Abstract
Zhilong Huoxue Tongyu capsule (ZL) is wildly used as a Chinese patent medicine for the treatment of cardiovascular diseases. Clinical studies have found that it can significantly improve hypertension and heart failure. However, its precise molecular mechanisms remain incompletely understood. The aim of this study was to investigate the effect of ZL on myocardial fibrosis (MF) in in vivo and its potential mechanisms. We established a hypertensive MF model by subcutaneous pumping of angiotensin II (Ang II) into mice, and validated in vivo whether ZL can reduce systolic blood pressure (SBP) and inhibit MF, including the use of echocardiography and various pathological staining techniques. Mechanistically, Western blot, qRT-PCR, and various immunostaining techniques were used to verify whether ZL can regulate TGF-β1/Smad3/Erbb4-IR/miR-29b pathway, and in vivo overexpression of Erbb4-IR was used to clarify whether it plays a key role in this pathway. ZL significantly reduced SBP in hypertensive MF mice, improved cardiac function and MF. Deposition of various collagen, expression of inflammatory factors, and activation of TGF-β1/Smad3 pathway is inhibited due to the intervention of ZL. In addition, ZL significantly reduced expression of Erbb4-IR and increased the expression of miR-29b. Mechanistically, after overexpression of Erbb4-IR in vivo, the regulatory effect of ZL on TGF-β1/Smad3/Erbb4-IR/miR-29b was reversed. Our results initially demonstrated ZL could exert cardioprotective effects in hypertensive MF mice. The pharmacological mechanism of ZL may be related to its regulation on TGF-β1/Smad3/Erbb4-IR/miR-29b pathway.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-10631-9.
Keywords: Zhilong Huoxue Tongyu capsule, Hypertensive, Heart failure, Myocardial fibrosis, TGF-β1/Smad3/Erbb4-IR/miR-29b pathway
Subject terms: Drug discovery, Cardiology
Introduction
Cardiovascular disease has emerged as the leading cause of death worldwide. Hypertension is the most common cause of chronic heart disease1and long-term hypertension is one of the main causes of myocardial fibrosis (MF). Pathologically, MF is the main common feature of chronic heart disease, manifesting as excessive proliferation of cardiac fibroblasts and excessive deposition of extracellular matrix (ECM)2,3. MF is a major cause of heart failure and is directly related to the risk of cardiovascular death4–6. The conventional treatment of hypertensive MF can relieve the clinical symptoms, but effective drugs to prevent MF are currently lacking due to the various underlying factors and unclear mechanism7,8. Therefore, researching the underlying mechanisms is essential to developing effective drugs for the prevention and treatment of MF.
Mir−29b functions as a downstream effector miRNA in the Smad3 signaling pathway9. Transforming growth factor-beta1 (TGF-β1) and angiotensin II (Ang II) activate Smad3 to down-regulate miR-29b, leading to fibrosis10. Non-coding RNAs (ncRNAs) play a regulatory role in the transcription and translation of effector proteins, with microRNAs (miRNAs) and long non-coding RNA (lncRNA) currently being the focus of research11. Our previous study employed a model of renal fibrosis and revealed that Erbb4-IR (np_5318) is a novel Smad3-dependent lncRNA involved in renal injury, and miR-29b is the direct target genes of Erbb4-IR12. In addition, TGF-β1/Erbb4-IR has been proven to be a specific therapeutic target for chronic kidney disease in Smads-mediated renal fibrosis12–14. Considering the opposite regulatory roles of Erbb4-IR and miR-29b in fibrosis, inhibiting Erbb4 IR and promoting miR-29b may contribute to fibrosis. According to previous studies15,16the TGF-β1/Smad3/Erbb4-IR/miR-29b pathway is hypothesized to play a pivotal role in the regulation of MF.
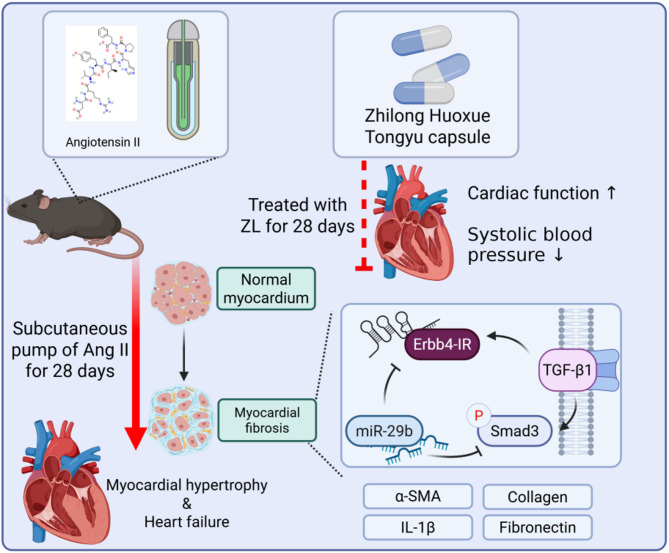
Traditional Chinese medicine (TCM) offers unique advantages in the treatment of MF. Many animal-based models and clinical studies have demonstrated that TCM is effective in treating cardiovascular diseases. Zhilong Huoxue Tongyu capsule (ZL) is a TCM preparation used in the treatment of cardiovascular and cerebrovascular diseases and was granted a Chinese patent in 2011 (Patent No.: 200810147774.1). Previous studies have shown its regulatory effects on the release of inflammatory factors, protecting the vascular endothelium and promoting angiogenesis by regulating PI3K/AKT1/Nrf2 axis, NLRP3 signaling pathway, and Lats1/Yap pathway17–20. ZL includes five components that provide a rich pharmacodynamic material basis, and is composed of Huangqi (Astragalus membranaceus Fisch. ex Bunge.), Guizhi (Cinnamomum cassia (L.) J.Presl), Daxueteng (Sargentodoxa cuneata (Oliv.) Rehder & E.H.Wilson), Earthworm and Leech, the plant name has been checked with the http://www.worldfloraonline.org. More importantly, the long-term toxicity of ZL was observed using the maximum tolerated dose (81.6 g/kg/d) in mice, indicating no obvious effect on the physiological function and no obvious long-term toxic effects21,22. This study established an Ang II-induced MF model to explore whether ZL can inhibit MF caused by sustained hypertension by balancing the TGF-β1/Smad3/Erbb4-IR/miR- 29b pathway. The process and regulatory mechanism of this study are briefly shown in Fig. 1.
Fig. 1.
Graphical abstract.
Methods
Drug Preparation
ZL was prepared in the pharmaceutical preparation room of the Affiliated Traditional Chinese Medicine Hospital of Southwest Medical University. ZL was characterized by ultra-high-performance liquid chromatography coupled with high-resolution mass spectrometry23.
