Abstract
Astronauts face significant stress in space, and understanding its neurobiological basis is key to assessing risk and resilience. Analogue environments, like the Antarctic Concordia Station, replicate isolated, confined, and extreme (ICE) conditions. This study assessed brain structure changes in 25 crewmembers who spent 12 months at Concordia, with MRI scans conducted before, immediately after, and five months post-mission. The study included 25 controls scanned over a similar interval and 4 “flying phantom” individuals who were scanned at all sites. Gray matter in the temporal and parietal lobes, hippocampus, pallidum, and thalamus as well as global white matter decreased during the mission in crewmembers, with all but the thalamus returning to baseline after five months. Brain ventricle volume increased, and better sleep correlated with less brain volume loss, highlighting its potentially protective role. These findings emphasize the importance of understanding mechanisms driving brain changes, particularly with growing interest in extended space missions in ICE environments.
Subject terms: Anatomy, Neurological manifestations
Introduction
Stress is a ubiquitous experience for humans with both positive and negative health consequences. When stress is long lasting, unexpected or extreme there can be deleterious effects on health and functioning1–3. Chronic stress strains both physiological and cognitive systems, which can degrade their functioning over time. While there is a wealth of knowledge about the impact of acute stressors on the human body and brain, determining the effects of extreme chronic stress is challenging due to ethical limitations on experimental manipulations. Thus, utilizing distinctive groups of participants who are exposed to chronic stressors can help elucidate the physiological and cognitive effects of enduring prolonged stress and shed light upon aspects of resilience to help identify potential countermeasures. Space exploration exposes astronauts to multiple environmental and psychological stressors, which can adversely affect astronaut health and cognitive performance if prolonged4, thereby potentially jeopardizing mission success. Understanding changes in biomarkers associated with risk or resilience under stressors is critical to sustaining mission success. Brain structure is particularly vulnerable to physical and psychological stressors5–10. Indeed, recent Magnetic Resonance Imaging (MRI) studies of astronauts’ brains post-flight have revealed transient structural changes relative to pre-flight baseline measurements11 suggesting that aspects of spaceflight affect brain structure. Brain changes include an upward shift of the brain12; ventricular13,14, cerebrospinal fluid (CSF)13,15,16 and gray matter volume changes14,15; alterations of brain white matter17; and increases in periventricular white matter hyperintensities18. Many of these changes are likely associated with effects of microgravity11; however, the extended exposure to an isolated, confined, and extreme (ICE) environment, which amplifies psychological stress, may further contribute to neuroanatomical changes.
As it takes a long time to study large numbers of astronauts before and after space missions, international space agencies support research in ICE analogues, such as Antarctic research stations, which share key features of exploration spaceflight like confinement, isolation, and threat-to-life19. High-fidelity space analog environments provide an opportunity to better understand the biological basis of stressors that affect astronauts. Individuals that spend the winter in Antarctica (i.e., ‘winter-over’) experience measurable physiological changes, including dyspnea, hypoxia, headaches, hypocapnia, reduced immune response, disrupted sleep, and altered circadian rhythms20. Psychological effects include depressed mood, irritability, insomnia, and cognitive impairment21. The only study of structural brain changes following a 14-month Antarctic winter-over indicates that significant brain changes occur, specifically in the frontal and temporal lobes and within subregions of the hippocampus, and they are associated with poorer cognition and lower levels of brain derived neurotrophic factor (BDNF22), suggesting a stress-induced physiological mechanism of action22. However, this study was small (N = 8) and the post-winter-over MRI was performed several weeks after the return from Antarctica, at which point some structural brain changes may have resolved.
ICE environments result in detrimental physiological changes and thus there is significant interest in helpful countermeasures that may increase resilience to these physiological changes. For example, over-wintering in Antarctic research stations results in decreased slow wave sleep, total sleep time and sleep efficiency and increased sleep fragmentation and lighter sleep stages23 culminating in a general worsening of sleep. These sleep disturbances likely affect performance, yet the specific neural mechanisms of sleep disruption are unclear and may be attributable to environmental stress24–26. Regular exercise is beneficial for sleep27 and cognitive performance28 and could therefore help reduce some of the negative effects of spending prolonged times in ICE environments. In fact, exercise is often used as a countermeasure to mitigate some of the effects of space travel in astronauts29. Altogether, the relationship between sleep, exercise and brain health in ICE is complex, appears to be rather heterogeneous and in need of better elucidation30.
The primary objective of this study was to investigate neuroanatomical brain changes, as measured using MRI, in two crews that completed winter-over missions in the Antarctic Concordia Station. Crewmembers underwent MRI before departure to Antarctica (Pre), immediately upon returning from Antarctica (Post1) and on average five-months after returning (Post2). Exercise, sleep, and cognitive performance were monitored during the winter-over. We hypothesized that crewmembers would experience a subtle decline in gray matter brain volume, as suggested by previous spaceflight11 and analogue studies19 after extended time in an ICE environment, but the reductions in gray matter brain volume would resolve post-mission. Moreover, based on extant findings of the utility of exercise29 and sleep23 as potential countermeasures of isolation, we hypothesized that more vigorous activity and better sleep would be associated with smaller changes in gray matter brain volume. Finally, we hypothesized that a decline in cognitive performance during the winter-over would be associated with a decline in gray matter brain volume based on previous report of the effects of ICE environments on cognitive performance31.
Results
Longitudinal brain changes after ICE
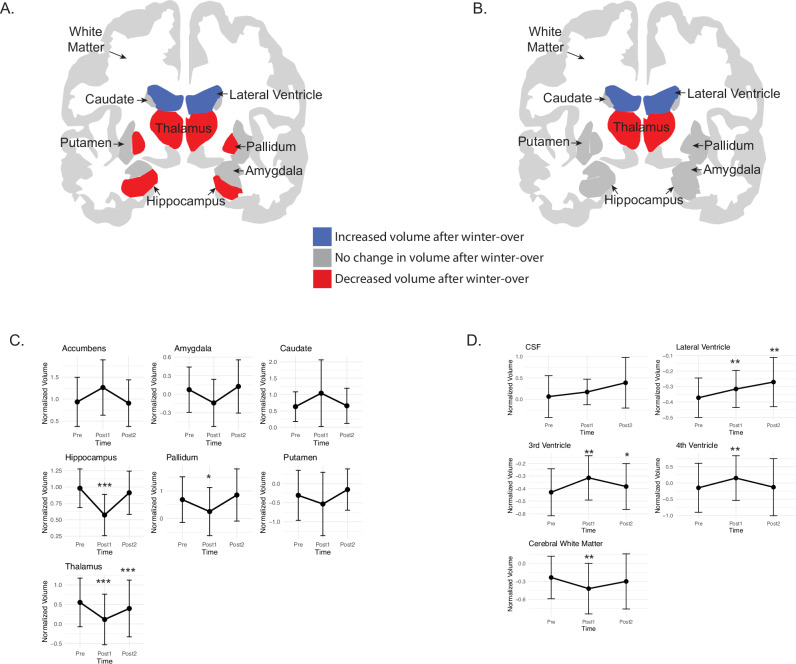
Cortical gray matter volume in the parietal [F(2, 40) = 25.54, p < 0.001; ηp2 = 0.40] and temporal [F(2, 40) = 10.87, p < 0.001; ηp2 = 0.35; Fig. 1A–C] lobe changed significantly over the ICE mission. There were no changes in frontal or occipital gray matter volume. Parietal [t(40) = 6.19, p < 0.01; ηp2 = 0.48] and temporal [t(40) = 4.66, p < 0.01; ηp2 = 0.35] lobe gray matter volume decreased from baseline (Pre) to post-mission (Post1), but returned to baseline levels by five months post mission (Post2; Fig. 1B). Subcortical gray matter volume in the hippocampus [F(2, 40) = 28.52, p < 0.001, ηp2 = 0.57], pallidum [F(2, 40) = 13.55, p < 0.001, ηp2 = 0.40], and thalamus [F(2, 40) = 47.29, p < 0.001, ηp2 = 0.70; Fig. 2A–C] changed over the ICE mission. There were no changes in gray matter volume in the accumbens, amygdala, caudate or putamen. Post-hoc analyses indicated hippocampus [t(40) = 7.28, p < 0.01; ηp2 = 0.56], pallidum [t(40) = 3.06, p < 0.01; ηp2 = 0.19] and thalamus [t(40) = 9.63, p < 0.01; ηp2 = 0.70] gray matter volume decreased from baseline (Pre) to post-ICE mission (Post1). Hippocampus and pallidum volumes returned to baseline levels after five months (Post2); however thalamus [t(40) = 3.36, p < 0.01; ηp2 = 0.43] gray matter volume remained significantly smaller than baseline at Post2 (Fig. 2B & C; Supplemental Table 1). Individual trajectories over time are shown in Supplemental Fig. 1.
