Abstract
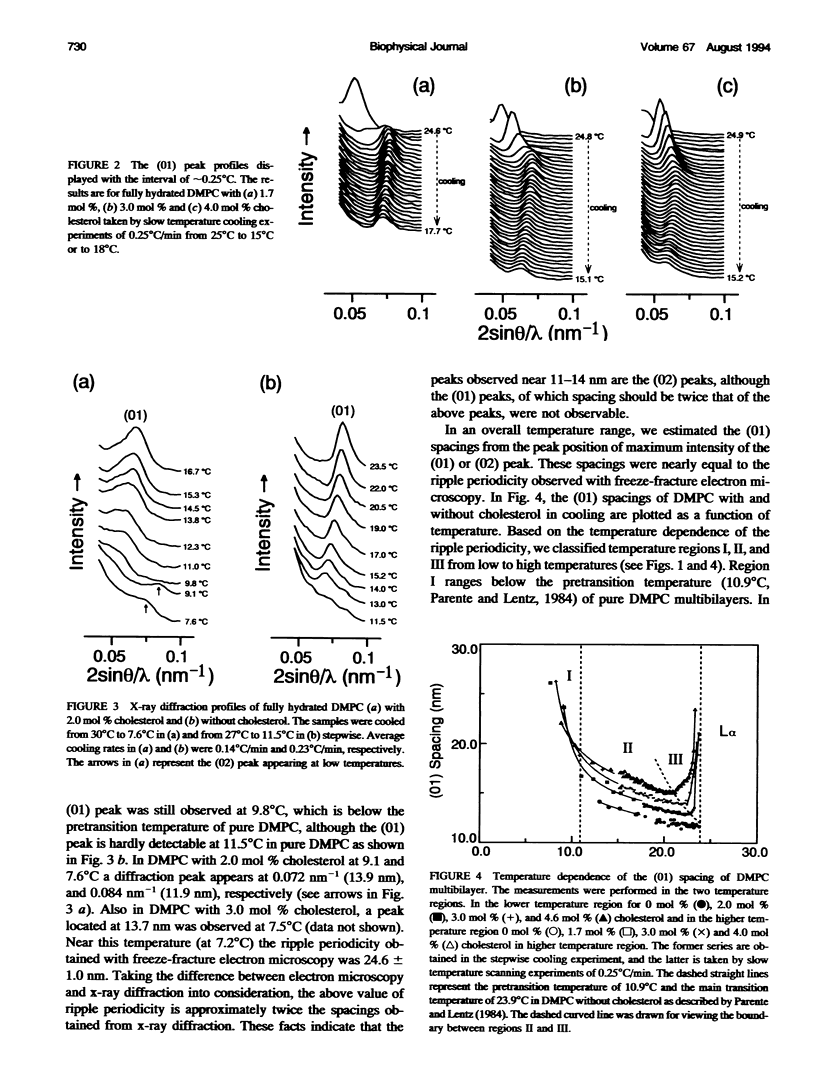
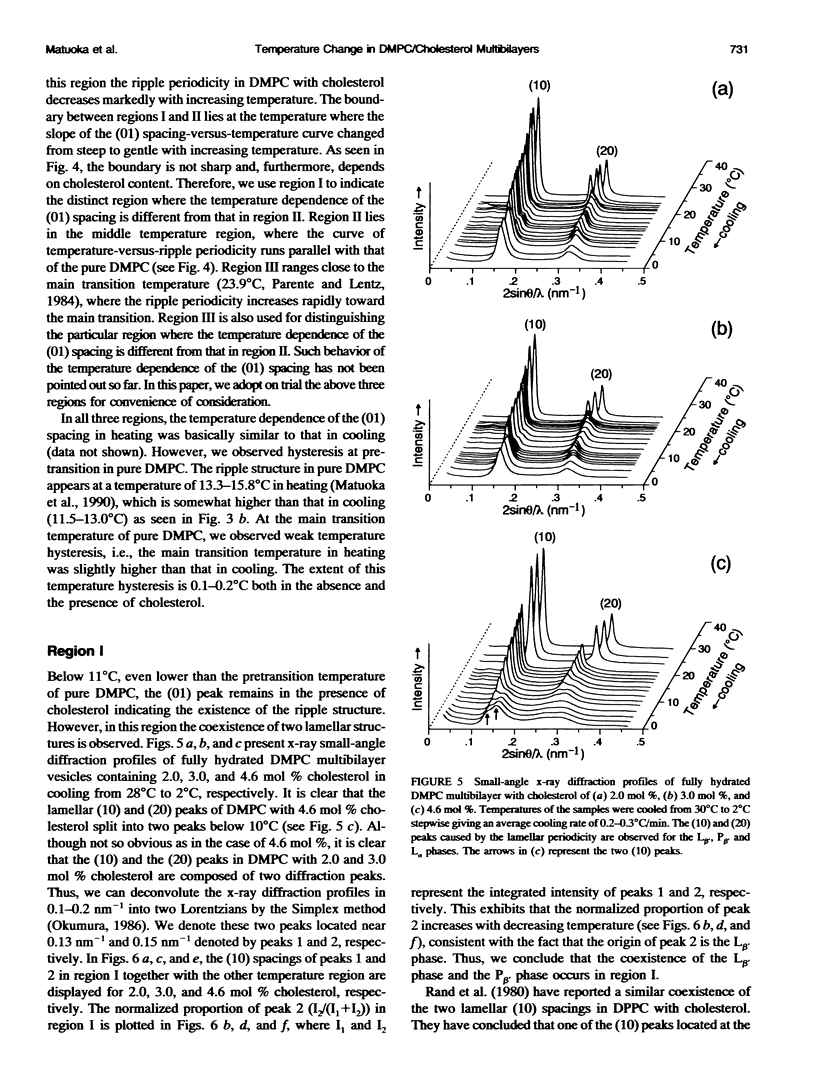
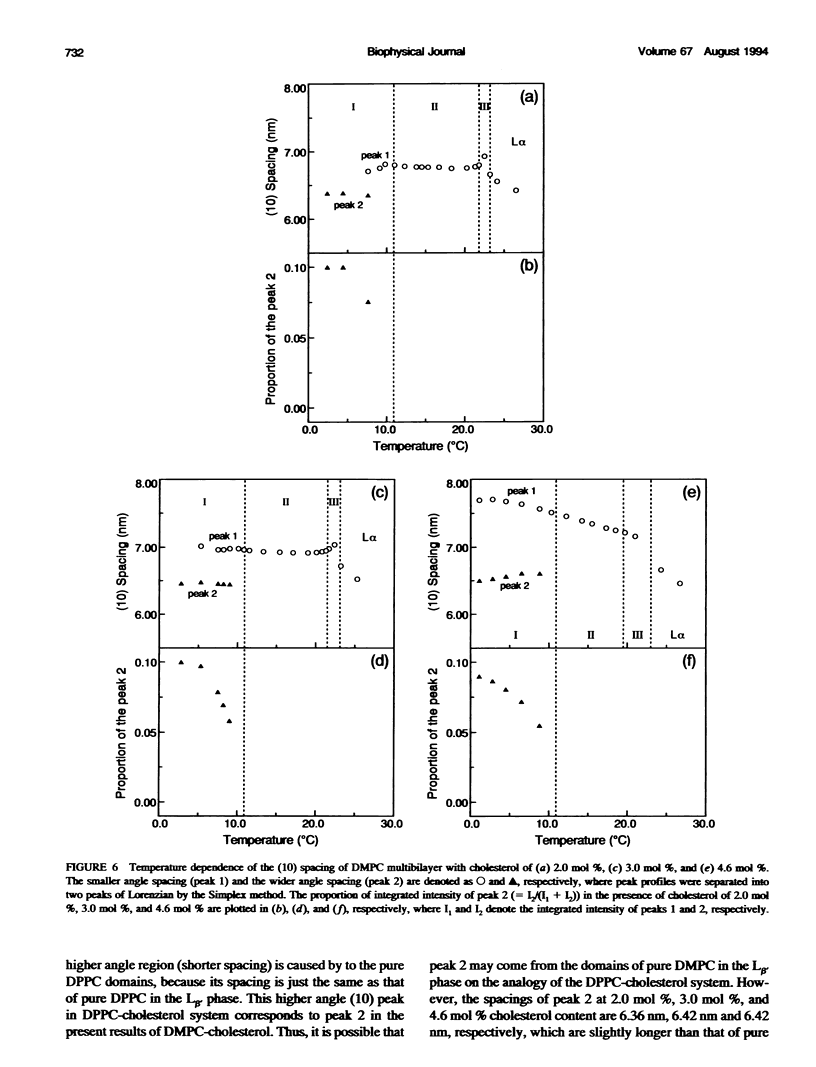
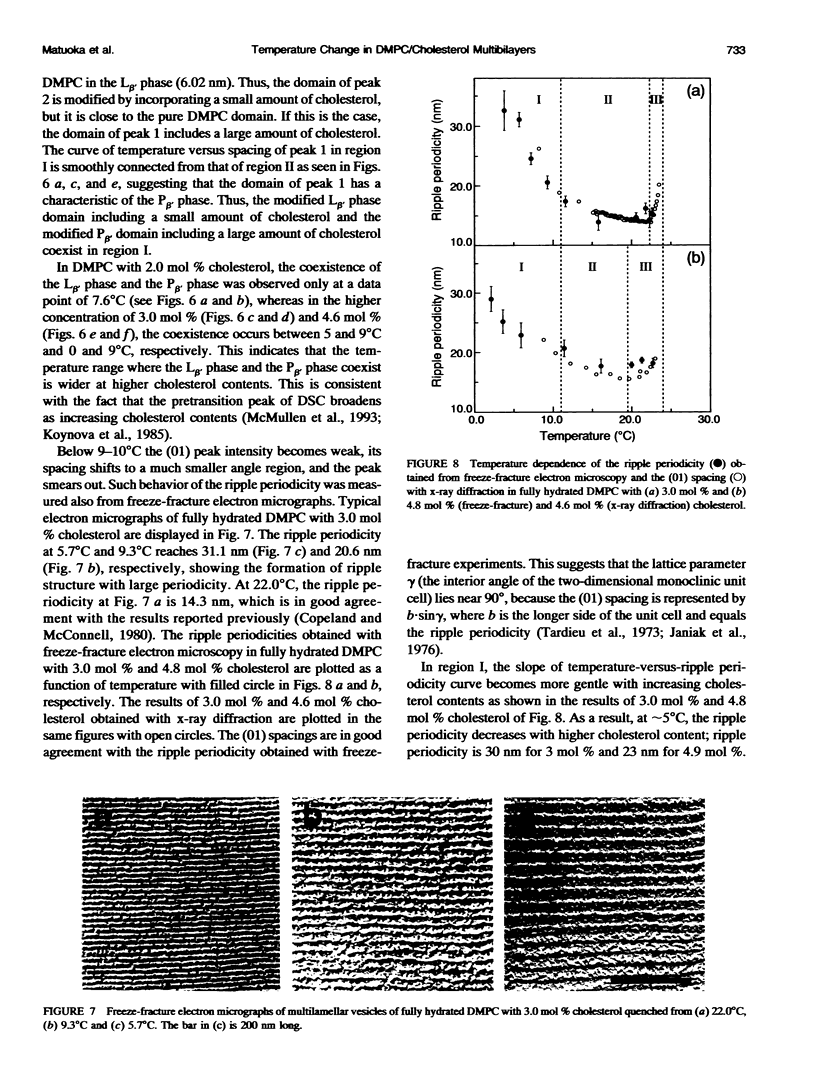
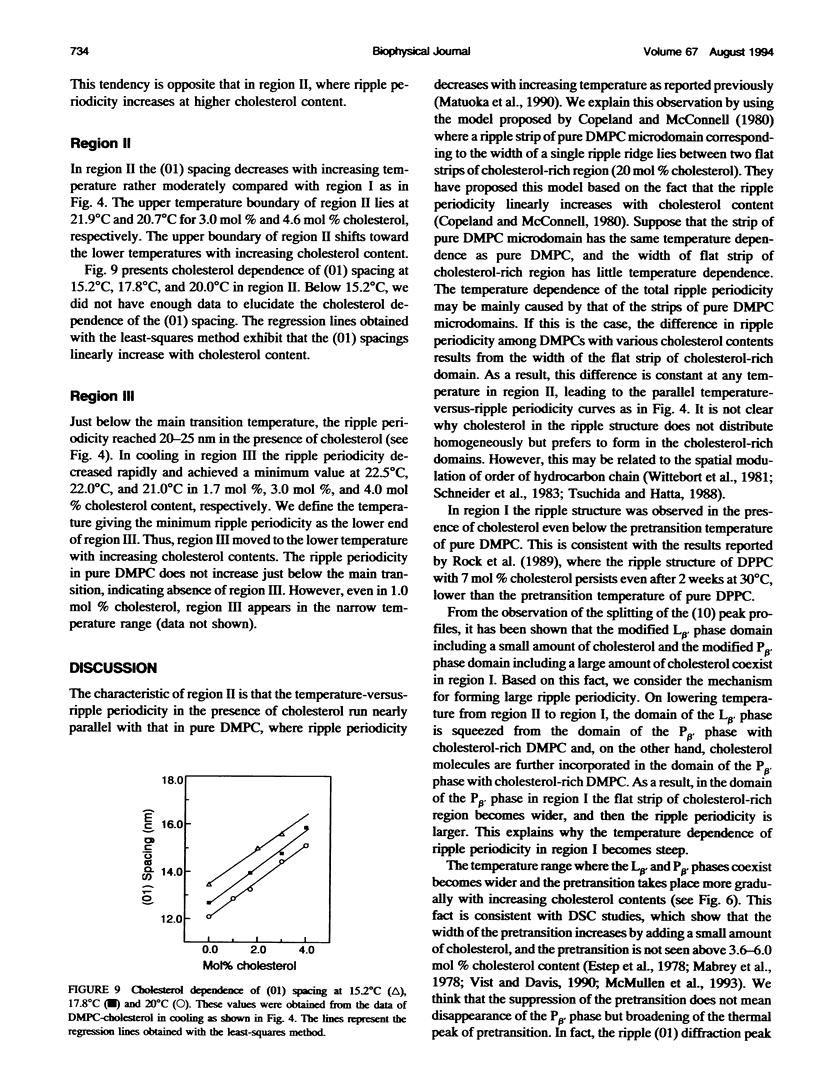
The ripple structure was studied as a function of temperature in fully hydrated dimyristoylphosphatidylcholine (DMPC)/cholesterol multibilayers using synchrotron x-ray small-angle diffraction and freeze-fracture electron microscopy. In the presence of cholesterol, the ripple structure appears below the pretransition temperature of pure DMPC multibilayers. In this temperature range the ripple periodicity is relatively large (25-30 nm) and rapidly decreases with increasing temperature. In this region, defined as region I, we observed coexistence of the P beta' phase and the L beta' phase. The large ripple periodicity is caused by the formation of the P beta' phase region in which cholesterol is concentrated and the L beta' phase region from which cholesterol is excluded. An increase in ripple periodicity also takes place in the narrow temperature range just below the main transition temperature. We define this temperature region as region III, where the ripple