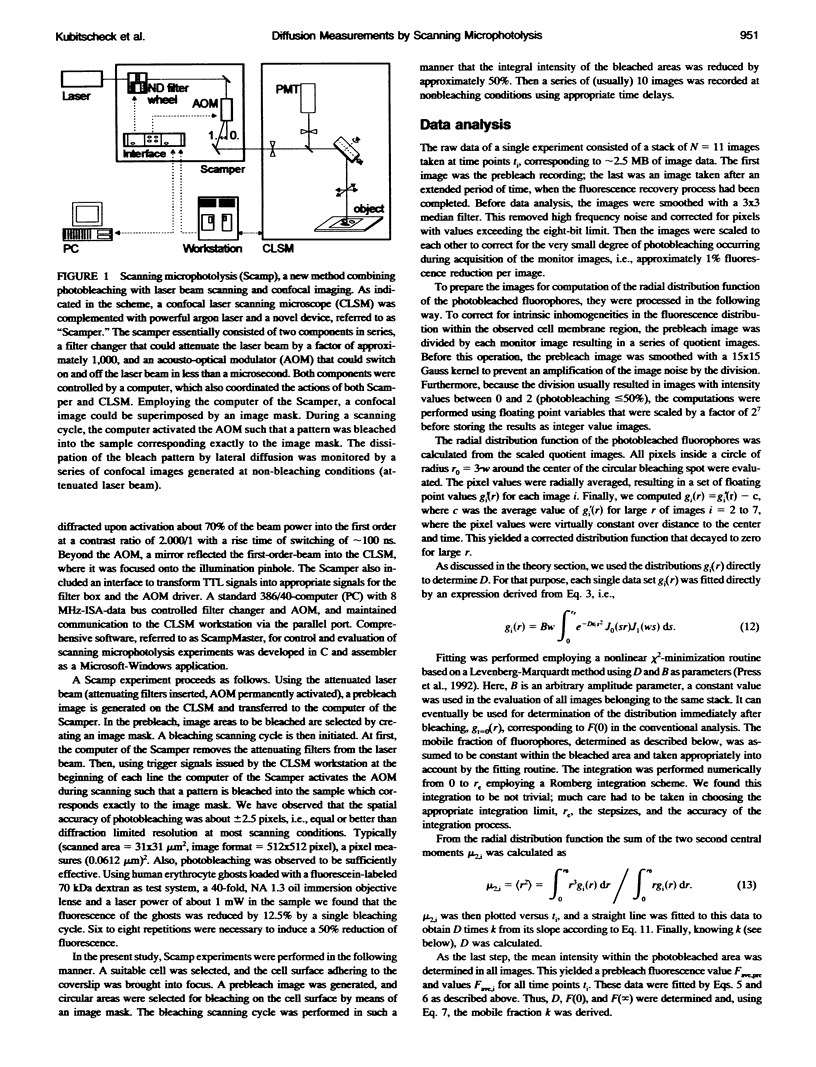
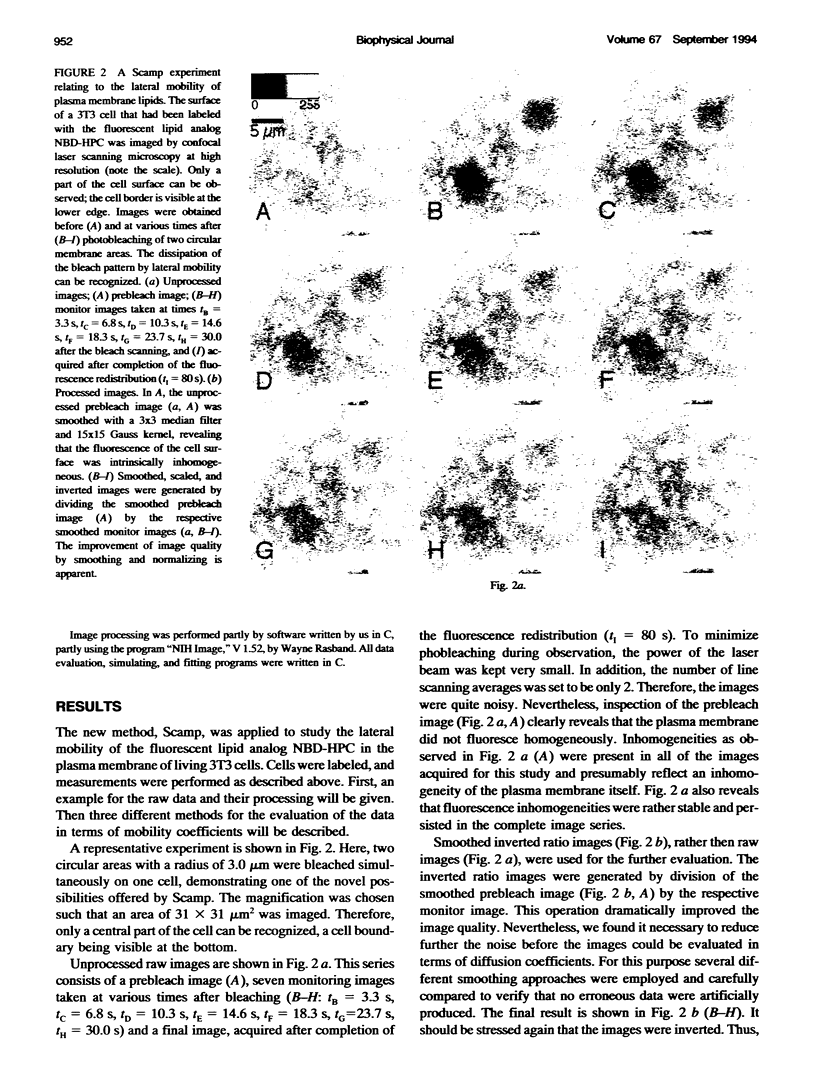
Abstract
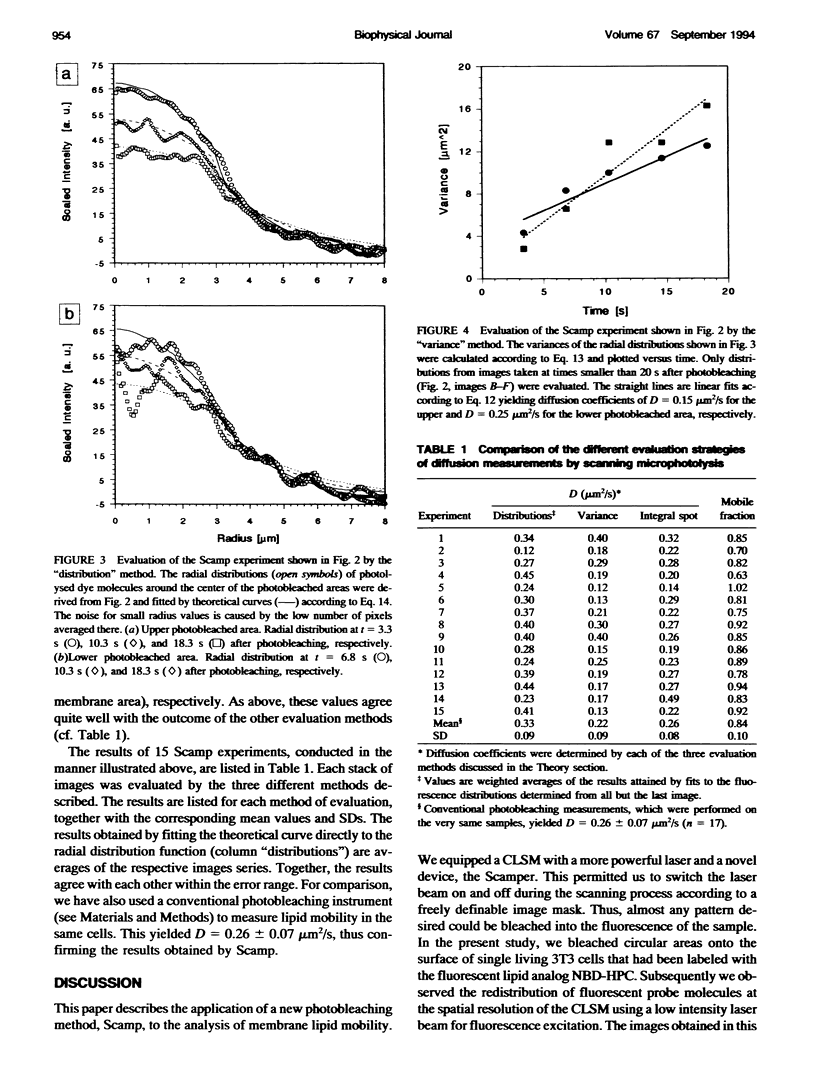
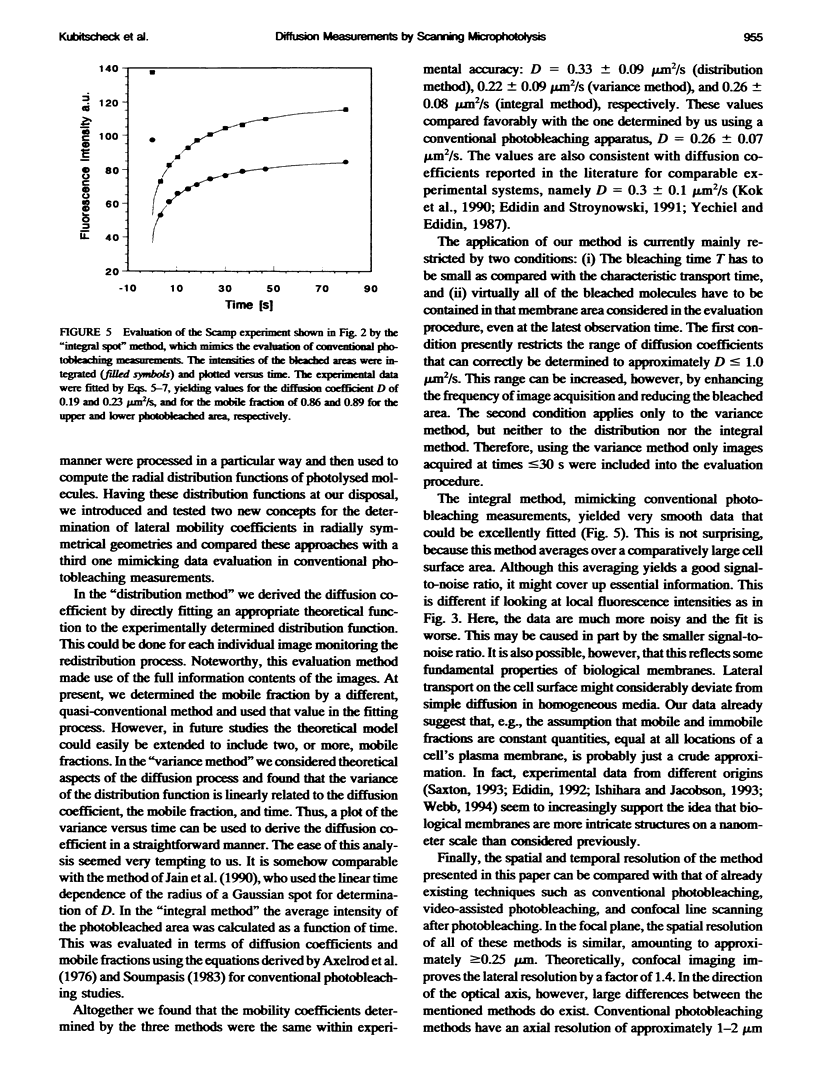
Fluorescence photobleaching methods have been widely used to study diffusion processes in the plasma membrane of single living cells and other membrane systems. Here we describe the application of a new photobleaching technique, scanning microphotolysis. Employing a recently developed extension module to a commercial confocal microscope, an intensive laser beam was switched on and off during scanning according to a user definable image mask. Thereby the location, geometry, and number of photolysed spots could be chosen arbitrarily, their size ranging from tens of micrometers down to the diffraction limit. Therewith we bleached circular areas on the surface of single living 3T3 cells labeled with the fluorescent lipid analog NBD-HPC. Subsequently, the fluorescence recovery process was observed using the attenuated laser beam for excitation. This yielded image stacks representing snapshots of the spatial distribution of fluorescent molecules. From these we computed the radial distribution functions of the photobleached dye molecules. The variance of these distributions