Fig. 1. Crewmembers show a transient decrease in gray matter volume in some cortical brain regions after a winter-over at the Concordia station.
Gray matter brain volume was lower in the temporal and parietal lobes immediately after winter-over (Post1; A) as compared to baseline but returned to baseline levels five months after return from Antarctica (Post2; B). Trajectory of change for each cortical gray matter volume is shown in C. Error bars reflect 95% confidence intervals. All gray matter volumes are normalized to flying phantoms who were scanned at each site. Sensitivity analyses, individual trajectories and analyses for control subjects who did not winter-over are shown in the Supplement Note 1. Significance values are represented as **p < 0.01; ***p < 0.001. All p-values shown are Bonferroni corrected. Images were created using ggseg82.
Fig. 2. Crewmembers show a decrease in gray matter volume in several subcortical brain regions and increase in ventricular volume after a winter-over at the Concordia station.
Gray matter brain volume was lower in Hippocampus, Pallidum and Thalamus immediately after winter-over (Post1, A and C) as compared to baseline. Hippocampus and Pallidum volumes returned to baseline levels five months after return from Antarctica (B, D), but Thalamus volume remained lower. Volume of the Lateral, 3rd and 4th Ventricles all increased after winter-over (A), (C). Volume of the Lateral and 3rd ventricles remained elevated five months after return from Antarctica (B, D). All volumes are normalized to flying phantoms who were scanned at each site. Sensitivity analyses, individual trajectories and analyses for control subjects who did not winter-over are shown in the Supplemental Note 1. Note: Total cerebral white matter volume also decreased from Pre to Post1, but is not highlighted in red. Significance values are represented as *p < 0.05, **p < 0.01; ***p < 0.001. All p-values shown are Bonferroni corrected. Error bars in C and D reflect 95% confidence intervals. Images were created using ggseg82.
Notably, volume of the Lateral [F(2,40) = 17.32, p = 3.78×10−6; ηp2 = 0.46], 3rd [F(2,40) = 21.28, p = 5.07×10-7 ;ηp2 = 0.52] and 4th ventricle [F(2,40) = 10.78, p = 1.7×10–4 ηp2 = 0.35] also changed significantly over the ICE mission (Fig. 2A & C, B & D; Supplemental Table 1). Volume of the CSF did not change. In the Lateral ventricle, post-hoc comparisons indicated that there was a significant volume increase from baseline to immediate post ICE mission (Post1), [t(40) = −5.24, p < 0.01, ηp2 = 0.49] and remained higher at Post2 when compared to baseline, [t(40) = −5.03, p < 0.01, ηp2 = 0.38]. Volume of the 3rd ventricle increased from baseline to Post1, [t(40) = −6.52, p < 0.01, ηp2 = 0.52] and remained elevated at Post2 as compared to baseline, [t(40) = −3.06, p = 0.01, ηp2 = 0.19]. Finally, volume of the 4th Ventricle increased from baseline to Post1, [t(40)= −4.38, p < 0.01, ηp2 = 0.32] but returned to baseline levels by Post 2 (Supplemental Table 1). Finally, cerebral white matter volume [F(2,40) = 15.47, p = 1.05×10−5; ηp2 = 0.44] also changed significantly over the ICE mission (Fig. 2D). Post-hoc comparisons indicated that there was a significant white matter volume decrease from baseline to immediate post ICE mission (Post1), [t(40) = 3.87 p < 0.01, ηp2 = 1.13] that returned to baseline levels at Post2. Prior to analysis with longitudinal Freesurfer, raw intracranial volume was compared by site for crewmembers. There were no differences in raw ICV over time [F(2,64) = 0.35, p = 0.70]. In addition, there were no differences in Euler values across time points [F(2,62) = 0.89, p = 0.41; Supplemental Fig. 2]. As indicated on the box plot there were two potential outliers. Removal of these data points did not change the pattern of results.
Several sensitivity analyses were completed to assess the validity of above results (see Supplemental Note 1). Data quality was high and did not differ by site (Supplemental Fig. 2). There were no time of day effects on gray matter volume (Supplemental Note 2; Supplemental Fig. 3) and age-trends were equivalent by site (Supplemental Fig. 4). Distribution of MRI volumes in the crew were largely symmetric and approximately normal across sex (Supplemental Fig. 5) and site (Supplemental Fig. 6) after implementing the longitudinal pipeline of Freesurfer and normalizing brain volume by the Flying Phantoms. Limiting analyses to only crew who completed baseline at Cologne did not change the results (Supplemental Figs. 7 & 8). Notably, unadjusted gray matter brain volume did not differ at baseline between crew and controls (Supplemental Fig. 9) nor were there any significant changes in brain volume in the control sample at Post1 or Post2 relative to baseline (Supplemental Figs. 10 & 11). In controls, lateral and 3rd ventricular volumes significantly changed over time (Supplemental Figs. 12 & 13). Volume in both ventricles remained similar between Pre and Post1, but declined by Post2 relative to Pre. Thus, ventricular volume changes in controls show the opposite pattern of change than those observed in crew.
Volumetric brain changes and exercise, sleep and cognition
In exploratory correlation analyses between pre-post winter-over brain volume changes and behavioral outcomes, we examined consistency in addition to the strength of effects (Fig. 3A). These associations were limited to cortical and subcortical regions showing significant change over time. We acknowledge that there are many correlations in the present analysis, and with the present sample size none of the reported correlations survive stringent multiple comparison correction (Supplemental Note 3). Nonetheless, we briefly highlight notable trends between brain change and mean behavioral and cognitive performance during winter-over.
Fig. 3. Effect size (Pearson’s r) between change (Post1 – Pre) in regional brain volume and average sleep, exercise, self-reported arousal measures, and cognitive performance during the winter-over.
