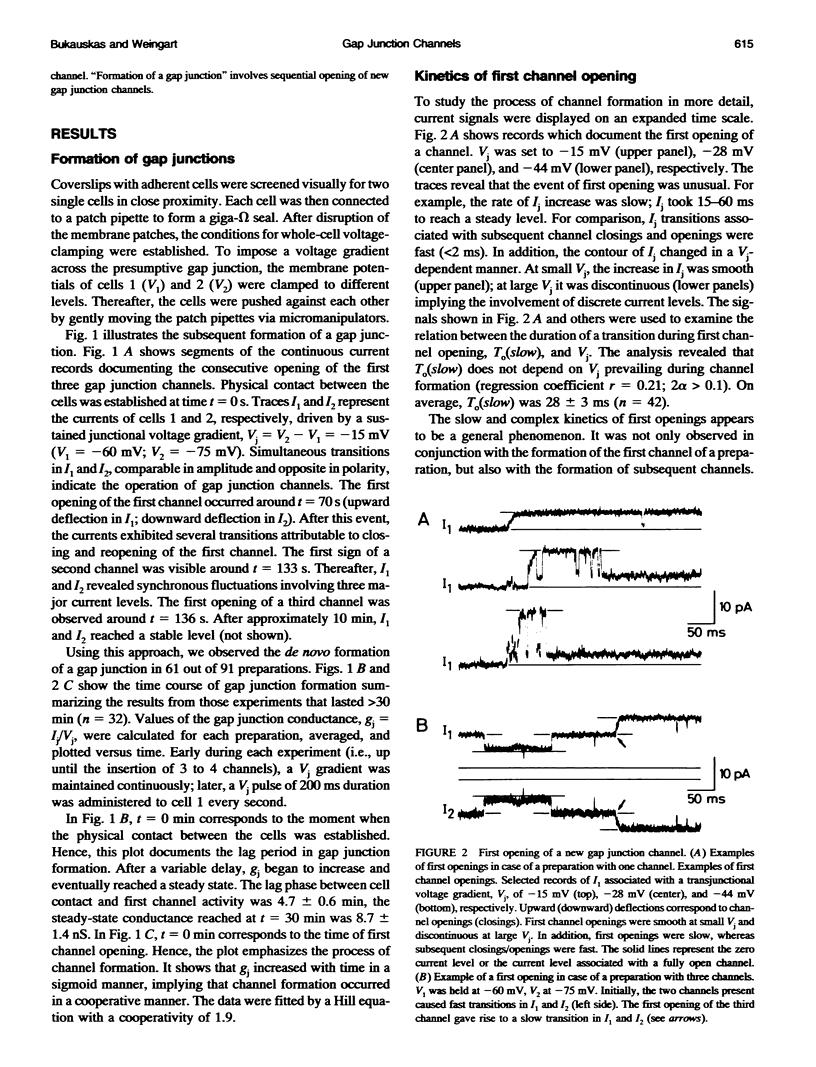
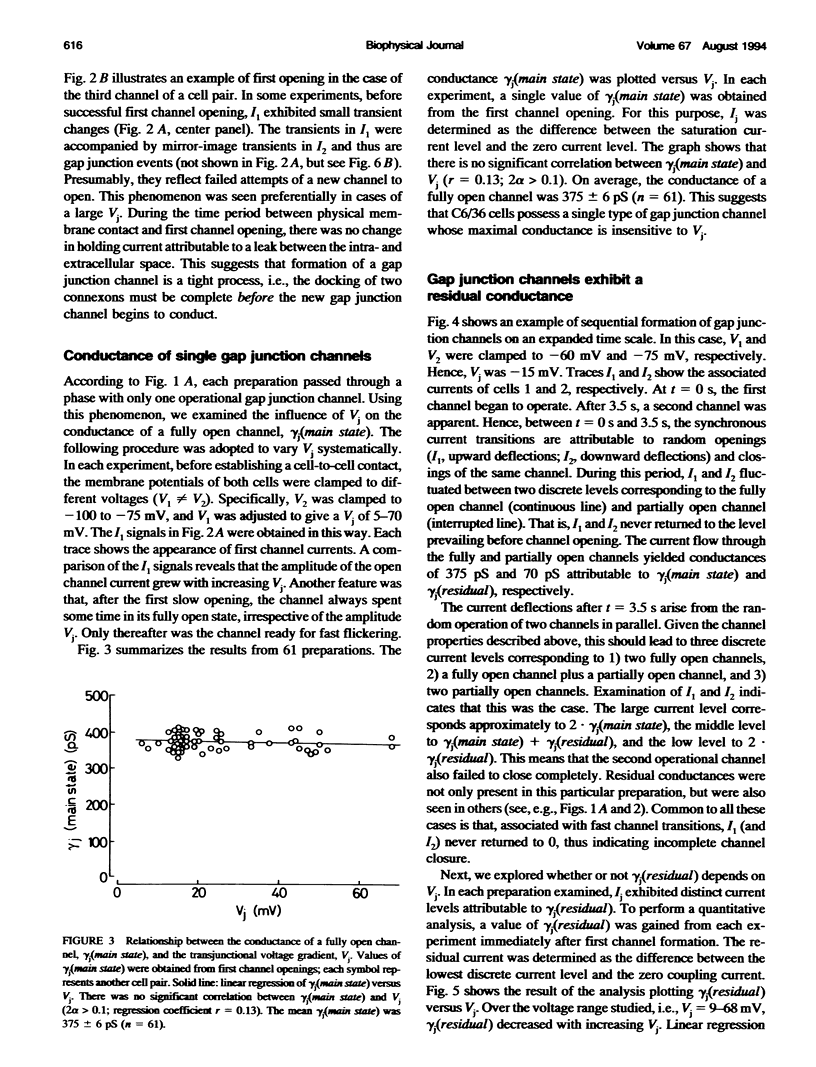
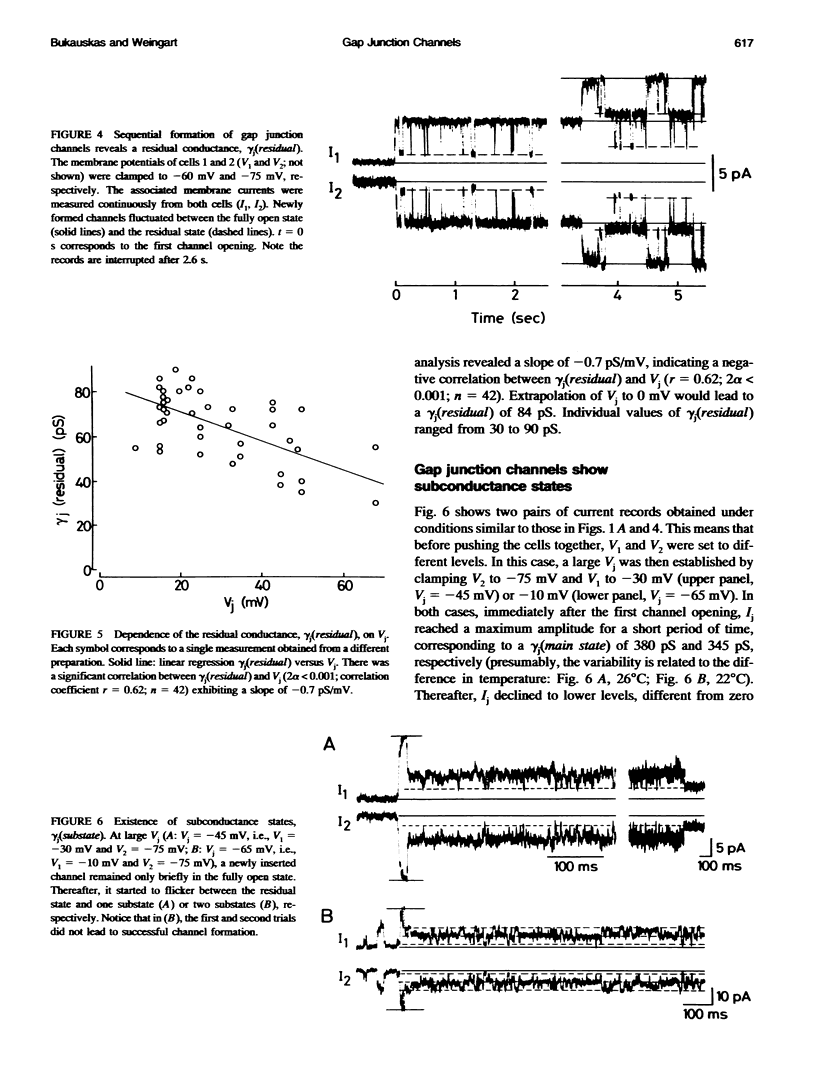
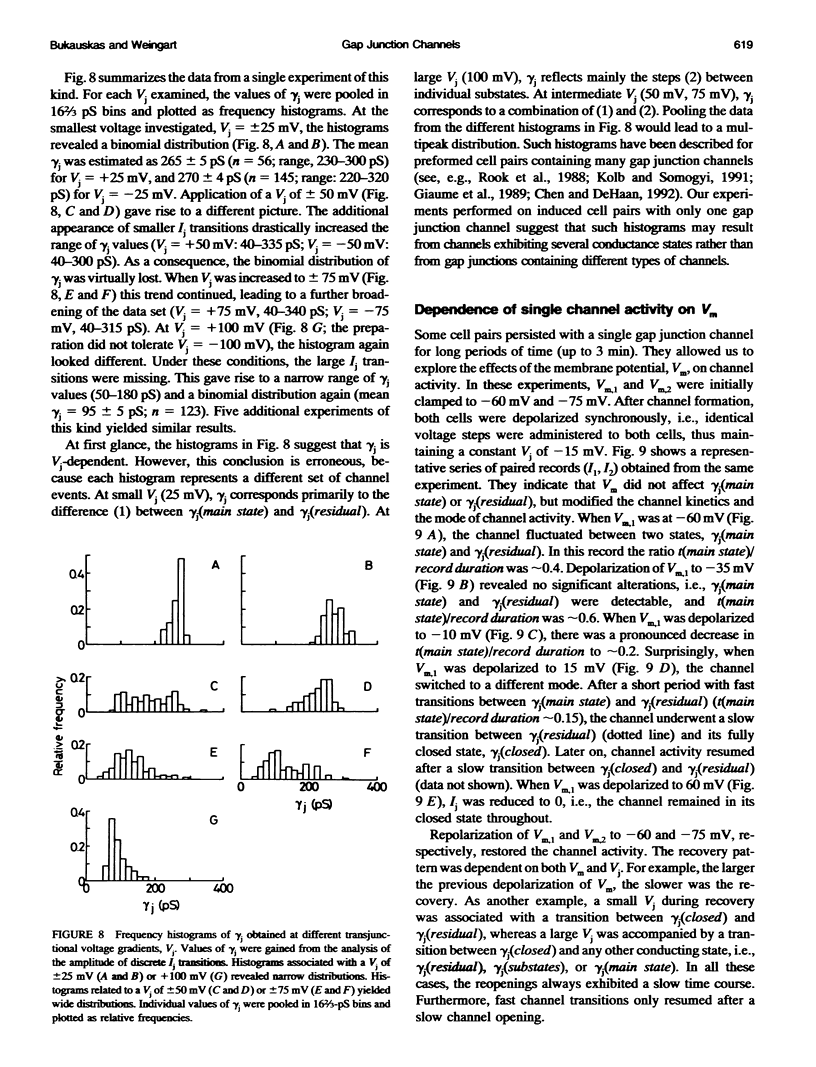
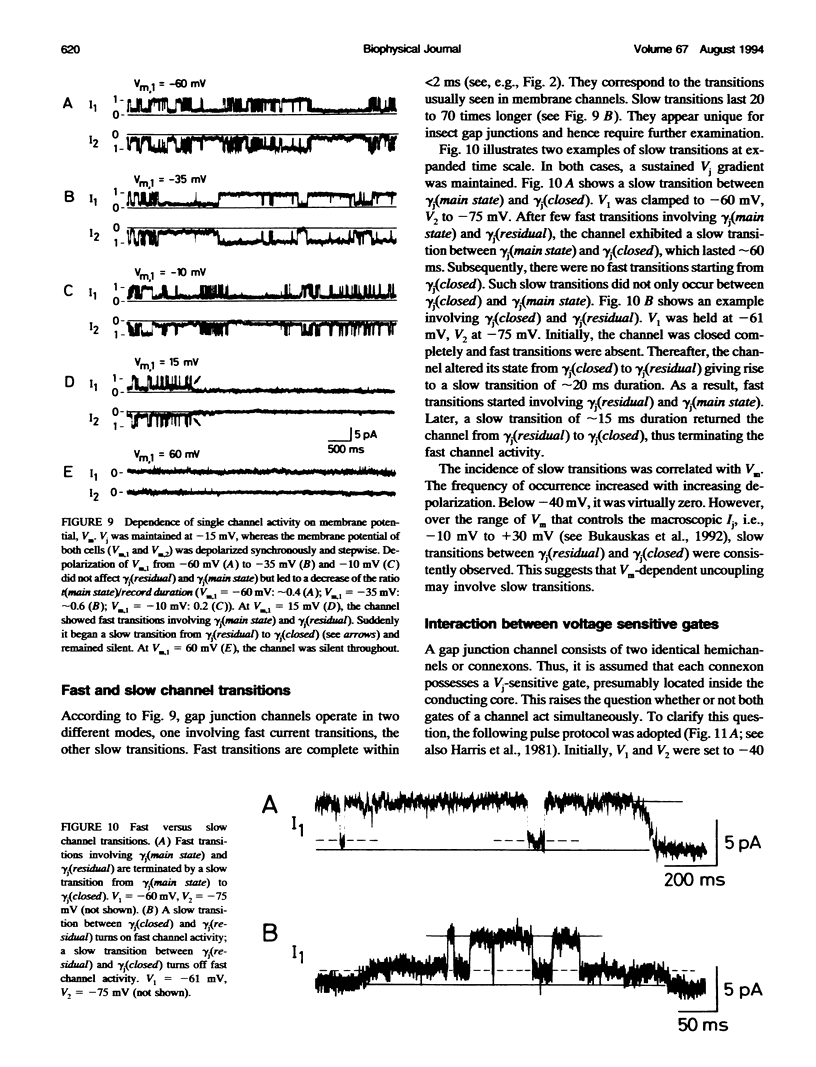
De novo formation of cell pairs was used to examine the gating properties of single gap junction channels. Two separate cells of an insect cell line (clone C6/36, derived from the mosquito Aedes albopictus) were pushed against each other to provoke formation of gap junction channels. A dual voltage-clamp method was used to control the voltage gradient between the cells (Vj) and measure the intercellular current (Ij). The first sign of channel activity was apparent 4.7 min after cell contact. Steady-state coupling reached after 30 min revealed a conductance of 8.7 nS. Channel formation involved no leak between the intra- and extracellular space. The first opening of a newly formed channel was slow (25-28 ms). Each preparation passed through a phase with only one operational gap junction channel. This period was exploited to examine the single channel properties. We found that single channels exhibit several conductance states with different conductances gamma j; a fully open state (gamma j(main state)), several substates (gamma j(substates)), a residual state (gamma j(residual)) and a closed state (gamma j(closed)). The gamma j(main state) was 375 pS, and gamma j(residual) ranged from 30 to 90 pS. The transitions between adjacent substates were 1/7-1/4 of gamma j(main state). Vj had no effect on gamma j(main state), but slightly