Animals
Forty SPF-grade male C57BL/6J mice weighing 20 ± 1 g were purchased from Chengdu Dashuo Experimental Animal Co., Ltd (Certification No. SYXK Chuan 2020-030). The animals were kept in the Animal Experiment Center of Southwest Medical University under a 12 h dark/light cycle and room temperature ranging from 19–22℃, and humidity ranging from 40 to 60%. All experimental procedures were performed in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals, and the experimental plan was approved by the Animal Research Committee of Southwest Medical University (Approval Number. SWMU2020317). The study is reported in accordance with ARRIVE guidelines.
Experimental animal protocols
All mice were randomly assigned into two large groups. Mice in the first group were adaptively fed for 1 week and then randomly equally divided into the control group, Ang II group, Ang II + ZL (6.24 g/kg/d, equivalent to 10 times the clinical equivalent dose) group, and Ang II + Fosinopril (2.6 × 10–3 g/kg/d, equivalent to 1 time the clinical equivalent dose) group. Medications were administrated by gavage at a volume of 0.1 mL/10 g. Model mice were anesthetized by inhalation of isoflurane (RWD, R510-22-10) under mask oxygen, and the upper neck and back of the upper limbs were disinfected after hair removal. A 0.5 cm incision was made, an osmotic pump (Alzet, Model 2004) was implanted subcutaneously in the scapula segment, and the skin was sutured. AngII (Sigma, A9525) was continuously pumped at a dose of 1.46 mg/kg/d for 28 days. Osmotic pumps were filled with normal saline in the control group and AngII solution in the other groups. Data were collected up to the 28th day of the experiment, and all mice were sacrificed under anesthesia by air injection. The method of model building refers to our published literature15.
The second batch of mice was used in another in vivo experiment. The mice were infected with adeno-associated virus (AAV) by tail vein injection 2 weeks prior to modeling. The AAV was produced by Tsingke Biotechnology Co.,Ltd., including gene synthesis and AAV9 packaging, yielding AAV9 [ssAAV. CMV. Erbb4-IR. WPRE. SV40pA] with a titer of 1E + 12GC/mL, totaling 500 µl. The sequence is described in the supplementary file for Erbb4-IR and our previously published research. Fifty mice were randomly and equally divided into the control group, AngI group, Ang II + AAV9-NC group, Ang II + AAV9-Erbb4-IR group, and Ang II + AAV9-Erbb4-IR + ZL (6.24 g/kg/d) group. Two weeks before modeling, tail vein injection was performed, and qRT-PCR was used to determine that Erbb4-IR was successfully transfected, Each medicated group was administrated by gavage at a volume of 0.1 mL/10 g. The model was established using the same methods as previously described.
Determination of systolic blood pressure
The systolic blood pressure (SBP) of the mice was measured non-invasively at the tail artery (Softron, BP-98 A). The mouse was placed in the pressure box, the tail was placed into the pressure cylinder, and the sleeve temperature was set at 37℃. The mouse was allowed to quiet down, and the SBP was measured three times to obtain an average value. SBP was measured once every 7 days after modeling, for a total of 5 times (including the reference value of the preoperative measurement). The SBP of the model mice was expected to rise significantly 7 days after operation, reaching and maintaining 150 mmHg or above within 14 days.
Measurement of cardiac function
On the 28th day of modeling, all experimental mice were anesthetized with inhaled isoflurane. The anterior chest wall was depilated and the mice were fixed on the thermostatic table. The ultrasound imaging system (FUJIFILM, Vevo 3100) was used for transthoracic echocardiography. The limbs of the mice made contact with the electrodes of the constant temperature platform to monitor the changes in cardiac electrical signals. The ultrasonic probe was used to scan the short axis of the level of the middle papillary muscle of the mouse chest wall in M mode to observe the heart and obtain images for analysis of cardiac function. Three images were captured from each mouse, and the average value of the data was calculated for subsequent analysis, including left ventricular internal diameter diastolic (LVIDd), left ventricular internal diameter systolic (LVIDs), left ventricular fractional shortening (FS), and ejection fraction (EF). After completing the cardiac ultrasound examination, all mice were sacrificed by air injection under deep anesthesia. Heart and blood samples were collected for subsequent experiments.
Pathological staining
All methods of pathological staining were described in our previously published literature24. Heart samples were immersed in 4% paraformaldehyde for over 48 h, then embedded and sectioned into 4 μm-thick slices. The sections were stained with hematoxylin and eosin (H&E) (Solarbio, G1120), Masson (Solarbio, G1340) and Sirius Red (Solarbio, G1472) for pathologic analysis. Images of the stained sections were obtained by light microscopy (OLYMPUS, SZ61TR).
Quantitative real-time PCR (qRT-PCR)
RNA extraction kit (TOYOBO, NPK-201 F), reverse transcription kit (TOYOBO, FSQ-201), and reaction reagents (TOYOBO, KMM-101) were used to prepare for PCR, and all operations were carried out according to the manufacturer’s instructions. A fluorescence quantitative PCR instrument (ROche, LightCycler 480II) was used for routine melting curve analysis to determine the Ct value. β-actin was used as an internal reference for control, and the relative expression levels of target genes were determined using the 2-ΔΔCt method. Operations were performed with reference to our previously published literature20. The primer sequences are listed in Table S1.
Immunohistochemical staining
The methods of immunohistochemical staining were described in our previously published literature24. The 4 μm heart sections were routinely deparaffinized with xylene, hydrated with descending gradient ethanol, washed with distilled water, incubated with 3% H2O2 drip for 10 min at room temperature, washed 3 times with PBS for 3 min each time, and blocked with normal goat immune serum drip. The samples were then incubated for 10 min at room temperature and primary antibody drip (dilution 1:200) was added before incubation at 4℃ overnight. The slices were recovered to room temperature and washed the next day. Secondary antibodies (dilution 1:200) were added to each section the next day and the sections were incubated for 10 min at room temperature and washed again. DAB was used for color development and the reaction time was controlled under the microscope. The quantification analysis for positively stained areas was carried out using Image Pro 6.0 software. The information on antibodies is shown in Table S2.
Immunofluorescence staining
Paraffin sections were dewaxed and antigens were retrieved. Subsequently, 3% hydrogen peroxide was added to inactivate endogenous enzymes. After washing, goat serum was used for blocking, followed by incubation with primary antibodies (dilution 1:100) at 4℃ overnight. The next day, the primary antibodies were recovered and the slides were washed with PBS. Subsequently, the slides were incubated with fluorescent secondary antibodies (cy3)-labeled goat anti-mouse IgG (dilution 1:100) for 1 h at room temperature in the dark. Then, the samples were washed and further incubated with another set of primary antibodies following the same steps. Fluorescence (FITC)-labeled goat anti-IgG (dilution 1:100) was used for secondary antibody incubation. Finally, the anti-fluorescence quencher containing DAPI was added. An upright fluorescence microscope (OLYMPUS, OlyVIA) was used to observe the slides and acquired images. The mean gray value was also calculated by Image-J software. The methods of immunofluorescence staining were described in our previously published literature24. The information on antibodies is shown in Table S1.