Cells in the table are labeled by positive (green) and negative (red) associations between change in brain volume and average behavior/performance. Positive correlation coefficients indicate that high values in the behavior/performance domain were associated with gray matter volume preservation, whereas negative correlation coefficients indicate high values in the behavior/performance domain were associated with gray matter volume loss. Cognition Tasks: PVT Psychomotor Vigilance Test, BART Balloon Analog Risk Test, DSST Digit Symbol Substitution Test, MRT Matrix Reasoning Test, ERT Emotion Recognition Test, LOT Line Orientation Test, AM Abstract Matching Test, NBCK N-back Working Memory Test, VOLT Visual Object Learning Test, MP Motor Praxis Test.
Better sleep was consistently associated with gray matter volume preservation in the temporal lobe, parietal lobe, and hippocampus, suggesting a protective effect of sleep against volume loss: time in bed, night sleep, total sleep time and sleep efficiency during the winter-over were correlated with smaller decreases in brain volume in the crew. The level of general waking activity (i.e., sedentary, light, moderate, or vigorous) showed no clear pattern with brain volume changes. However, gym use with presumably the highest levels of physical exertion was consistently associated with brain volume preservation. Survey responses indicating “better” outcomes or responses were in general related with more cortical and subcortical brain volume loss in the temporal lobe, hippocampus and pallidum, but the opposite was true in the parietal lobe and thalamus. Finally, the exploratory associations between gray matter volume change and cognitive performance indicated that higher speed and accuracy on the PVT, ERT, AM, NBCK, and MP were consistently associated with volume loss across cortical and subcortical regions, whereas LOT accuracy and speed were associated with volume preservation. No clear patterns emerged for BART, DSST, MRT, and VOLT.
Discussion
Using longitudinal high-resolution structural MRI in crewmembers who wintered over in Antarctica, we found that extended exposure to an ICE environment resulted in reduced gray matter brain volume. The observed reduction in gray matter volume was diffuse, but most pronounced in the temporal and parietal cortices, hippocampus, pallidum and thalamus. Importantly, brain volume in all regions, except the thalamus, returned to baseline levels five months after return from Antarctica. Gray matter volume reductions were accompanied by a nominal increase in CSF and a significant increase in lateral, 3rd and 4th ventricular volume; with lateral, 3rd ventricular volume remaining elevated at five months follow-up. In exploratory correlational analyses, better sleep—in both total time and efficiency—was associated with less pronounced reductions in gray matter volume as were some aspects of physical activity, including gym use. Brain-behavior associations indicated that a greater reduction in brain volume was puzzlingly associated with better performance across several cognitive domains. Altogether the current data provides evidence for transient anatomical brain alterations during prolonged ICE exposure and indicates that most of these gray matter changes return to baseline levels 5 months after returning from Antarctica and may be associated with patterns of sleep, wake activity levels, and cognitive performance.
A transient reduction in gray matter volume during ICE exposure could be due to a myriad of factors. It is possible that gray matter volume change is a direct product of a long-term stress response to the ICE environment. The neuronal response to stress is complex and dynamic, comprising a range of molecular changes that are coordinated at several levels to monitor function and determine an optimal response to threatening challenges32. The stress response may help bring the brain and body back to homeostasis, thus shielding individuals from acute alterations and maintaining their ability to function33. Thus, the stress response during an extended winter-over mission may be crucial for an individual’s adaptation to this new environment and may trigger physiological changes in the brain to optimize an individual’s experience. Brain anatomy studies of extended stays in ICE environments are rare22,34, and challenge our ideas on the adaptive nature of the brain. Nonetheless, the present findings of ICE-associated gray matter volume reduction in our sample align with a recent investigation of anatomical volume changes in eight Antarctic explorers22. Stahn et al.22, found lower brain volume in two regions of the frontal cortex and focal changes in the hippocampus and parahippocampus after 14 months in another Antarctic station (Neumayer). Moreover, volume reductions in the hippocampus were associated with lower levels of BDNF and lowered performance on tasks requiring spatial abilities, selective attention and resolution of response conflicts, which specifically target the hippocampus35,36. We found volumetric reductions in several structures, including the hippocampus, after 12 months in a similar environment in a sample three times larger. Notably, Stahn et al.22 used high resolution T1- and T2-weighted MRI scans to segment the hippocampus while the present data was limited to only T1 weighted images. But, unlike Stahn et al.22, we did not find gray matter reductions in the frontal cortex, although we did not specifically analyze the dorsolateral or orbitofrontal cortex. It is possible that finer grain parcellations may show convergent results and future studies in larger samples can better elucidate focal cortical changes. Nonetheless, we replicate the transient changes in the hippocampus and show that it had robust associations with levels of sleep. Importantly, the reported gray matter changes within the hippocampus were transient as volume was fully recovered five-months post-mission. This was the case for most regions except the thalamus, which remained at lower volume 5 months after the winter-over.
The thalamus is a key relay station in the brain, serving as a central hub that receives incoming sensory information and then directs it to the appropriate cortical areas for further processing37. Beyond its role in sensory integration, it also helps regulate states of arousal, attention, and consciousness by interacting closely with various cortical and subcortical regions38. Chronic stress7, stress-related disorders39 and microgravity17,40 have been associated with structural and functional changes in the thalamus. In general, our findings suggest that spending a significant time in an ICE environment may contribute to subtle decreases in thalamic size or changes in its internal structure. Over time, such changes could potentially influence how information is relayed and integrated, which may have downstream effects on cognition, mood, and emotional regulation.
We also found a transient decrease in a global measurement of cerebral white matter, which aligns with one previous diffusion weighted MRI study in participants of the MARS500 project34. Other spaceflight analogues (e.g. head-down tilt best rest) report limited white matter changes41 that differ from spaceflight-associated white matter changes17,42. Importantly, diffusion MRI provides much higher resolution of cerebral white matter than the global measure used in the current study and has been implement in astronauts17,42. While preliminary the present findings indicate that ICE environments also have measurable effects on brain white matter microstructure.
Finally, we also found a concomitant increase in the volume of the lateral and 3rd ventricles in crew suggesting possible volumetric redistribution to the fluid compartments of the brain. This redistrubtion may be attritutable to several aspects of the ICE enviroment including relative deprivation, monotony or the hypoxic environment at the Concordia station. Previous work has found that monotonous sensory stimulation, boredom, as well as isolation and confinement, are severe stressors that can lead to a variety of negative outcomes43,44; however, the extent to which this type of deprivation results in brain changes, and changes in cognitive performance remains unclear. Spaceflight13 and prolonged hypoxia45 are known to alter fluid dynamics in the ventricles of the brain likely due to compensatory mechanisms and there is significant interindividual variability in this response46. In spaceflight there is a reduced capability for CSF resorption which increases pressure on the gray matter leading to volume reduction, which would not have been a contributor to changes in vetricular volume in the present study. But, it is possible that redistribution between brain compartments in the ICE environment may be associated with the hypoxic environment at Concordia station; however these changes were smaller than those seen in astronauts. As such additional studies need to better elucidate those changes reported here since they are likely of different origin than what has been reported in spaceflight. It is also possible that age-related changes47 in brain volume could explain some of the present findings, but the crews that wintered-over were relatively young. Changes in nutrition48 and hydration status49 can also affect brain volume, but these were not measured as part of the present study, so the potential impact of these physiological changes remains unknown. Nonetheless, monitoring brain structure after long-duration missions seems informative and could be of critical importance when considering exposure of humans to long duration ICE environments.