affected gamma j (residual). The lj transitions involving gamma j(closed) were slow (15-60 ms), whereas those not involving gamma j(closed) were fast (< 2 ms). An increase in Vj led to a decrease in open channel probability. Depolarization of the membrane potential (Vm) increased the incidence of slow transitions leading to gamma j(closed). We conclude that insect gap junctions possess two gates, a fast gate controlled by Vj and giving rise to gamma j(substates) and gamma j(residual), and a slow gate sensitive to Vm and able to close the channel completely.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abney J. R., Braun J., Owicki J. C. Lateral interactions among membrane proteins. Implications for the organization of gap junctions. Biophys J. 1987 Sep;52(3):441–454. doi: 10.1016/S0006-3495(87)83233-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. V., Barrio L. C., Bargiello T. A., Spray D. C., Hertzberg E., Sáez J. C. Gap junctions: new tools, new answers, new questions. Neuron. 1991 Mar;6(3):305–320. doi: 10.1016/0896-6273(91)90241-q. [DOI] [PubMed] [Google Scholar]
- Bennett M. V., Verselis V. K. Biophysics of gap junctions. Semin Cell Biol. 1992 Feb;3(1):29–47. doi: 10.1016/s1043-4682(10)80006-6. [DOI] [PubMed] [Google Scholar]
- Brink P. R., Fan S. F. Patch clamp recordings from membranes which contain gap junction channels. Biophys J. 1989 Sep;56(3):579–593. doi: 10.1016/S0006-3495(89)82705-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukauskas F. F., Kempf C., Weingart R. Cytoplasmic bridges and gap junctions in an insect cell line (Aedes albopictus). Exp Physiol. 1992 Nov;77(6):903–911. doi: 10.1113/expphysiol.1992.sp003657. [DOI] [PubMed] [Google Scholar]