Fluorescence in situ hybridization (FISH)
The FISH kit was customized by Guangzhou Baiwei Biotechnology Co., Ltd., and the sequences are listed in Table S1. In addition, 6 μm cardiac tissue sections were deparaffinized, and the reaction solution was prepared at a ratio of 1:39 between the probe and hybridization solution. The samples and the reaction solution were hybridized overnight (16–20 h) at 37 °C, washed, sealed with 20 µL of anti-fluorescent attenuating sealant containing DAPI, and observed under an inverted fluorescence microscope. Positive areas were calculated using Aipathwell software.
Western blot analysis
Myocardial tissues were removed from the − 80 °C refrigerator and processed using RIPA lysate containing inhibitors and phosphatase inhibitors. After centrifugation, the supernatant was collected and tested for protein concentration using the BCA Protein Assay Kit (Beyotime, P0012S). After determining the protein concentration, the samples were added to a gel, subjected to SDS-PAGE gel electrophoresis to separate the proteins, and transferred to a PVDF membrane. The membrane was then closed for 1 h using skimmed milk, and incubated with the following primary antibodies. After washing the next day, the secondary antibodies (1:5000) were added and incubated at room temperature for 2 h. After washing again, a chemiluminescence imaging system (ChemiScope 6200, Clinx, China) was employed to acquire images, and the grayscale values of the bands were analyzed using ImageJ software. The methods of western blot analysis were described in our previously published literature25. All antibody information is shown in Table S1.
Statistical analysis
GraphPad Prism 9.1.2 software was used for statistical analysis. Data were presented as mean ± standard deviation (SD). Among them, in order to compare the differences between multiple groups, the one-way ANOVA was used when the data conformed to the normal distribution, and the rank-sum test was used when the data did not conform to the normal distribution. Statistical significance was defined as *p < 0.05, **p < 0.01.
Results
ZL reduces SBP and improves cardiac function in MF mice
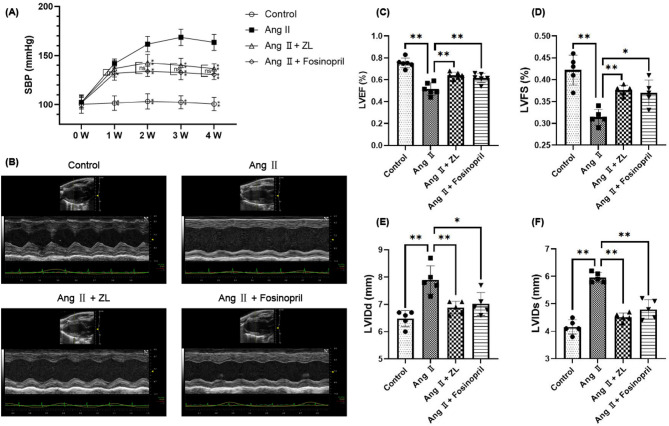
The basic SBP of all mice was 101.41 ± 7.33 mmHg after one week of adaptive feeding. No statistically significant difference in SBP was found between the groups (P > 0.05). Seven days after subcutaneous implantation of the pump, the mean value of SBP in the Ang II group increased to more than 140 mmHg, indicating that Ang II significantly constricted blood vessels and raised blood pressure in mice. However, ZL did not significantly reduce SBP (P > 0.05), whereas Fosinopril showed a significant decrease in SBP in the model mice (P < 0.05). On days 14, 21, and 28, the SBP of the AngII group was maintained above 150 mmHg. Compared with the Ang II group, the use of ZL and Fosinopril decreased SBP in model animals (P < 0.01), but no significant difference was observed between the two drug groups (P > 0.05), as shown in Fig. 2A. All experimental animals were examined by echocardiography before being sacrificed, and the results are shown in Fig. 2 (B-F). Compared with the control group, the AngII group demonstrated a thinner anterior ventricular wall, compensatory hypertrophy of the posterior wall, weakened anterior wall motion, and significantly decreased LVEF and LVFS (P < 0.01), with significantly increased LVIDds and LVIDs (P < 0.01). These results indicated that under the induction of pressure load for 28 days, the LVEF and LVFS of the AngII group were significantly lower than those of the control group. The model mice showed cardiac structural changes characterized by left ventricular hypertrophy and decreased cardiac function. Compared with the AngII group, ZL and Fosinopril significantly increased the LVEF and LVFS of model mice (P < 0.05 or P < 0.01). In contrast, the LVIDd and LVIDs of the model mice were reduced (P < 0.05 or P < 0.01), showing no significant difference between the two groups (P > 0.05).
Fig. 2.
Effect of ZL on cardiac function on hypertensive MF mice. (A) Measurements of average SBP. Performed three times per mouse per week in the morning (n = 10), the results were presented as mean ± SD. *P < 0.05, **P < 0.01 vs. Ang II group; ns, not significant. (B) Original image of heart detected by Color Doppler echocardiography. Color Doppler echocardiography demonstrated the changes of (C) LVEF, (D) LVFS, (E) LVIDd and (F) LVIDs, the measured data on the 28th day after the osmotic pump implantation were used for statistical analysis (n = 5), the results were presented as mean ± SD, *P < 0.05, **P < 0.01.