is linearly related to the diffusion constant, time, and the mobile fraction of the diffusing species. Furthermore, we compared directly the theoretically expected and measured distribution functions, and could thus determine the diffusion coefficient from each single image. The results of these two new evaluation methods (D = 0.3 +/- 0.1 micron 2/s) agreed well with the outcome of conventional fluorescence recovery measurements. We show that by scanning microphotolysis information on dynamical processes such as diffusion of lipids or proteins can be acquired at the superior spatial resolution of a confocal laser scanning microscope.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cooper M. S., Cornell-Bell A. H., Chernjavsky A., Dani J. W., Smith S. J. Tubulovesicular processes emerge from trans-Golgi cisternae, extend along microtubules, and interlink adjacent trans-golgi elements into a reticulum. Cell. 1990 Apr 6;61(1):135–145. doi: 10.1016/0092-8674(90)90221-y. [DOI] [PubMed] [Google Scholar]
- Edidin M., Stroynowski I. Differences between the lateral organization of conventional and inositol phospholipid-anchored membrane proteins. A further definition of micrometer scale membrane domains. J Cell Biol. 1991 Mar;112(6):1143–1150. doi: 10.1083/jcb.112.6.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara A., Jacobson K. A closer look at how membrane proteins move. Biophys J. 1993 Nov;65(5):1754–1755. doi: 10.1016/S0006-3495(93)81231-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson K., Derzko Z., Wu E. S., Hou Y., Poste G. Measurement of the lateral mobility of cell surface components in single, living cells by fluorescence recovery after photobleaching. J Supramol Struct. 1976;5(4):565(417)–576(428). doi: 10.1002/jss.400050411. [DOI] [PubMed] [Google Scholar]
- Kapitza H. G., McGregor G., Jacobson K. A. Direct measurement of lateral transport in membranes by using time-resolved spatial photometry. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4122–4126. doi: 10.1073/pnas.82.12.4122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppel D. E. Fluorescence redistribution after photobleaching. A new multipoint analysis of membrane translational dynamics. Biophys J. 1979 Nov;28(2):281–291. doi: 10.1016/S0006-3495(79)85176-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusumi A., Sako Y., Yamamoto M. Confined lateral diffusion of membrane receptors as studied by single particle tracking (nanovid microscopy). Effects of calcium-induced differentiation