- Bukauskas F. F., Weingart R. Multiple conductance states of newly formed single gap junction channels between insect cells. Pflugers Arch. 1993 Apr;423(1-2):152–154. doi: 10.1007/BF00374973. [DOI] [PubMed] [Google Scholar]
- Bukauskas F., Kempf C., Weingart R. Electrical coupling between cells of the insect Aedes albopictus. J Physiol. 1992 Mar;448:321–337. doi: 10.1113/jphysiol.1992.sp019044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt J. M., Spray D. C. Single-channel events and gating behavior of the cardiac gap junction channel. Proc Natl Acad Sci U S A. 1988 May;85(10):3431–3434. doi: 10.1073/pnas.85.10.3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanson M., Chandross K. J., Rook M. B., Kessler J. A., Spray D. C. Gating characteristics of a steeply voltage-dependent gap junction channel in rat Schwann cells. J Gen Physiol. 1993 Nov;102(5):925–946. doi: 10.1085/jgp.102.5.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanson M., Roy C., Spray D. C. Voltage-dependent gap junctional conductance in hepatopancreatic cells of Procambarus clarkii. Am J Physiol. 1994 Feb;266(2 Pt 1):C569–C577. doi: 10.1152/ajpcell.1994.266.2.C569. [DOI] [PubMed] [Google Scholar]
- Chen Y. H., DeHaan R. L. Multiple-channel conductance states and voltage regulation of embryonic chick cardiac gap junctions. J Membr Biol. 1992 Apr;127(2):95–111. doi: 10.1007/BF00233282. [DOI] [PubMed] [Google Scholar]