periodicity increases dramatically toward the main transition temperature. In region II, between regions I and III, the ripple periodicity decreases gradually with temperature. This behavior is quite similar to that of pure DMPC. Temperature-versus-ripple periodicity curves are parallel among pure DMPC and DMPCs with various cholesterol contents. We explain this behavior in terms of a model proposed by other workers.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Copeland B. R., McConnel H. M. The rippled structure in bilayer membranes of phosphatidylcholine and binary mixtures of phosphatidylcholine and cholesterol. Biochim Biophys Acta. 1980 Jun 20;599(1):95–109. doi: 10.1016/0005-2736(80)90059-0. [DOI] [PubMed] [Google Scholar]
- Estep T. N., Mountcastle D. B., Biltonen R. L., Thompson T. E. Studies on the anomalous thermotropic behavior of aqueous dispersions of dipalmitoylphosphatidylcholine-cholesterol mixtures. Biochemistry. 1978 May 16;17(10):1984–1989. doi: 10.1021/bi00603a029. [DOI] [PubMed] [Google Scholar]
- Falkovitz M. S., Seul M., Frisch H. L., McConnell H. M. Theory of periodic structures in lipid bilayer membranes. Proc Natl Acad Sci U S A. 1982 Jun;79(12):3918–3921. doi: 10.1073/pnas.79.12.3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebhardt C., Gruler H., Sackmann E. On domain structure and local curvature in lipid bilayers and biological membranes. Z Naturforsch C. 1977 Jul-Aug;32(7-8):581–596. doi: 10.1515/znc-1977-7-817. [DOI] [PubMed] [Google Scholar]
- Hawton MH, Keeler WJ. van der Waals energy of lecithins in the ripple phase. Phys Rev A Gen Phys. 1986 May;33(5):3333–3340. doi: 10.1103/physreva.33.3333. [DOI] [PubMed] [Google Scholar]
- Hicks A., Dinda M., Singer M. A. The ripple phase of phosphatidylcholines: effect of chain length and cholesterol. Biochim Biophys Acta. 1987 Sep 18;903(1):177–185. doi: 10.1016/0005-2736(87)90167-2. [DOI] [PubMed] [Google Scholar]
- Janiak M. J., Small D. M., Shipley G. G. Nature of the Thermal pretransition of synthetic phospholipids: dimyristolyl- and dipalmitoyllecithin. Biochemistry. 1976 Oct 19;15(21):4575–4580. doi: 10.1021/bi00666a005. [DOI] [PubMed] [Google Scholar]
- Janiak M. J., Small D. M., Shipley G. G. Temperature and compositional dependence of the structure of hydrated dimyristoyl lecithin. J Biol Chem. 1979 Jul 10;254(13):6068–6078. [PubMed] [Google Scholar]