Sleep duration and efficiency were associated with brain volume conservation, especially for the temporal cortex and hippocampus. Both adequate sleep duration and quality are of paramount importance for daytime neurobehavioral function50. Recent studies have shown that glymphatic spaces open up during sleep and contribute to clearance of metabolic products, some of which have been implicated in the genesis of neurodegenerative disease (e.g., β-amyloid and tau-protein)51. Animal models show that chronic sleep loss is associated with cell death of select populations of neurons, and that the likelihood of irreversible degeneration increases with the duration and severity of sleep loss52. Notably, short term sleep deprivation is associated with thalamic gray matter volume decline in men53 and smaller hippocampal volume in patients with chronic sleep disturbance54. That daytime sleep was the only investigated sleep variable associated with cortical gray matter volume loss may be explained by the possibility that those sleeping during the day likely tried to make up sleep loss during the night (nighttime and daytime sleep were negatively correlated with r = -0.74). If replicated in future studies, these findings stress the importance for the brain of sufficient sleep in environments that are stressing the brain in other ways (e.g., isolation, confinement, threat-to-life, hypoxia). The observation that ISS astronauts regularly sleep less than the recommended 7-8 h per day is of potential concern considering our findings55–57.
We used wrist-actigraphy to objectively assess sleep-wake cycles and wake activity levels. A unique proximity feature of these actigraphs provided us with information on where and for how long a crewmember spent their time, including the gym. Our data suggest that gym use was most consistently associated with less gray matter volume loss. These findings reflect the typically observed beneficial effects of exercise on brain health58,59, as more gym time was associated with less gray matter volume change. Clearly one of the goals of performing research at Concordia station, which is situated at an altitude of 3200 m with an air pressure of ~645 hPa, is to determine if certain countermeasures are effective in a chronic hypobaric and hypoxic environment. The present findings suggest that spending more time in the gym, where presumably higher intensity level activity occurs, results in protective effects on the brain. However, specific exercises were not tracked while crewmembers were in the gym. Overall, this association suggests that certain levels of activity likely need to be achieved in hypoxic environments for beneficial effects to the brain and that future countermeasure studies should carefully consider the environmental conditions experienced in analogues. Indeed, exercise with the Advanced Resistive Exercise Device (ARED), elevated CO2 levels and a cephalad fluid shift have all been implicated in the development of the Spaceflight-Associated Neuro-Ocular Syndrome (SANS)60.
The associations between volume change and cognitive performance change generally showed an unexpected and puzzling effect where less reduction in brain volume was associated with poorer performance levels in several cognitive domains, but these effects were generally small. Typically, larger volume and more preservation of brain volume is associated with better cognitive performance61. We speculate that conserving neural resources through brain volume reduction is adaptive for maintaining optimal levels of performance during extended periods of stress. Additional work is needed to confirm and interpret this hypothesis.
The current study has several limitations. First, the physical and psychological effects of ICE environments are likely heterogeneous and non-specific, thus individual differences likely contribute to risk and resiliency under these conditions. Hence, changes in brain anatomy could be a consequence of many factors in an ICE environment. Longitudinal MRI studies of individuals in Antarctica are challenging but we measured brain volume in crewmembers immediately upon return from the winter-over, thereby improving the likelihood of capturing ICE-related brain changes. Moreover, we collected data from human phantoms at each study site and implemented rigorous methods to overcome site effects before study implementation by matching the MRI acquisition protocols. Yet, practicalities of implementing the study design resulted in substantial confounds between MRI scanner and time point and controls could only be scanned at one location, thus creating additional limitations. Future endeavors can address these limitations by acquiring data on the same scanner across all time points or implementing portable low-field scanning62 in the Antarctic. By design these studies include small sample sizes due to the physical limitations of the Antarctic stations, therefore our correlative analyses are exploratory in nature. None of these associations survive multiple comparison corrections. Another limitation is that we limited our main examination to gray matter volume data. We included a coarse measure of white matter volume, which also showed a transient change in crewmembers, but, white matter parcellation of T1-weighted images is not ideal for precise parcellation of white matter bundles. Hence, we considered these finding to be rather preliminary and suggest that more refined measures (e.g., diffusion MRI) of brain white matter be assessed in ICE. ICE environment examined here could also affect brain structural and functional connectivity, as well as blood oxygenation level dependent response to activation by cognitive tasks. Such measures were obtained in this study but are beyond the scope of this manuscript. Our anatomical findings will nonetheless help guide analyses of the connectivity and functional data. Finally, our sample was of mostly healthy young adults, which included few women, who volunteered to endure the stressful environment. They likely underestimate the effects of harsh environments on populations who did not volunteer to be exposed to it and are not young and healthy. Additional methodological limitations include the lack of availability of laboratory (e.g., blood work) values for crew participants and a lack of detailed stressful life event information for control subjects during the study period. These data should be interpreted with caution and it should be emphasized that associations reported in this study do not necessarily represent cause and effect.
In conclusion, life in space and on Earth entails exposure to and requires resilience to stress. Elucidating the effects of prolonged stress in extreme conditions highlights significant, but transient brain changes relevant for optimal physiological and cognitive functioning. Recent interest in extended space exploration, where individuals will be subjected to extreme isolation and confinement, compels elucidating mechanisms contributing to transient and chronic changes in brain anatomy. The current data and future studies in ICE environments, including space travel, reemphasize the need to identify specific countermeasures that may mitigate changes in brain anatomy when individuals are isolated. Moreover, the relevance of the current findings has broadened with the increase in isolation in the general population63–65. Our findings may be informative to the population at large, many of whom have suffered chronic stress over the past years.
Methods
Concordia station as an ICE environment
The French/Italian Concordia station is located at Dome C on the Antarctic Plateau at 75° 06’ S, 123° 23’ E at an altitude of 3200 m (4000 m equator equivalent). Concordia is 685 miles inland from Dumont d’Urville (French coastal station) and 750 miles inland from Mario Zucchelli Station at Terra Nova Bay (Italian coastal station). This area is one of the most hostile places on Earth. Air pressure averages 645 hPa (i.e., chronic hypobaric hypoxia). Access to and rescue from Concordia station is limited to the austral summer (November to February) due to extreme weather conditions (average temperature -76°F in winter, very low humidity, very little precipitation). The landscape is flat and barren.
The sun does not rise for several weeks during the Antarctic winter. Concordia station consists of two buildings and a container housing the generators. One building is designated as the “calm building”, which houses laboratories, private quarters, a hospital, a radio room, and a weather station. The other building is designated as the “noisy building”, which houses workshops and technical offices, storage shelves, a gym, a video room, kitchen and restaurant, and a meeting/common room. Each crewmember has his/her own private quarters.
Participants
Two separate crews wintered over at Concordia station in 2015 (N = 13) and 2016 (N = 12). Crewmembers averaged 37.2 (SD = 12.1) years at baseline. Twenty of the crewmembers were male and five were female. All crewmembers underwent medical and psychological screening prior to selection. Selection process for participants has been detailed previously66. Crewmembers also received psychological training to prepare them for possible psychological and social issues that an ICE environment may cause67. Individual crewmembers arrived at and departed from Concordia station during the Austral summer and stayed an average of 12.7 months (range 10.0–14.4 months).
