Abstract
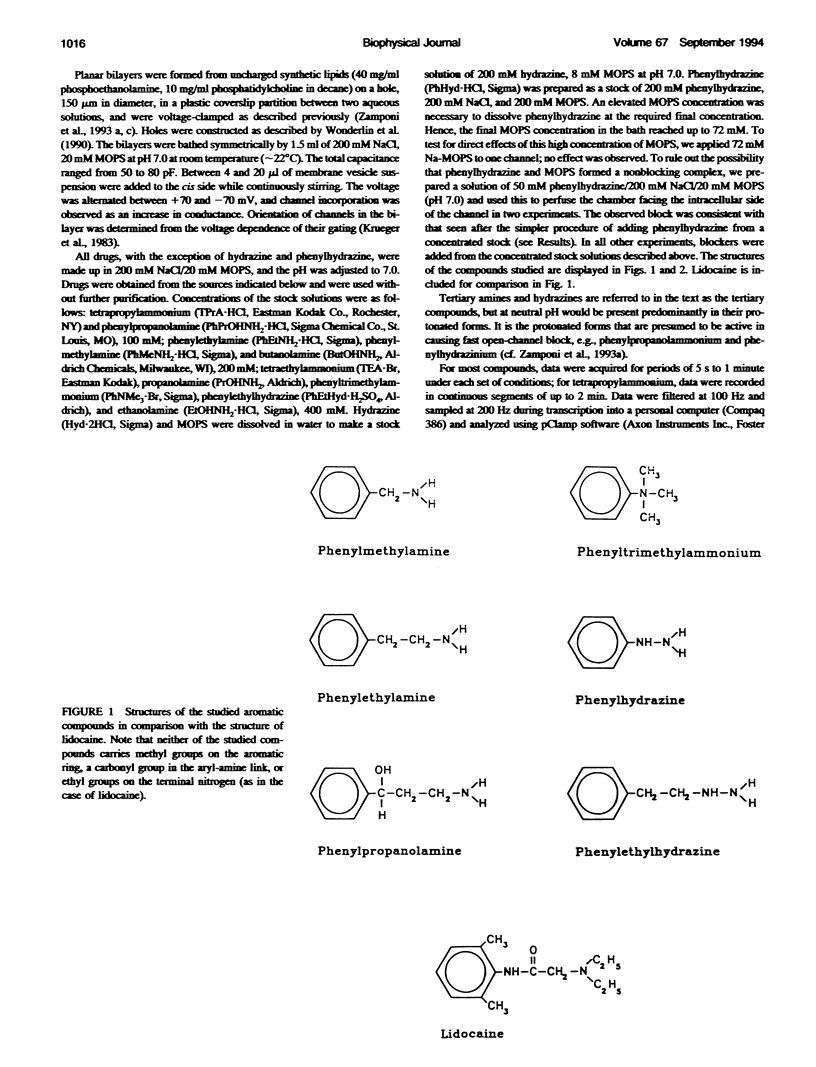
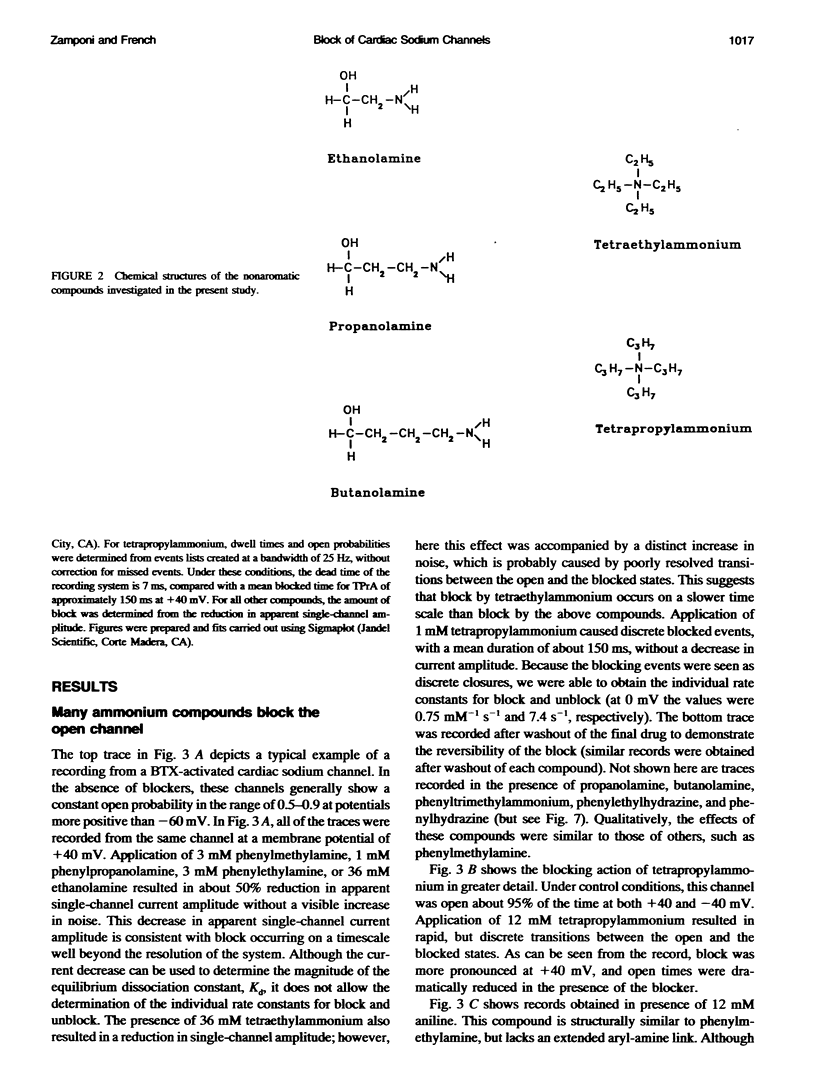