- Mabrey S., Mateo P. L., Sturtevant J. M. High-sensitivity scanning calorimetric study of mixtures of cholesterol with dimyristoyl- and dipalmitoylphosphatidylcholines. Biochemistry. 1978 Jun 13;17(12):2464–2468. doi: 10.1021/bi00605a034. [DOI] [PubMed] [Google Scholar]
- Marder M., Frisch H. L., Langer J. S., McConnell H. M. Theory of the intermediate rippled phase of phospholipid bilayers. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6559–6561. doi: 10.1073/pnas.81.20.6559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matuoka S., Kato S., Akiyama M., Amemiya Y., Hatta I. Temperature dependence of the ripple structure in dimyristoylphosphatidylcholine studied by synchrotron X-ray small-angle diffraction. Biochim Biophys Acta. 1990 Oct 5;1028(2):103–109. doi: 10.1016/0005-2736(90)90145-e. [DOI] [PubMed] [Google Scholar]
- McCullough WS, Scott HL. Statistical-Mechanical Theory of the Ripple Phase of Lipid Bilayers. Phys Rev Lett. 1990 Aug 13;65(7):931–934. doi: 10.1103/PhysRevLett.65.931. [DOI] [PubMed] [Google Scholar]
- McMullen T. P., Lewis R. N., McElhaney R. N. Differential scanning calorimetric study of the effect of cholesterol on the thermotropic phase behavior of a homologous series of linear saturated phosphatidylcholines. Biochemistry. 1993 Jan 19;32(2):516–522. doi: 10.1021/bi00053a016. [DOI] [PubMed] [Google Scholar]
- Parente R. A., Lentz B. R. Phase behavior of large unilamellar vesicles composed of synthetic phospholipids. Biochemistry. 1984 May 22;23(11):2353–2362. doi: 10.1021/bi00306a005. [DOI] [PubMed] [Google Scholar]
- Tardieu A., Luzzati V., Reman F. C. Structure and polymorphism of the hydrocarbon chains of lipids: a study of lecithin-water phases. J Mol Biol. 1973 Apr 25;75(4):711–733. doi: 10.1016/0022-2836(73)90303-3. [DOI] [PubMed] [Google Scholar]
- Tenchov B. G., Yao H., Hatta I. Time-resolved x-ray diffraction and calorimetric studies at low scan rates: I. Fully hydrated dipalmitoylphosphatidylcholine (DPPC) and DPPC/water/ethanol phases. Biophys J. 1989 Oct;56(4):757–768. doi: 10.1016/S0006-3495(89)82723-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wack DC, Webb WW. Synchrotron x-ray study of the modulated lamellar phase P beta ' in the lecithin-water system. Phys Rev A Gen Phys. 1989 Sep 1;40(5):2712–2730. doi: 10.1103/physreva.40.2712. [DOI] [PubMed] [Google Scholar]