Control individuals underwent similar screening as the crew66, but did not travel to, or winter-over, in Antarctica. These individuals were selected from employees of the German Aerospace Center (DLR) in Cologne. Individuals were excluded for pregnancy and contraindication for MRI. Twenty-five healthy control participants matched as well as possible according to age and sex to the crew were enrolled at the DLR in Cologne (CGN), Germany, over a similar timeframe as the Crew. Controls were 36.9 (SD = 10.2) years old at baseline. Twenty-one of the control participants were male and four were female.
Four non-crew individuals (‘flying phantoms’) were enrolled and traveled to each study site. Data were used to standardize measurement acquisition by MRI site. Flying phantoms included four male participants (authors MB, AJE, RCG, KP) mean age=46.5; SD = 14.3 years. Phantom scanning occurred in Cologne, followed by Christchurch, New Zealand (CHR) then Hobart, Australia (HOB).
This study was approved by the institutional IRB of NASA Johnson Space Center, the University of Pennsylvania, and by Ärztekammer Nordrhein, Cologne, Germany. All participants provided written informed consent after receiving a complete description of the study.
Neuroimaging procedure and analysis
T1-weighted structural MRIs were acquired to measure changes in gray matter, cerebrospinal fluid and ventricular volume in the brain. Imaging parameters (Supplemental Table 2) were harmonized and optimized across sites. Baseline MRI scans (Pre) of the crew were completed 1.5 months (range 0.0−3.4 months) before arriving at Concordia station at DLR in CGN. A second scan (Post1) was performed within 16 days after crewmembers departed from Concordia station (range 7–24 days after departure and 10.7–16.2 months after the baseline scan) either in CHR or HOB. The crew was scanned again in CGN one more time (Post2) 4.9 months (range 3.9–6.3 months) after their departure from Concordia station. All participants provided usable data for at least one time point.
Controls were scanned at CGN. Post1 scans occurred 13.6 months (range 12.5–15.2 months) after the first scan and Post2 scans occurred 7.4 months (range 5.7–9.7 months) after the Post1 scan. Flying phantoms were scanned twice at CGN (October 2015 and May 2017) and once at HOB and CHR (September 2015).
Where possible pre-mission (Pre) and follow-up (Post2) MRI scans were collected at CGN. Two baseline scans were performed in HOB and one baseline scan was performed in CHR instead of CGN. One crewmember had no Pre scan, another crewmember was only scanned at the Pre time point, and 5 crewmembers did not have a Post2 scan.
For crewmembers, 24 pre-mission (Pre), 24 post-mission (Post1), and 19 follow-up (Post2) scans passed quality assurance. For controls, 25 pre-mission (Pre), 21 post-mission (Post1), and 15 follow-up (Post2) scans were included in the final analysis.
Freesurfer (version 7.4) was used to extract bilateral subcortical and cortical gray matter volumes. Subcortical gray matter brain regions measured included: accumbens, amygdala, caudate, hippocampus, pallidum, putamen, and thalamus. Cortical gray matter regions were combined within each lobe (e.g., Frontal, Parietal, Temporal and Occipital) to be comparable with published space flight data42 and to reduce multiple comparisons. White matter brain regions were not evaluated. Images were further automatically processed with the longitudinal stream68 in FreeSurfer. This stream creates an unbiased within-subject template space and image69 using robust, inverse consistent registration70. Several processing steps, such as skull stripping, Talairach transforms, atlas registration as well as spherical surface maps and parcellations are then initialized with common information from the within-subject template, significantly increasing reliability and statistical power68. Once the longitudinal stream completed, volume from cortical gray matter regions of interest were extracted using the Desikan-Killiany (lh/rh.aparc.annot) atlas. Volume of subcortical gray matter brain regions were extracted using the default Freesurfer subcortical region segmentation. Total cerebral white matter volume was also extracted from the Freesurfer output. Using data from the flying phantoms, values were normalized (z-transformed) for each site. Data quality was assessed using Euler values extracted from Freesurfer. Euler values offer a robust measurement of data QA71.
Alertness and mood survey, cognitive testing and wrist-actigraphy
The crew completed alertness and mood surveys and the Cognition72 test battery at baseline and monthly while at Concordia station. The alertness and mood surveys assessed self-reported aspects of sleep time, sleep quality, physical and mental fatigue, stress level, workload, and emotional distress with 11-point Likert-type scales. On the survey, the crew entered the time they tried to fall asleep and woke up, which was used as an estimate of their sleep duration. They then indicated their status on the following thirteen 11-point Likert scales (anchors are provided in parenthesis after each question; the middle point was labeled “neutral”): (1) What was the quality of your sleep? (good-poor); (2) What was today’s workload? (very high-very low); How are you feeling right now? (3) (not sleepy at all-very sleepy); (4) (happy-unhappy); (5) (sick-healthy); (6) (energetic-physically exhausted); (7) (mentally sharp-mentally fatigued); (8) (not stressed at all-very stressed); (9) (tired-fresh, ready to go); (10) (very depressed-not depressed at all); (11) (very bored-not bored at all); (12) (not lonely at all-very lonely); (13) What is your current everyday life like? (very monotonous-not monotonous at all). All scores were keyed for analysis such that higher numbers indicated better/positive outcomes.
Cognition is a cognitive test battery that was developed for NASA and emphasizes tests that have either been used extensively in spaceflight or that assess cognitive domains of particular interest in spaceflight73,74, such as executive functions, spatial orientation, emotion recognition, and risk decision making72,73,75,76. The 10 Cognition tests were modified to reflect the high aptitude and motivation of astronauts, and the brain regions primarily recruited by each test have been previously established. Importantly, Cognition has 15 unique stimulus sets (i.e., versions) that allow for repeated administration without the need to re-use stimulus sets. One standard speed and accuracy outcome for each of the 10 Cognition tests was measured. Correction factors to adjust for practice and stimulus set difficulty effects have been established and were applied prior to analyzing the cognitive data31. A detailed overview of Cognition can be found in Basner et al.72. All 10 tests were always administered in the same order.
Test order, abbreviations and names for Cognition: (1) MP=Motor Praxis Test; (2) VOLT=Visual Object Learning Test; (3) NBCK = N-back Working Memory Test; (4) AM=Abstract Matching Test; (5) LOT=Line Orientation Test; (6) ERT=Emotion Recognition Test; (7) MRT= Matrix Reasoning Test; (8) DSST = Digit Symbol Substitution Test; (9) BART = Balloon Analog Risk Test; (10) PVT = Psychomotor Vigilance Test.
Concordia crewmembers were asked to continuously wear a Bluetooth® enabled actigraph (Actigraph Link, Actigraph, Pensacola, FL) on the wrist of the non-dominant arm (Supplemental Fig. 14). The methodology is validated to provide reliable estimates on wake and sleep times and activity levels77,78. Average sleep probability and average activity levels were calculated for each day of the mission. Actigraphy is a non-invasive method to gather objective sleep-wake data over prolonged periods of time based on wrist movement. Sleep periods were automatically scored with the ActiLife software (version 6.13.4; settings: Cole-Kripke79 and “use Actigraph algorithm”). The automatic scoring was visually inspected and sleep periods that were obviously scored incorrectly were corrected manually. Epochs within a sleep period that had 0 activity counts in all 3 axes were scored as SLEEP. Epochs within a sleep period that had >0 activity counts in at least one of the 3 axes were scored as WAKE (see Fig. S2 below for axis orientation).