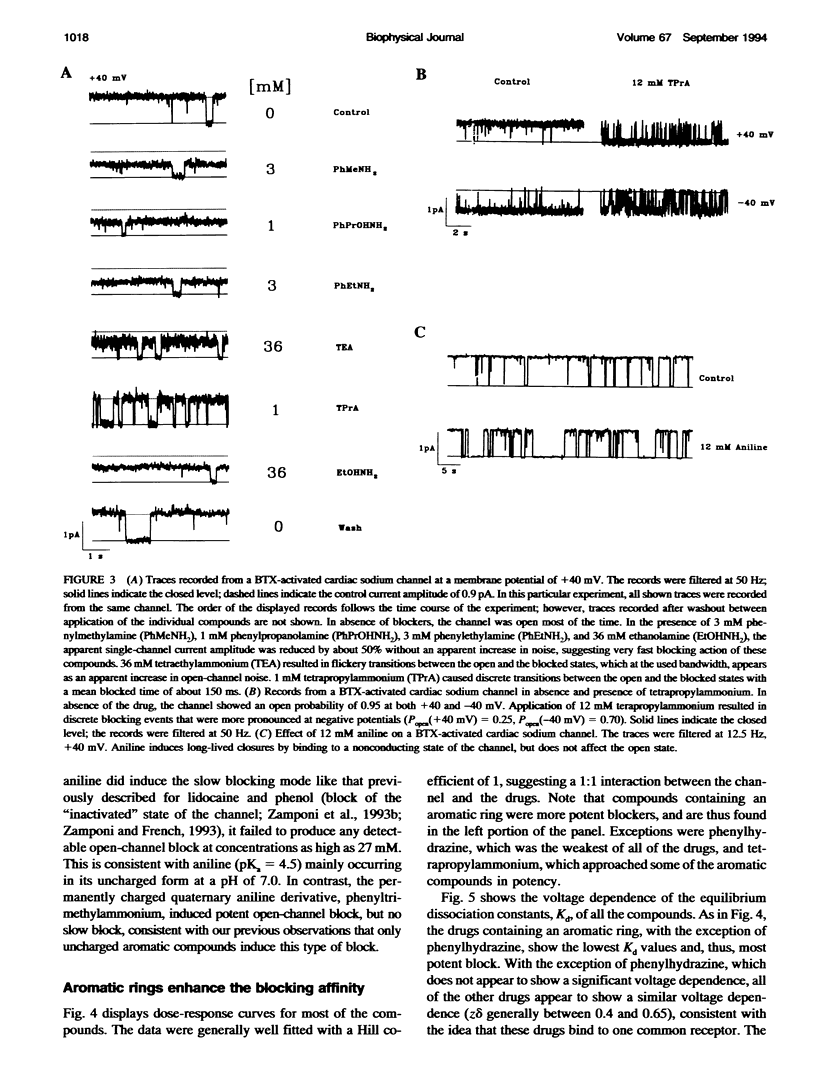