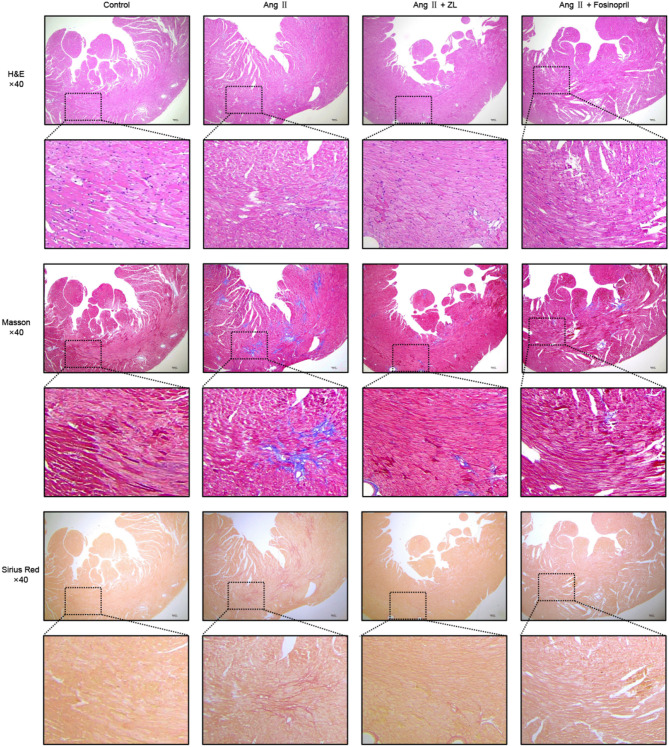
ZL ameliorates myocardial injury in MF mice
H&E staining revealed a normal myocardial structure in the control group. In the AngII group, the myocardial cells were significantly hypertrophic, demonstrating a disordered arrangement and thickened myocardial microvascular intima-media. After intervention with ZL or Fosinopril, the above pathological changes were improved to varying degrees. Masson staining revealed no obvious blue staining of collagen fibers in the myocardium of the control group, while collagen tissue was stained blue in the intima-media of myocardial microvessels. In the AngII group, significantly greater blue staining of collagen fibers connected into networks was observed around the myocardial interstitium and blood vessels compared to the Control group. After the intervention with ZL or Fosinopril, the above pathological changes were improved to varying degrees. Sirius red staining also supported the above results. The results are displayed in Fig. 3.
Fig. 3.
Histological effects of ZL on hearts tissue of hypertensive MF mice. H&E staining, Masson staining and Sirius Red staining were used to demonstrate the pathological features of ZL inhibition of MF. Under the microscope, 40× images were acquired to show histological changes across the entire vessel cross-section, scale bar = 100 μm.
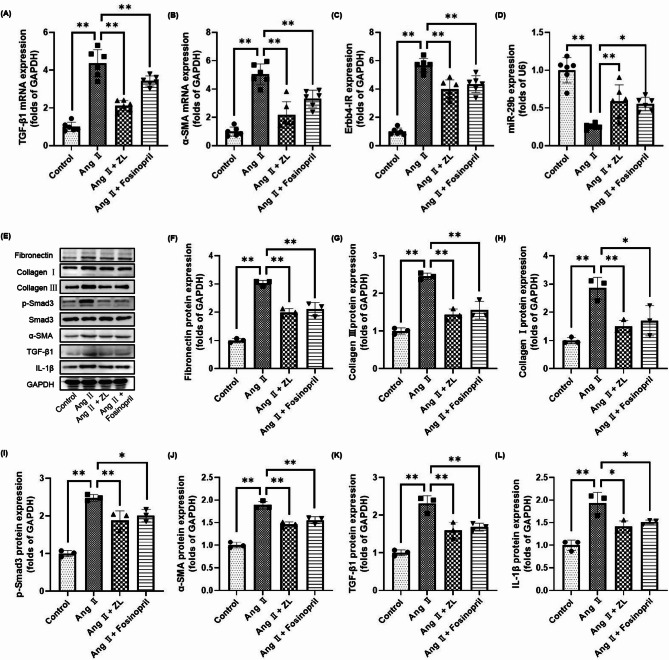
ZL inhibits MF by interfering with the TGF-β1/Smad3/Erbb4-IR/miR-29b pathway
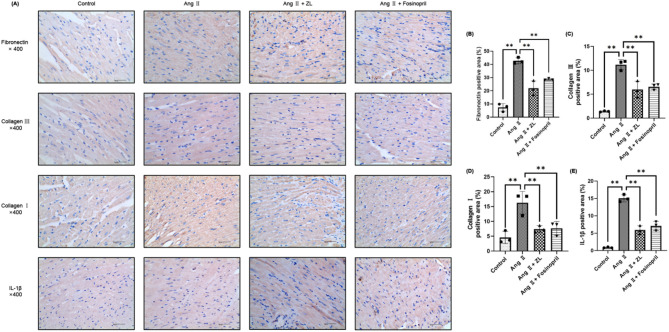
As shown in Fig. 4, the pathological changes of MF are closely related to collagen deposition and the expression of inflammatory factors in myocardial tissue. Furthermore, the expression of collagen-related proteins and inflammatory factors in myocardial tissue was investigated in each group. Compared with the Control group, the expression of Fibronectin, Collagen III, Collagen I, and IL-1β (yellow-brown area) was significantly increased in the Ang II group (P < 0.01). The increase in positive areas suggested that pressure overload caused excessive deposition of collagen and the release of inflammatory factors in the myocardium. ZL and Fosinopril were found to inhibit the expression of Fibronectin, Collagen III, Collagen I, and IL-1β in myocardial tissue to varying degrees compared with the Ang II group (P < 0.05 or P < 0.01). As shown in Fig. 5, the qRT-PCR results indicated that the mRNA levels of TGF-β1 and α-SMA in the heart tissue of the AngII group were significantly up-regulated compared with the control group (P < 0.01). Moreover, the level of Erbb4-IR was significantly up-regulated (P < 0.01) and the level of miR-29b was significantly down-regulated (P < 0.01). Compared with the AngII group, the expression levels of TGF-β1, α-SMA, and Erbb4-IR in myocardial tissue were down-regulated by varying degrees by ZL and Fosinopril (P < 0.01). The expression of miR-29b was significantly up-regulated (P < 0.05 or P < 0.01). Western blotting results indicated that the protein expression levels of Fibronectin, Collagen I, Collagen III, p-Smad3, α-SMA, TGF-β1 and IL-1β in the heart tissue of AngII group were significantly up-regulated compared with the control group (P < 0.01). These protein expressions were inhibited by ZL and Fosinopril to varying degrees (P < 0.05 or P < 0.01).
Fig. 4.
Effect of ZL on cardiac fibrosis-associated protein deposition on hpertensive MF mice. (A) Immunohistochemistry was used to demonstrate the expression of fibrosis-and inflammation-related proteins in the myocardium. Gray value analysis of immunohistochemistry Fibronectin (B), Collagen III (C), Collagen I (D) and IL-1β (E) were made and three fields of view (×400) were randomly selected for each slice for gray value analysis (n = 3), scale bar = 40 μm, the results were presented as mean ± SD, **P < 0.01.
Fig. 5.
Effect of ZL on related target genes in cardiac tissue of hypertensive MF mice. The expression of TGF-β1 (A), α-SMA (B), Erbb4-IR (C) and miR-29b (D) in different groups was detected by qRT-PCR (n = 6), the results were presented as mean ± SD, *P < 0.05**, P < 0.01. (E) Western blotting bands, the expressions of Fibronectin (F), Collagen III (G), Collagen I (H), p-Smad3 (I), α-SMA (J), TGF-β1 (K) and IL-1β (L) from different groups were detected by western blotting (n = 3), the results were presented as mean ± SD, *P < 0.05, **P < 0.01.