All epochs not falling within a sleep period were also scored as WAKE. Average sleep probability was determined based on epochs with sleep-wake scoring stratified by month of the year (February to November), weekday (Sunday to Monday), and time of day (1440 one-minute epochs). These averages were used for single imputation of epochs classified as off-wrist. Time in bed (TIB) and Sleep Efficiency (SE) were determined based on scored sleep periods only (i.e., not using imputed sleep probability outside sleep periods). SE was defined as SLEEP/(WAKE + SLEEP). Average activity levels were calculated for each day of the mission based on WAKE epochs outside of sleep periods. Activity outcomes were only generated if at least 60 epochs were classified as WAKE in a given 24-h period. First, the vector magnitude (VM) was calculated for each epoch using Eq. (1):
| 1 |
In addition to average activity levels, the percentage of time spent in sedentary activity (VM ≤ 99), in light activity (VM 100-2019), in moderate activity (VM 2020−5998), and in vigorous activity (VM > 5998) were determined according to Troiano et al.80. Several crewmembers chose to take off the actiwatch during the night. Six crewmembers were excluded from the analysis because they had 36.2% or less wear-time during the period 9 pm – 9 am. Two crewmembers in year 2 stopped data acquisition in April 2016. The remaining 17 crewmembers (9 from year 1 and 8 from year 2) contributed to the analysis. They had at least 69.8% wear-time during the period 9 pm – 9 am (average wear-time: 88.6%) and at least 75.6% wear-time during the period 9 am – 9 pm (average wear-time: 89.3%). A Bluetooth® proximity feature of the actiwatches was also used to determine the amount of time each crewmember spent in the station’s gym based on how often a beacon placed in the gym was detected by the actiwatch.
Statistical analyses
Since the MRI data were collected in a longitudinal, multi-site manner, Longitudinal Freesurfer (v7.4)68 was utilized to measure change over time. All raw gray matter volume measures were normalized and converted to Z-scores based upon the gray matter volume of the Flying Phantoms at each site. All raw gray matter volume data is presented in Supplemental Table 3.
Standard linear mixed-effects models were used to analyze changes in gray matter, CSF or ventricular volume over time and to account for missing data points. Participant was included as a random effect.
For changes in gray matter brain, CSF and ventricular volume, statistical significance was set at an alpha-level of 0.05 and p-values were adjusted for multiple comparisons using Bonferroni corrections. All statistics were computed in R (version 4.3.1) and figures were generated using R packages ‘ggplot2’81 or ‘ggseg’82 and effects sizers were calculated using ‘effectsize’ package83.
Exploratory correlation analyses were used to associate changes in total cortical or total subcortical gray matter brain volume with average survey responses, cognitive performance, sleep duration and efficiency, daytime physical activity, and exercise frequency. Correlations between Pre to Post1 changes in gray matter brain volume and behavioral values observed during the winter-over (i.e., averaged across multiple observations) were analyzed in an exploratory framework. An exploratory approach was taken since the study included a limited number of subjects and timepoints, but a large number of behavioral and cognitive assessments.
For each behavioral measure (cognitive, sleep, etc.), an average of all values collected during the winter-over period was calculated. To account for age effects across subjects, age, age squared, and age cubed were regressed out of the means for behavioral and imaging data, resulting in the final data set used.
A positive correlation between gray matter volume for a given brain region and a behavioral outcome indicates that better brain change (less decline) was associated with a higher average behavioral outcome. Negative correlations indicate the opposite: volume loss associated with increase in the behavioral outcome. Detailed analysis for each of these behavioral outcomes are beyond the scope of this manuscript and will be reported elsewhere.
Supplementary information
Acknowledgements
We thank, first and foremost, all study participants who generously volunteered their time and efforts to complete the assessments. Furthermore, we also thank Institut Polaire Français Paul-Émile Victor (IPEV) and Programma Nazionale di Ricerche in Antartide (PNRA) as well as the European Space Agency (ESA) for logistical support. We also thank the MR technologists and research coordinators at all sites who made sure that high quality data were acquired. This work was supported by the National Aeronautics and Space Administration grant 80NSSC19K1046 (MB); the German Aerospace Center, DLR (BJ).
Author contributions
MB, DD, BJ, and RCG conceived the study. MB, DD, BJ, and RCG designed the study. DRR, MB, KR, JCB, TMM, and RCG accessed and verified the data. DRR and KR completed neuroimaging analysis. TMM completed phenotypic analysis with guidance from MB. MB, KR, AS, JN, EH, AJE, MMA, MGS, CWJ, DAG, BH, FPG, HCG, HG, TRM and RCG assisted with study setup and data collection. DRR, TMM and KR completed the statistical analysis under the guidance of RTS and JCB. DRR and MB produced the first draft of the manuscript. All authors reviewed and commented on the manuscript. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Data availability
The raw data supporting the conclusions of this manuscript can be requested by qualified researchers from NASA’s Life Science Data Archive (https://lsda.jsc.nasa.gov/).
Code availability
Statistical code is available here: https://github.com/upenn/nasa_concordia.git.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: David Roalf, Mathias Basner.
Supplementary information
The online version contains supplementary material available at 10.1038/s41526-025-00497-6.