- Chow I., Poo M. M. Formation of electrical coupling between embryonic Xenopus muscle cells in culture. J Physiol. 1984 Jan;346:181–194. doi: 10.1113/jphysiol.1984.sp015015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow I., Young S. H. Opening of single gap junction channels during formation of electrical coupling between embryonic muscle cells. Dev Biol. 1987 Aug;122(2):332–337. doi: 10.1016/0012-1606(87)90298-3. [DOI] [PubMed] [Google Scholar]
- Dahl G., Levine E., Rabadan-Diehl C., Werner R. Cell/cell channel formation involves disulfide exchange. Eur J Biochem. 1991 Apr 10;197(1):141–144. doi: 10.1111/j.1432-1033.1991.tb15892.x. [DOI] [PubMed] [Google Scholar]
- DeVries S. H., Schwartz E. A. Hemi-gap-junction channels in solitary horizontal cells of the catfish retina. J Physiol. 1992 Jan;445:201–230. doi: 10.1113/jphysiol.1992.sp018920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaume C., Randriamampita C., Trautmann A. Arachidonic acid closes gap junction channels in rat lacrimal glands. Pflugers Arch. 1989 Jan;413(3):273–279. doi: 10.1007/BF00583541. [DOI] [PubMed] [Google Scholar]
- Harris A. L., Spray D. C., Bennett M. V. Kinetic properties of a voltage-dependent junctional conductance. J Gen Physiol. 1981 Jan;77(1):95–117. doi: 10.1085/jgp.77.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi A. Isolation of a Singh's Aedes albopictus cell clone sensitive to Dengue and Chikungunya viruses. J Gen Virol. 1978 Sep;40(3):531–544. doi: 10.1099/0022-1317-40-3-531. [DOI] [PubMed] [Google Scholar]