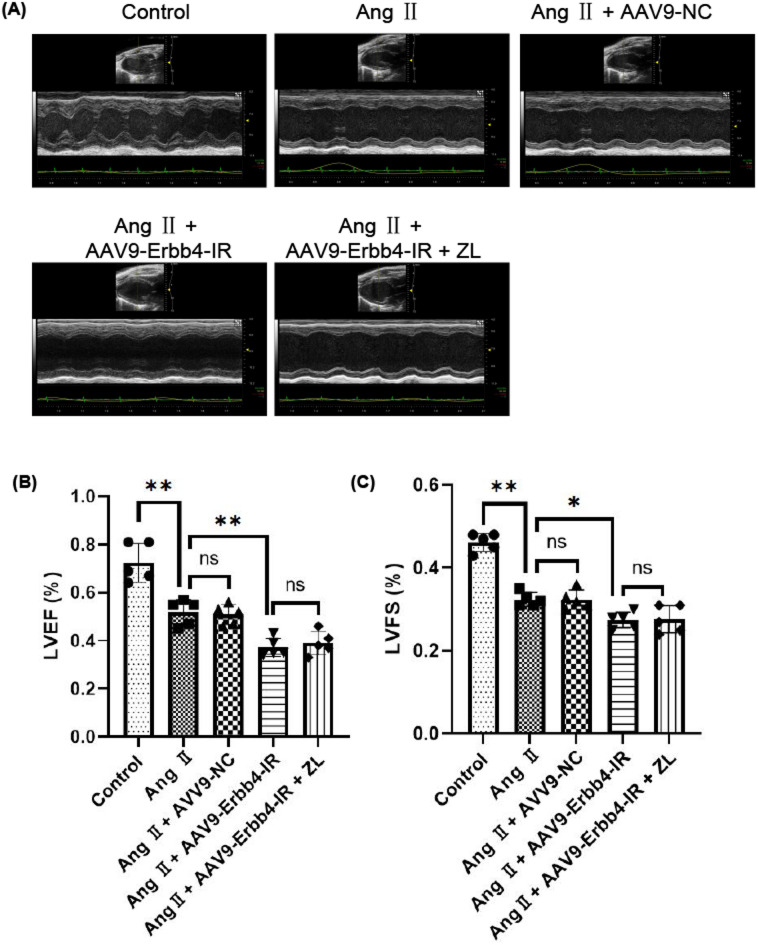
Effect of overexpression of Erbb4-IR on cardiac function on hypertensive MF mice
After establishing the animal models, all experimental animals were subjected to echocardiography before being sacrificed, as shown in Fig. 6. The over-expression of Erbb4-IR significantly reduced the cardiac function of mice, resulting in significant changes in LVEF and LVFS (P < 0.05). ZL treatment could not reverse the cardiac function damage caused by the Ang II with over-expression of Erbb4-IR (P > 0.05). These results suggested that overexpression of Erbb4-IR can reduce cardiac function in mice under the action of Ang II. However, ZL failed to significantly reverse Erbb4-IR-induced cardiac dysfunction in this animal model, suggesting that Erbb4-IR may be a key therapeutic target for the treatment of cardiac dysfunction.
Fig. 6.
Effect of overexpression of Erbb4-IR on cardiac function on hypertensive MF mice. (A) Original image of heart detected by Color Doppler echocardiography. Color Doppler echocardiography demonstrated the changes of (B) LVEF, (C) LVFS, the measurement data of AAV9 injection for 2 weeks and the 28th day after osmotic pump implantation were used for statistical analysis (n = 5), the results were presented as mean ± SD, *P < 0.05, **P < 0.01; ns, not significant.
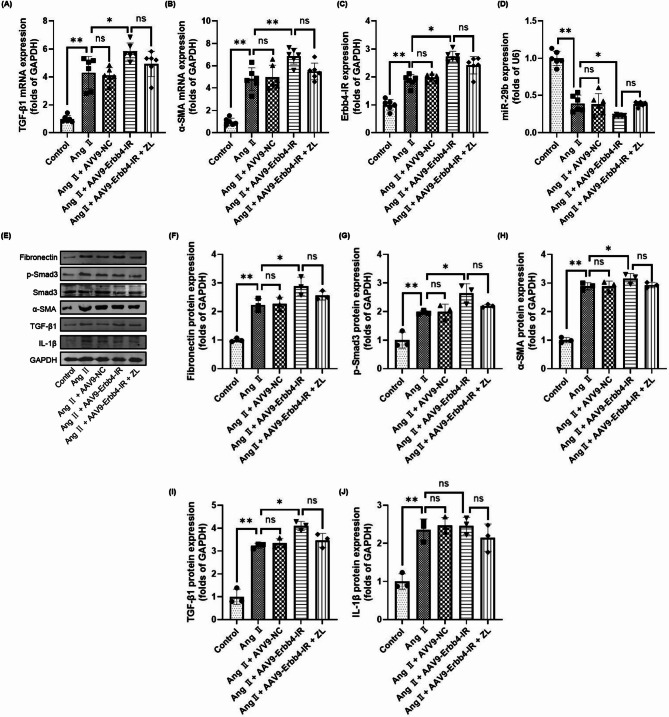
Effect of overexpression of Erbb4-IR on target genes and proteins related to the TGF-β1/Smad3/Erbb4-IR/miR-29b pathway in MF mice
The qRT-PCR results revealed that compared with the control group, the mRNA levels of TGF-β1 and α-SMA in the cardiac tissues of the other groups were significantly up-regulated (P < 0.01), and the level of Erbb4-IR was significantly up-regulated (P < 0.01), as shown in Fig. 7. The level of miR-29b was down-regulated (P < 0.01). Overexpression of Erbb4-IR further up-regulated the expression levels of TGF-β1, α-SMA, and Erbb4-IR in cardiac tissue (P < 0.01) and down-regulated the miR-29b levels (P < 0.05). The changes in the above indicators caused by overexpression of Erbb4-IR could not be reversed by ZL (P > 0.05). Western blotting results also showed that overexpression of Erbb4-IR increased the protein expression levels of Fibronectin, p-Smad3, α-SMA, and TGF-β1 (P < 0.05), but had no significant effect on IL-1β (P > 0.05). The changes in all indicators caused by Erbb4-IR overexpression could not be reversed by ZL (P > 0.05).
Fig. 7.