References
- 1.McEwen, B. S. Stellar E. Stress and the individual: Mechanisms leading to disease. Arch. Intern Med.153, 2093–2101 (1993). [PubMed] [Google Scholar]
- 2.Marin, M.-F. et al. Chronic stress, cognitive functioning and mental health. Neurobiol. Learn Mem.96, 583–595 (2011). [DOI] [PubMed] [Google Scholar]
- 3.Miller, G. E., Chen, E. & Zhou, E. S. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychol. Bull.133, 25 (2007). [DOI] [PubMed] [Google Scholar]
- 4.Garrett-Bakelman, F. E. et al. The NASA Twins Study: A multidimensional analysis of a year-long human spaceflight. Science364, eaau8650 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sheline, Y. I. 3D MRI studies of neuroanatomic changes in unipolar major depression: the role of stress and medical comorbidity. Biol. Psychiatry48, 791–800 (2000). [DOI] [PubMed] [Google Scholar]
- 6.Golkar, A. et al. The influence of work-related chronic stress on the regulation of emotion and on functional connectivity in the brain. PLoS ONE9, e104550 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Magalhães, R. et al. The dynamics of stress: a longitudinal MRI study of rat brain structure and connectome. Mol. Psychiatry23, 1998–2006 (2018). [DOI] [PubMed] [Google Scholar]
- 8.Stranahan, A. M., Khalil, D. & Gould, E. Social isolation delays the positive effects of running on adult neurogenesis. Nat. Neurosci.9, 526–533 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schloesser, R., Lehmann, M., Martinowich, K., Manji, H. & Herkenham, M. Environmental enrichment requires adult neurogenesis to facilitate the recovery from psychosocial stress. Mol. Psychiatry15, 1152–1163 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gould, E., McEwen, B. S., Tanapat, P., Galea, L. A. & Fuchs, E. Neurogenesis in the dentate gyrus of the adult tree shrew is regulated by psychosocial stress and NMDA receptor activation. J. Neurosci.17, 2492–2498 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hupfeld, K. E., McGregor, H. R., Reuter-Lorenz, P. A. & Seidler, R. D. Microgravity effects on the human brain and behavior: Dysfunction and adaptive plasticity. Neurosci. Biobehav Rev.122, 176–189 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roberts, D. R. et al. Effects of spaceflight on astronaut brain structure as indicated on MRI. N. Engl. J. Med.377, 1746–1753 (2017). [DOI] [PubMed] [Google Scholar]
- 13.Van Ombergen, A. et al. Brain ventricular volume changes induced by long-duration spaceflight. Proc. Natl. Acad. Sci.116, 10531–10536 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hupfeld, K. E. et al. The impact of 6 and 12 months in space on human brain structure and intracranial fluid shifts. Cereb. Cortex Commun.1, tgaa023 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kramer, L. A. et al. Intracranial effects of microgravity: a prospective longitudinal MRI study. Radiology295, 640–648 (2020). [DOI] [PubMed] [Google Scholar]
- 16.Roberts, D. et al. Prolonged microgravity affects human brain structure and function. Am. J. Neuroradiol.40, 1878–1885 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee, J. K. et al. Spaceflight-associated brain white matter microstructural changes and intracranial fluid redistribution. JAMA Neurol.76, 412–419 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alperin, N., Bagci, A. M. & Lee, S. H. Spaceflight-induced changes in white matter hyperintensity burden in astronauts. Neurology89, 2187–2191 (2017). [DOI] [PubMed] [Google Scholar]
- 19.Stahn, A. C. & Kühn, S. Extreme environments for understanding brain and cognition. Trends Cogn. Sci.26, 1–3 (2022). [DOI] [PubMed] [Google Scholar]
- 20.Palinkas, L. A. Effects of physical and social environments on the health and well-being of Antarctic winter-over personnel. Environ. Behav.23, 782–799 (1991). [Google Scholar]
- 21.Palinkas, L. A. & Suedfeld, P. Psychological effects of polar expeditions. Lancet371, 153–163 (2008). [DOI] [PubMed] [Google Scholar]
- 22.Stahn, A. C. et al. Brain changes in response to long Antarctic expeditions. N. Engl. J. Med.381, 2273–2275 (2019). [DOI] [PubMed] [Google Scholar]
- 23.Zivi, P., De Gennaro, L. & Ferlazzo, F. Sleep in isolated, confined, and extreme (ICE): A review on the different factors affecting human sleep in ICE. Front Neurosci.14, 551460 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mairesse, O. et al. Preparing for Mars: human sleep and performance during a 13 month stay in Antarctica. Sleep42, zsy206 (2019). [DOI] [PubMed] [Google Scholar]
- 25.Collet, G. et al. Altitude and seasonality impact on sleep in Antarctica. Aerosp. Med. Hum. Perform.86, 392–396 (2015). [DOI] [PubMed] [Google Scholar]
- 26.Tellez, H. F. et al. Exercise during short-term and long-term continuous exposure to hypoxia exacerbates sleep-related periodic breathing. Sleep39, 773–783 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kelly, S. et al. Widespread white matter microstructural differences in schizophrenia across 4322 individuals: results from the ENIGMA Schizophrenia DTI Working GroupI. Mol Psychiatry23, 1261–1269 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chang, Y.-K., Labban, J. D., Gapin, J. I. & Etnier, J. L. The effects of acute exercise on cognitive performance: a meta-analysis. Brain Res.1453, 87–101 (2012). [DOI] [PubMed] [Google Scholar]
- 29.Schneider, S. et al. Exercise as a countermeasure to psycho-physiological deconditioning during long-term confinement. Behav. Brain Res.211, 208–214 (2010). [DOI] [PubMed] [Google Scholar]
- 30.Schneider, M. L. & Kwan, B. M. Psychological need satisfaction, intrinsic motivation and affective response to exercise in adolescents. Psychol. Sport Exerc14, 776–785 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nasrini, J. et al. Cognitive performance during confinement and sleep restriction in NASA’s Human Exploration Research Analog (HERA). Front Physiol.11, 394 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Joëls, M. & Baram, T. Z. The neuro-symphony of stress. Nat. Rev. Neurosci.10, 459–466 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Diniz, C. R. A. F. & Crestani, A. P. The times they are a-changin’: a proposal on how brain flexibility goes beyond the obvious to include the concepts of “upward” and “downward” to neuroplasticity. Mol. Psychiatry28, 977–992 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brem, C. et al. Changes of brain DTI in healthy human subjects after 520 days isolation and confinement on a simulated mission to Mars. Life Sci. Space Res.24, 83–90 (2020). [DOI] [PubMed] [Google Scholar]
- 35.Oehrn, C. R. et al. Human hippocampal dynamics during response conflict. Curr. Biol.25, 2307–2313 (2015). [DOI] [PubMed] [Google Scholar]
- 36.Manohar, S. G., Pertzov, Y. & Husain, M. Short-term memory for spatial, sequential and duration information. Curr. Opin. Behav. Sci.17, 20–26 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Keun, J. T. B. et al. Structural assessment of thalamus morphology in brain disorders: a review and recommendation of thalamic nucleus segmentation and shape analysis. Neurosci. Biobehav Rev.131, 466–478 (2021). [DOI] [PubMed] [Google Scholar]
- 38.Ward, L. M. The thalamus: gateway to the mind. Wiley Interdiscip. Rev. Cogn. Sci.4, 609–622 (2013). [DOI] [PubMed] [Google Scholar]
- 39.Yoshii, T. The role of the thalamus in post-traumatic stress disorder. Int. J. Mol. Sci.22, 1730 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jillings, S. et al. Prolonged microgravity induces reversible and persistent changes on human cerebral connectivity. Commun. Biol.6, 46 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roberts, D. et al. Structural brain changes following long-term 6 head-down tilt bed rest as an analog for spaceflight. Am. J. Neuroradiol.36, 2048–2054 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hasan, K. M. et al. Brain quantitative MRI metrics in astronauts as a unique professional group. J. Neuroimaging28, 256–268 (2018). [DOI] [PubMed] [Google Scholar]