- Keane R. W., Mehta P. P., Rose B., Honig L. S., Loewenstein W. R., Rutishauser U. Neural differentiation, NCAM-mediated adhesion, and gap junctional communication in neuroectoderm. A study in vitro. J Cell Biol. 1988 Apr;106(4):1307–1319. doi: 10.1083/jcb.106.4.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewenstein W. R., Kanno Y., Socolar S. J. Quantum jumps of conductance during formation of membrane channels at cell-cell junction. Nature. 1978 Jul 13;274(5667):133–136. doi: 10.1038/274133a0. [DOI] [PubMed] [Google Scholar]
- Meyer R. A., Laird D. W., Revel J. P., Johnson R. G. Inhibition of gap junction and adherens junction assembly by connexin and A-CAM antibodies. J Cell Biol. 1992 Oct;119(1):179–189. doi: 10.1083/jcb.119.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman S., Carlile G., Evans W. H. Assembly of hepatic gap junctions. Topography and distribution of connexin 32 in intracellular and plasma membranes determined using sequence-specific antibodies. J Biol Chem. 1993 Jan 15;268(2):1260–1265. [PubMed] [Google Scholar]
- Ramanan S. V., Brink P. R. Multichannel recordings from membranes which contain gap junctions. II. Substates and conductance shifts. Biophys J. 1993 Oct;65(4):1387–1395. doi: 10.1016/S0006-3495(93)81193-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swenson K. I., Jordan J. R., Beyer E. C., Paul D. L. Formation of gap junctions by expression of connexins in Xenopus oocyte pairs. Cell. 1989 Apr 7;57(1):145–155. doi: 10.1016/0092-8674(89)90180-3. [DOI] [PubMed] [Google Scholar]