Effect of overexpression of Erbb4-IR on target genes and proteins related to the TGF-β1/Smad3/Erbb4-IR/miR-29b pathway in MF mice. The expression of TGF-β1 (A), α-SMA (B), Erbb4-IR (C) and miR-29b (D) in different groups was detected by qRT-PCR (n = 6), the results were presented as mean ± SD, *P < 0.05, **P < 0.01; ns, not significant. (E) Western blotting bands, the expressions of Fibronectin (F), p-Smad3 (G), α-SMA (H), TGF-β1 (I) and IL-1β (J) from different groups were detected by western blotting (n = 3), the results were presented as mean ± SD, *P < 0.05, **P < 0.01; ns, not significant.
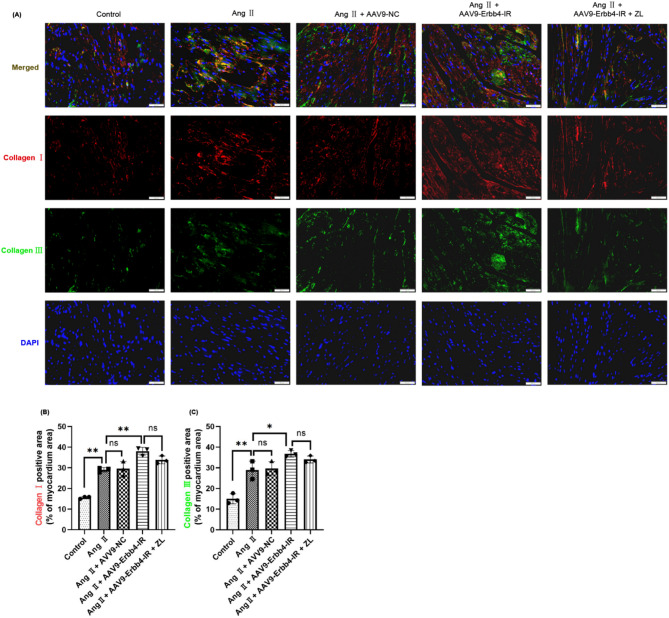
To further validate the synergistic effect of Erbb4-IR and miR-29b in the model mice, two non-coding RNAs were co-stained using FISH technology. The results are shown in Fig. 8. Compared with the control group, Erbb4-IR was significantly up-regulated in the other groups (P < 0.01), whereas miR-29b was significantly down-regulated (P < 0.01). Overexpression of Erbb4-IR further increased Erbb4-IR (P < 0.05) and downregulated the expression of miR-29b (P > 0.05). The increase of Erbb4-IR and the decrease of miR-29b caused by overexpression of Erbb4-IR could not be reversed by ZL treatment (P > 0.05). The pathological changes of MF are closely related to the expression of collagen in myocardial tissue. Moreover, an immunofluorescence assay was performed to investigate the expression of collagen in the myocardial tissue of each group under the overexpression of Erbb4-IR. The results are shown in Fig. 9. Compared with the Control group, Collagen I (red) and Collagen III (green) were significantly increased in the other groups (P < 0.01). The overexpression of Erbb4-IR significantly increased the expression of collagens (P < 0.05). However, ZL treatment could not reverse the increase of Collagen I and Collagen III caused by the overexpression of Erbb4-IR (P > 0.05).
Fig. 8.
Effect of Erbb4-IR overexpression on Erbb4-IR and miR-29b in cardiac tissues. (A) FISH imaging of Erbb4-IR and miR-29b. Cardiac tissue were stained with Erbb4-IR (green), miR-29b(red) and DAPI (blue) to mark the condition of target genes. Gray value analysis of Erbb4-IR (B) and miR-29b (C) were made and six fields of view (×200) were randomly selected for each slice for gray value analysis (n = 6), scale bar = 100 μm, the results were presented as mean ± SD, *P < 0.05, **P < 0.01; ns, not significant.
Fig. 9.
Effect of Erbb4-IR overexpression on fibrosis-related protein deposition in cardiac tissue. (A) Double immunofluorescence of Collagen I and Collagen III. Cardiac tissue were stained with Collagen I (red), Collagen III (green) and DAPI (blue) to mark the condition of myocardial tissue collagen deposition. Gray value analysis of Collagen I (B) and Collagen III (C) were made and three fields of view (×200) were randomly selected for each slice for gray value analysis (n = 3), scale bar = 50 μm, the results were presented as mean ± SD, *P < 0.05, **P < 0.01; ns, not significant.
Discussion
MF is a common pathological feature of most cardiovascular diseases and mainly presents as an excessive proliferation of CFs and excessive deposition of ECM26,27. MF is a significant cause of heart failure and is directly related to the risk of cardiovascular death28. The activation of the renin angiotensin aldosterone system (RAAS) system caused by continuous abnormal elevation of SBP is the main factor in the development of chronic heart failure and MF29. Many studies have been conducted on hypertensive heart disease and MF. However, the mechanism of MF formation is very complex and has not been fully elucidated. For example, hemodynamic disorders lead to pressure overload, long-term inflammatory response, metabolic toxins, RAAS activation, abnormal secretion of various growth factors and oxidative stress, and activation of multiple signaling pathways (including TGF-β/Smad, BMP, Wnt/β-catenin, MAPK, Rho); many other factors are involved in the formation and development of MF30–33. Furthermore, current studies have confirmed that the TGF-β/Smad pathway is the core pathway of MF34.
Our recent studies have confirmed that miR-29b is a downstream target miRNA of Smad310. TGF-β1 and AngII activate Smad3 to down-regulate miR-29b, leading to fibrosis of the heart, lungs, and kidneys. MiR-29b can specifically bind to the TGF-β1 coding sequence region and inhibit TGF-β1 production and Smad3 activation in a negative feedback manner to improve hypertensive myocardium and bleomycin-induced pulmonary fibrosis35–37. In renal inflammatory and fibrotic diseases, our research team has confirmed that Erbb4-IR is a target gene of Smad3, and knockout of Smad3 blocks TGF-β1 and advanced glycation end products to up-regulate Erbb4-IR-mediated fibrosis. The 3’UTR of the miR-29b genome sequence has a potential binding site for Erbb4-IR, and silencing Erbb4-IR can increase miR-29b expression12–14. Elucidating the regulatory role of the TGF-β1/Smad3/Erbb4-IR/miR-29b pathway in the heart holds great significance in understanding the mechanism of MF.
TCM has unique advantages in improving hypertension and MF38,39. Hypertension has no specific name in TCM and can be understood as Tou Yun (dizzy) or Xuan Yun (vertigo), which are consistent with the clinical manifestations of MF. ZL has long been used in the clinical treatment of cardiovascular diseases. Our previous studies have found that ZL can significantly improve cardiac function in patients with heart failure19. Some basic studies have shown that ZL has an excellent therapeutic effect on myocardial infarction, heart failure, and atherosclerosis in vitro and in vivo models20,24.