- 43.Stahn, A. C. & Kühn, S. Brains in space: the importance of understanding the impact of long-duration spaceflight on spatial cognition and its neural circuitry. Cogn. Process22, 105–114 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eastwood, J. D., Frischen, A., Fenske, M. J. & Smilek, D. The unengaged mind: Defining boredom in terms of attention. Perspect. Psychol. Sci.7, 482–495 (2012). [DOI] [PubMed] [Google Scholar]
- 45.Lawley, J. S. et al. Cerebral spinal fluid dynamics: effect of hypoxia and implications for high-altitude illness. J. Appl. Physiol.120, 251–262 (2016). [DOI] [PubMed] [Google Scholar]
- 46.Ross, R. The random nature of cerebral mountain sickness. Lancet325, 990–991 (1985). [DOI] [PubMed] [Google Scholar]
- 47.Resnick, S. M. et al. One-year age changes in MRI brain volumes in older adults. Cereb. Cortex10, 464–472 (2000). [DOI] [PubMed] [Google Scholar]
- 48.Croll, P. H. et al. Better diet quality relates to larger brain tissue volumes: The Rotterdam Study. Neurology90, e2166–e2173 (2018). [DOI] [PubMed] [Google Scholar]
- 49.Biller, A. et al. Responses of the human brain to mild dehydration and rehydration explored in vivo by 1H-MR imaging and spectroscopy. Am. J. Neuroradiol.36, 2277–2284 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Banks, S. & Dinges, D. F. Behavioral and physiological consequences of sleep restriction. J. Clin. Sleep. Med.3, 519–528 (2007). [PMC free article] [PubMed] [Google Scholar]
- 51.Chong, P. L., Garic, D., Shen, M. D., Lundgaard, I. & Schwichtenberg, A. J. Sleep, cerebrospinal fluid, and the glymphatic system: A systematic review. Sleep. Med. Rev.61, 101572 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Owen, J. E. & Veasey, S. C. Impact of sleep disturbances on neurodegeneration: Insight from studies in animal models. Neurobiol. Dis.139, 104820 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu, C., Kong, X. -z, Liu, X., Zhou, R. & Wu, B. Long-term total sleep deprivation reduces thalamic gray matter volume in healthy men. Neuroreport25, 320–323 (2014). [DOI] [PubMed] [Google Scholar]
- 54.Joo, E. Y., Kim, H., Suh, S. & Hong, S. B. Hippocampal substructural vulnerability to sleep disturbance and cognitive impairment in patients with chronic primary insomnia: magnetic resonance imaging morphometry. Sleep37, 1189–1198 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Barger, L. K. et al. Prevalence of sleep deficiency and use of hypnotic drugs in astronauts before, during, and after spaceflight: an observational study. Lancet Neurol.13, 904–912 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Panel, C. C. et al. Recommended amount of sleep for a healthy adult: a joint consensus statement of the American Academy of Sleep Medicine and Sleep Research Society. J. Clin. Sleep. Med.11, 591–592 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jones, C. W., Basner, M., Mollicone, D. J., Mott, C. M. & Dinges, D. F. Sleep deficiency in spaceflight is associated with degraded neurobehavioral functions and elevated stress in astronauts on six-month missions aboard the International Space Station. Sleep45, zsac006 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cotman, C. W., Berchtold, N. C. & Christie, L.-A. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci.30, 464–472 (2007). [DOI] [PubMed] [Google Scholar]
- 59.Hillman, C. H., Erickson, K. I. & Kramer, A. F. Be smart, exercise your heart: exercise effects on brain and cognition. Nat. Rev. Neurosci.9, 58–65 (2008). [DOI] [PubMed] [Google Scholar]
- 60.Marshall-Goebel, K., Damani, R. & Bershad, E. M. Brain physiological response and adaptation during spaceflight. Neurosurgery85, E815–E821 (2019). [DOI] [PubMed] [Google Scholar]
- 61.Oschwald, J. et al. Brain structure and cognitive ability in healthy aging: a review on longitudinal correlated change. Rev. Neurosci.31, 1–57 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Iglesias J. E., et al. Quantitative brain morphometry of portable low-field-strength MRI using super-resolution machine learning. Radiology. 2022:220522. [DOI] [PMC free article] [PubMed]
- 63.Jeste, D. V., Lee, E. E. & Cacioppo, S. Battling the modern behavioral epidemic of loneliness: suggestions for research and interventions. JAMA Psychiatry77, 553–554 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pai, N. & Vella, S.-L. The physical and mental health consequences of social isolation and loneliness in the context of COVID-19. Curr. Opin. Psychiatry35, 305–310 (2022). [DOI] [PubMed] [Google Scholar]
- 65.Lee, H., Yong, S. Y., Choi, H., Yoon, G. Y. & Koh, S. Association between loneliness and cognitive function, and brain volume in community-dwelling elderly. Front. Aging Neurosci.16, 1389476 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Van Ombergen, A., Rossiter, A. & Ngo-Anh, T. J. White Mars’–nearly two decades of biomedical research at the Antarctic Concordia station. Exp. Physiol.106, 6–17 (2021). [DOI] [PubMed] [Google Scholar]
- 67.Pagel, J. I. & Choukèr, A. Effects of isolation and confinement on humans-implications for manned space explorations. J. Appl. Physiol.120, 1449–1457 (2016). [DOI] [PubMed] [Google Scholar]
- 68.Reuter, M., Schmansky, N. J., Rosas, H. D. & Fischl, B. Within-subject template estimation for unbiased longitudinal image analysis. Neuroimage61, 1402–1418 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Reuter, M. & Fischl, B. Avoiding asymmetry-induced bias in longitudinal image processing. Neuroimage57, 19–21 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Reuter, M., Rosas, H. D. & Fischl, B. Highly accurate inverse consistent registration: a robust approach. Neuroimage53, 1181–1196 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rosen, A. F. et al. Quantitative assessment of structural image quality. Neuroimage169, 407–418 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Basner, M. et al. Development and validation of the cognition test battery for spaceflight. Aerosp. Med. Hum. Perform.86, 942–952 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gur, R. C. et al. A cognitive neuroscience-based computerized battery for efficient measurement of individual differences: standardization and initial construct validation. J. Neurosci. Methods187, 254–262 (2010). Mar 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Moore, T. M., Reise, S. P., Gur, R. E., Hakonarson, H. & Gur, R. C. Psychometric properties of the Penn Computerized Neurocognitive Battery. Neuropsychology29, 235–246 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gur, R. C. et al. Age group and sex differences in performance on a computerized neurocognitive battery in children age 8–21. Neuropsychology26, 251–265 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Moore, T. M. et al. Validation of the cognition test battery for spaceflight in a sample of highly educated adults. Aerosp. Med. Hum. Perform.88, 937–946 (2017). [DOI] [PubMed] [Google Scholar]
- 77.Ancoli-Israel, S. et al. The role of actigraphy in the study of sleep and circadian rhythms. Sleep26, 342–392 (2003). [DOI] [PubMed] [Google Scholar]
- 78.Basner, M., Rao, H., Goel, N. & Dinges, D. F. Sleep deprivation and neurobehavioral dynamics. Curr. Opin. Neurobiol.23, 854–863 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cole, R. J., Kripke, D. F., Gruen, W., Mullaney, D. J. & Gillin, J. C. Automatic sleep/wake identification from wrist activity. Sleep15, 461–469 (1992). [DOI] [PubMed] [Google Scholar]
- 80.Troiano, R. P. et al. Physical activity in the United States measured by accelerometer. Med Sci. Sports Exerc40, 181 (2008). [DOI] [PubMed] [Google Scholar]
- 81.Wickham, H., Chang, W. & Wickham, M. H. Package ‘ggplot2’. Creat. Elegant Data Visual. Using Gramm. Graph. Version2, 1–189 (2016). [Google Scholar]
- 82.Mowinckel, A. M. & Vidal-Piñeiro, D. Visualization of brain statistics with R packages ggseg and ggseg3d. Adv. Methods Pract. Psychol. Sci.3, 466–483 (2020). [Google Scholar]
- 83.Ben-Shachar et al. effectsize: Estimation of Effect Size Indices and Standardized Parameters. J. Open Source Softw.5, 2815 (2020).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusions of this manuscript can be requested by qualified researchers from NASA’s Life Science Data Archive (https://lsda.jsc.nasa.gov/).
Statistical code is available here: https://github.com/upenn/nasa_concordia.git.