in cultured epithelial cells. Biophys J. 1993 Nov;65(5):2021–2040. doi: 10.1016/S0006-3495(93)81253-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters R., Peters J., Tews K. H., Bähr W. A microfluorimetric study of translational diffusion in erythrocyte membranes. Biochim Biophys Acta. 1974 Nov 15;367(3):282–294. doi: 10.1016/0005-2736(74)90085-6. [DOI] [PubMed] [Google Scholar]
- Saxton M. J. Lateral diffusion in an archipelago. Single-particle diffusion. Biophys J. 1993 Jun;64(6):1766–1780. doi: 10.1016/S0006-3495(93)81548-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsay T. T., Jacobson K. A. Spatial Fourier analysis of video photobleaching measurements. Principles and optimization. Biophys J. 1991 Aug;60(2):360–368. doi: 10.1016/S0006-3495(91)82061-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willem J., ter Beest M., Scherphof G., Hoekstra D. A non-exchangeable fluorescent phospholipid analog as a membrane traffic marker of the endocytic pathway. Eur J Cell Biol. 1990 Oct;53(1):173–184. [PubMed] [Google Scholar]
- Yechiel E., Edidin M. Micrometer-scale domains in fibroblast plasma membranes. J Cell Biol. 1987 Aug;105(2):755–760. doi: 10.1083/jcb.105.2.755. [DOI] [PMC free article] [PubMed] [Google Scholar]