Studies have shown that AngII is a hormone that regulates water and salt metabolism and SBP, regulates the RAAS system as a significant active substance and plays a vital role in the acute and chronic regulation of systemic arterial SBP40. Continuous in vivo pumping of AngII will cause a constant increase in host SBP, leading to MF after two weeks. In this study, the experimental mice exhibited significantly decreased cardiac function. Masson staining and immunohistochemistry were performed, revealing myocardial fibrosis and collagen expression in the model mice, confirming that the model was successfully established. Through drug intervention, each drug group’s cardiac function and MF degree were significantly improved, indicating that ZL could improve cardiac function and inhibit MF. The TGF-β1/Smad3 signaling pathway is the core pathway of multiple organ fibrosis. In this study, the expression of TGF-β1, Smad3, and collagen-related mRNAs and proteins in the myocardial tissue of model mice was significantly increased due to continuous pumping of AngII. ZL is a prescription consisting of various TCM components, which exerts its therapeutic effect by acting on multiple targets and signaling pathways. Erbb4-IR is speculated to be involved in the regulation of this pathway and plays a role in promoting MF. In the in vivo experiment, ZL inhibited MF in hypertensive mice. The improvement in cardiac function was closely related to the inhibition of TGF-β1 expression, Smad3 activation, Erbb4-IR expression, and up-regulation of miR-29b. Erbb4-IR can be induced by TGF-β1 through a Smad3-dependent mechanism to promote fibrosis; moreover, miR-29b is also a miRNA that inhibits MF by targeting the TGF-β/Smad signaling pathway12–14. However, whether Erbb4-IR can regulate TGF-β1/Smad3/Erbb4-IR/miR-29b pathway in MF remains to be explored. Additionally, overexpression of Erbb4-IR with AVV9-Erbb4-IR reversed the therapeutic effect of ZL on MF by down-regulating TGF-β1/Smad3 and up-regulating miR-29b. More importantly, the current findings are limited to mice and may not be generalizable to larger animals. Furthermore, as the experiments were exclusively observational, the target molecule likely acts as an upstream regulator of TGF-β. Given the lack of structure-activity relationship studies, the specific target molecule of ZL within the pathway described therefore remains unknown and requires further investigation.
Conclusion
In conclusion, ZL could effectively inhibit Ang II-induced MF and improve cardiac function in vivo, which was closely related to the balance of TGF-β1/Smad3/Erbb4-IR/miR-29b pathway. In addition, overexpression of Erbb4 IR in vivo demonstrates its pivotal role in regulating the pathway. This study provides a new way for the regulation of hypertensive MF and further enriches the scientific implication of using TCM to treat cardiovascular diseases.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Home for Researchers editorial team (www.home-for-researchers.com) for language editing service and Mr. Wayhow Chen for his support of this study.
Author contributions
Qiuyue Liu: Writing - original draft, Resources, Formal analysis, Data curation. Dingshan Zhang and Raoqiong Wang: Writing - original draft, Validation, Methodology, Formal analysis. Shihan Sun: Visualization, Validation, Methodology. Yuanyuan Li: Software, Methodology, Formal analysis. Yuanyuan Li and Zhenxun Wan: Visualization, Formal analysis, Data curation. Yumei Qian and Xinrui Yang: Software, Methodology, Formal analysis. Gang Luo, Mingtai Chen and Mengnan Liu: Writing - review & editing, Validation, Supervision, Investigation.
Funding
This work was supported by the National Natural Science Foundation of China (No. 82074378), Project of Office of Science & Technology and talent work of Luzhou (2023JYJ029, 2022JYJ104), 2024 Traditional Chinese Medicine Guangdong Provincial Laboratory Project (HQCML-C-2024005), Shenzhen Science and Technology Program (JCYJ20230807094603007, JCYJ20240813152440051), Shenzhen Medical Research Fund (A2403028) and the Project of Southwest Medical University (2023ZYYQ04, 2024ZKZ007). The funder had no role in the study design, data analysis, or decision to publish.
Data availability
The data used for this study are available from the corresponding author upon reasonable request.
Declarations
Competing interests
The authors declare no conflicts of interest, financial or otherwise.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contribute to this research equally: Qiuyu Liu, Dingshan Zhang, Raoqiong Wang, Shihan Sun and Yuanyuan Li.
Contributor Information
Gang Luo, Email: 1982luogang@163.com.
Mingtai Chen, Email: zyycardio@foxmail.com.
Mengnan Liu, Email: liumengnan@swmu.edu.cn.
References
- 1.Zhao, D., Liu, J., Wang, M., Zhang, X. & Zhou, M. Epidemiology of cardiovascular disease in china: current features and implications. Nat. Rev. Cardiol.16, 203–212 (2019). [DOI] [PubMed] [Google Scholar]
- 2.Czubryt, M. P. & Hale, T. M. Cardiac fibrosis: pathobiology and therapeutic targets. Cell. Signal.85, 110066 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.López, B. et al. Diffuse myocardial fibrosis: mechanisms, diagnosis and therapeutic approaches. Nat. Rev. Cardiol.18, 479–498 (2021). [DOI] [PubMed] [Google Scholar]
- 4.Guo, Y. et al. Entanglement of GSK-3β, β-catenin and TGF-β1 signaling network to regulate myocardial fibrosis. J. Mol. Cell. Cardiol.110, 109–120 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ravassa, S. et al. Phenotyping of myocardial fibrosis in hypertensive patients with heart failure. Influence on clinical outcome. J. Hypertens.35, 853–861 (2017). [DOI] [PubMed] [Google Scholar]
- 6.Piek, A., de Boer, R. A. & Silljé, H. H. W. The fibrosis-cell death axis in heart failure. Heart Fail. Rev.21, 199–211 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu, M. et al. Hypertensive heart disease and myocardial fibrosis: how traditional Chinese medicine can help addressing unmet therapeutical needs. Pharmacol. Res.185, 106515 (2022). [DOI] [PubMed] [Google Scholar]
- 8.Lee, V. et al. Sacubitril/valsartan versus Valsartan in regressing myocardial fibrosis in hypertension: a prospective, randomized, open-label, blinded endpoint clinical trial protocol. Front. Cardiovasc. Med.10, 1248468 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu, B. H. et al. Deletion of Smad3 prevents renal fibrosis and inflammation in type 2 diabetic nephropathy. Metabolism103, 154013 (2020). [DOI] [PubMed] [Google Scholar]
- 10.Liu, G. X. et al. Disruption of Smad7 promotes ANG II-mediated renal inflammation and fibrosis via Sp1-TGF-β/Smad3-NF.κB-dependent mechanisms in mice. PLoS One. 8, e53573 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nemeth, K., Bayraktar, R., Ferracin, M. & Calin, G. A. Non-coding RNAs in disease: from mechanisms to therapeutics. Nat. Rev. Genet.25, 211–232 (2024). [DOI] [PubMed] [Google Scholar]
- 12.Feng, M. et al. TGF-β mediates renal fibrosis via the Smad3-Erbb4-IR long noncoding RNA Axis. Mol. Ther.26, 148–161 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou, Q. et al. Identification of novel long noncoding RNAs associated with TGF-β/Smad3-mediated renal inflammation and fibrosis by RNA sequencing. Am. J. Pathol.184, 409–417 (2014). [DOI] [PubMed] [Google Scholar]
- 14.Zhang, Y. Y. et al. LRNA9884, a novel Smad3-Dependent long noncoding RNA, promotes diabetic kidney injury in db/db mice via enhancing MCP-1-Dependent renal inflammation. Diabetes68, 1485–1498 (2019). [DOI] [PubMed] [Google Scholar]
- 15.Li, J. C. et al. Angiotensin II mediates hypertensive cardiac fibrosis via an Erbb4-IR-dependent mechanism. Mol. Therapy - Nucleic Acids. 33, 180–190 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu, M. et al. Zhilong Huoxue Tongyu capsule down regulates Erbb4-IR and inhibits cardiac fibroblast fibrosis in mice via TGFB1/SMAD3 signaling pathway. Pharmacol. Clin. Chin. Materia Med.38, 70–75 (2022). [Google Scholar]
- 17.Chen, J. Y. Guizhi Decoction and Guishao in different proportions regulate TGF-β1/Smads signal pathway and chronic inflammation improve myocardial fibrosis in salt-sensitive hypertensive rats. Chin. J. Experimental Prescriptions. 26, 50–58 (2019). [Google Scholar]
- 18.Wang, J., Li, F., Zhou, X. L. & Zeng, Q. N. Clinical observation on the treatment of myocardial fibrosis in chronic heart failure with modified Zhenwu Decoction. Chin. J. Experimental Prescriptions. 24, 173–178 (2018). [Google Scholar]
- 19.Zhao, X. et al. Zhilong Huoxue Tongyu capsule improves myocardial ischemia/reperfusion injury via the PI3K/AKT/Nrf2 axis. PLoS One. 19, e0302650 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu, M. et al. Zhilong Huoxue Tongyu Capsule Alleviated the Pyroptosis of Vascular Endothelial Cells Induced by ox-LDL through miR-30b-5p/NLRP3, Evid Based Complement Alternat Med. 3981350 (2022). (2022). [DOI] [PMC free article] [PubMed]
- 21.Leask, A. Getting to the heart of the matter: new insights into cardiac fibrosis. Circ. Res.116, 1269–1276 (2015). [DOI] [PubMed] [Google Scholar]
- 22.Oparil, S. et al. Hypertension, Nat. Rev. Dis. Primers4, 18014 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mazhar, M. et al. Zhilong Huoxue Tongyu capsules ameliorate early brain inflammatory injury induced by intracerebral hemorrhage via Inhibition of canonical NFкβ signalling pathway. Front. Pharmacol.13, 850060 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu, M. et al. Zhilong Huoxue Tongyu capsule inhibits rabbit model of hyperlipidemia and atherosclerosis through NF-κB/NLRP3 signaling pathway. Heliyon9, e20026 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu, M. et al. Network Pharmacology and in vitro experimental verification reveal the mechanism of the Hirudin in suppressing myocardial hypertrophy. Front. Pharmacol.13, 914518 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hinderer, S. & Schenke-Layland, K. Cardiac fibrosis - A short review of causes and therapeutic strategies. Adv. Drug Deliv. Rev.146, 77–82 (2019). [DOI] [PubMed] [Google Scholar]
- 27.Wysoczynski, M. et al. Myocardial reparative properties of cardiac mesenchymal cells isolated on the basis of adherence. J. Am. Coll. Cardiol.69, 1824–1838 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fan, Z. & Guan, J. Antifibrotic therapies to control cardiac fibrosis. Biomater. Res.20, 13 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Whaley-Connell, A. T. et al. Salt loading exacerbates diastolic dysfunction and cardiac remodeling in young female Ren2 rats. Metabolism62, 1761–1771 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dobaczewski, M., Chen, W. & Frangogiannis, N. G. Transforming growth factor (TGF)-β signaling in cardiac remodeling. J. Mol. Cell. Cardiol.51, 600–606 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li, Y. et al. Histologic and hemodynamic correlates of right ventricular function in a pressure overload model: a study using Three-Dimensional speckle tracking echocardiography. Ultrasound Med. Biol.44, 467–476 (2018). [DOI] [PubMed] [Google Scholar]
- 32.Craig, J. C. et al. Impaired hemodynamic response to exercise in patients with peripheral artery disease: evidence of a link to inflammation and oxidative stress. Am. J. Physiol. Regul. Integr. Comp. Physiol.323, R710–R719 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lytvyn, Y. et al. Renal hemodynamic function and RAAS activation over the natural history of type 1 diabetes. Am. J. Kidney Dis.73, 786–796 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pan, J. et al. Huangqi shengmai Yin ameliorates myocardial fibrosis by activating Sirtuin3 and inhibiting TGF-β/Smad pathway. Front. Pharmacol.12, 722530 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang, Y. et al. miR-29b as a therapeutic agent for angiotensin II-induced cardiac fibrosis by targeting TGF-β/Smad3 signaling. Mol. Ther.22, 974–985 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xiao, J. et al. miR-29 inhibits bleomycin-induced pulmonary fibrosis in mice. Mol. Ther.20, 1251–1260 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qin, W. et al. TGF-β/Smad3 signaling promotes renal fibrosis by inhibiting miR-29. J. Am. Soc. Nephrol.22, 1462–1474 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang, T., Jiang, X., Ruan, Y., Zhuang, J. & Yin, Y. Based on network Pharmacology and in vitro experiments to prove the effective Inhibition of myocardial fibrosis by Buyang Huanwu Decoction. Bioengineered13, 13767–13783 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lai, X. et al. Efficacy and safety of Chinese herbal medicine compared with Losartan for mild essential hypertension: A randomized, multicenter, Double-Blind, noninferiority trial. Circ. Cardiovasc. Qual. Outcomes. 15, e007923 (2022). [DOI] [PubMed] [Google Scholar]
- 40.Ding, H., Zhou, Y. & Huang, H. MiR-101a ameliorates AngII-mediated hypertensive nephropathy by Blockade of TGFβ/Smad3 and NF-κB signalling in a mouse model of hypertension. Clin. Exp. Pharmacol. Physiol.46, 246–254 (2019). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used for this study are available from the corresponding author upon reasonable request.