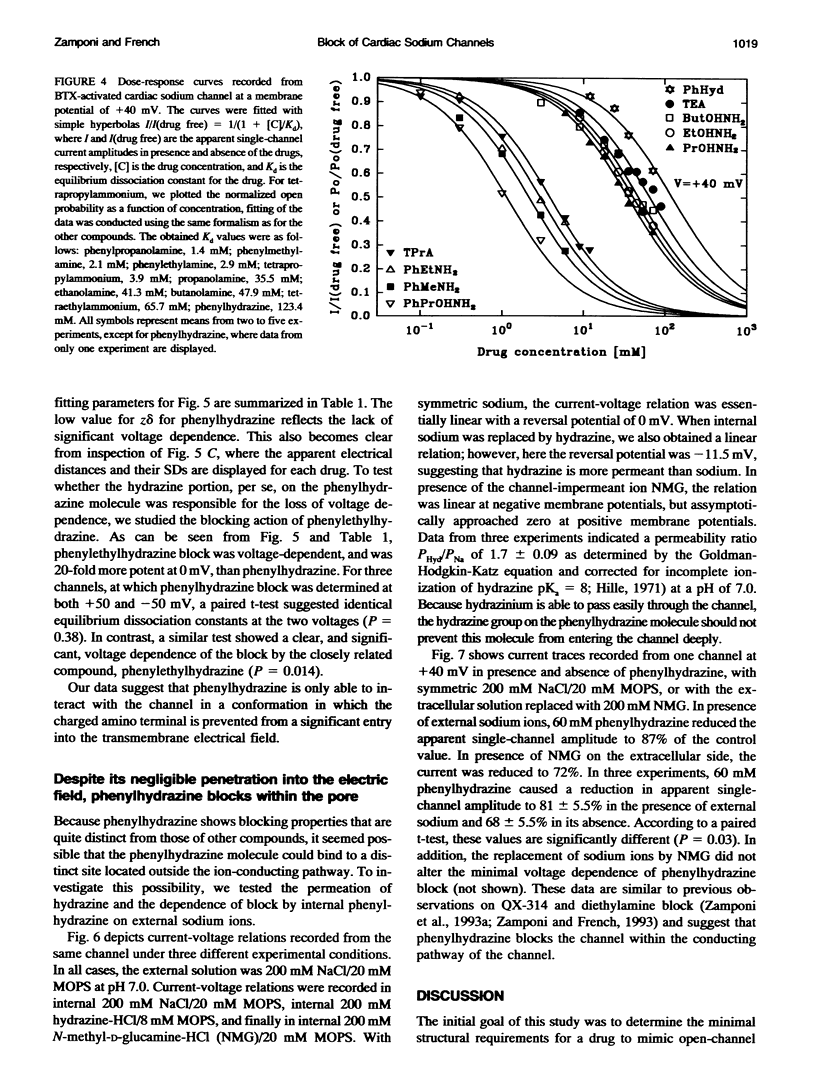
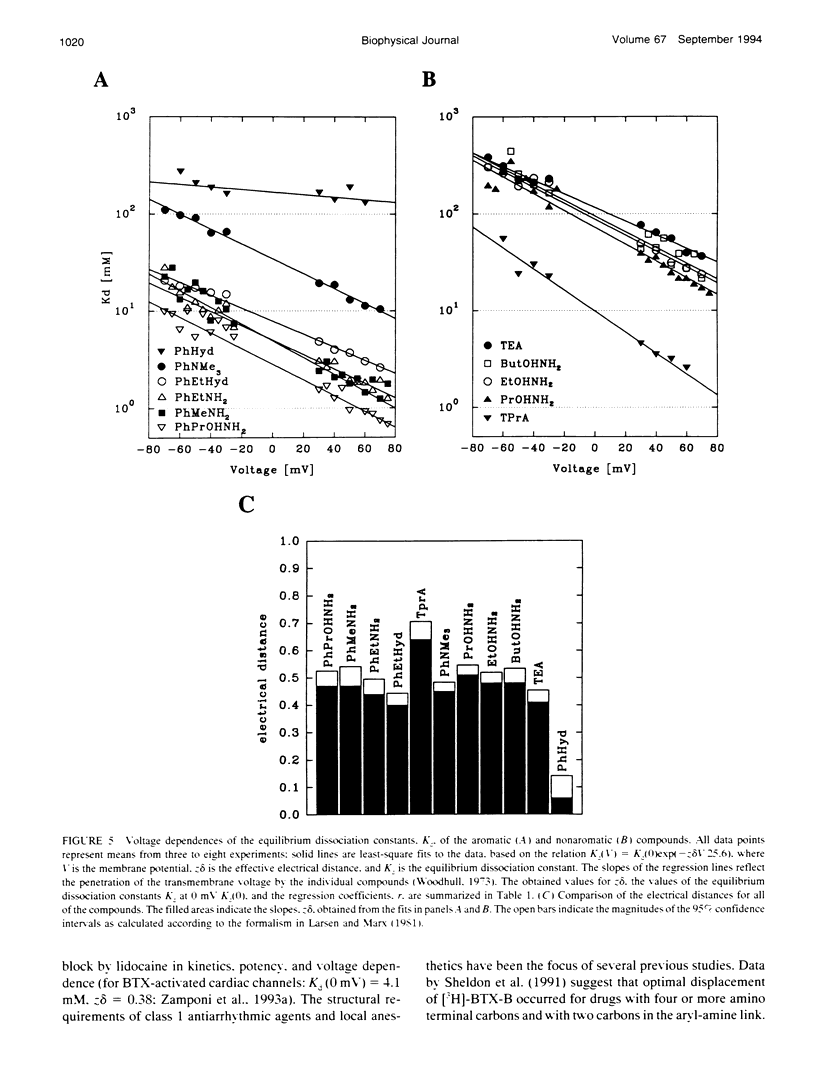
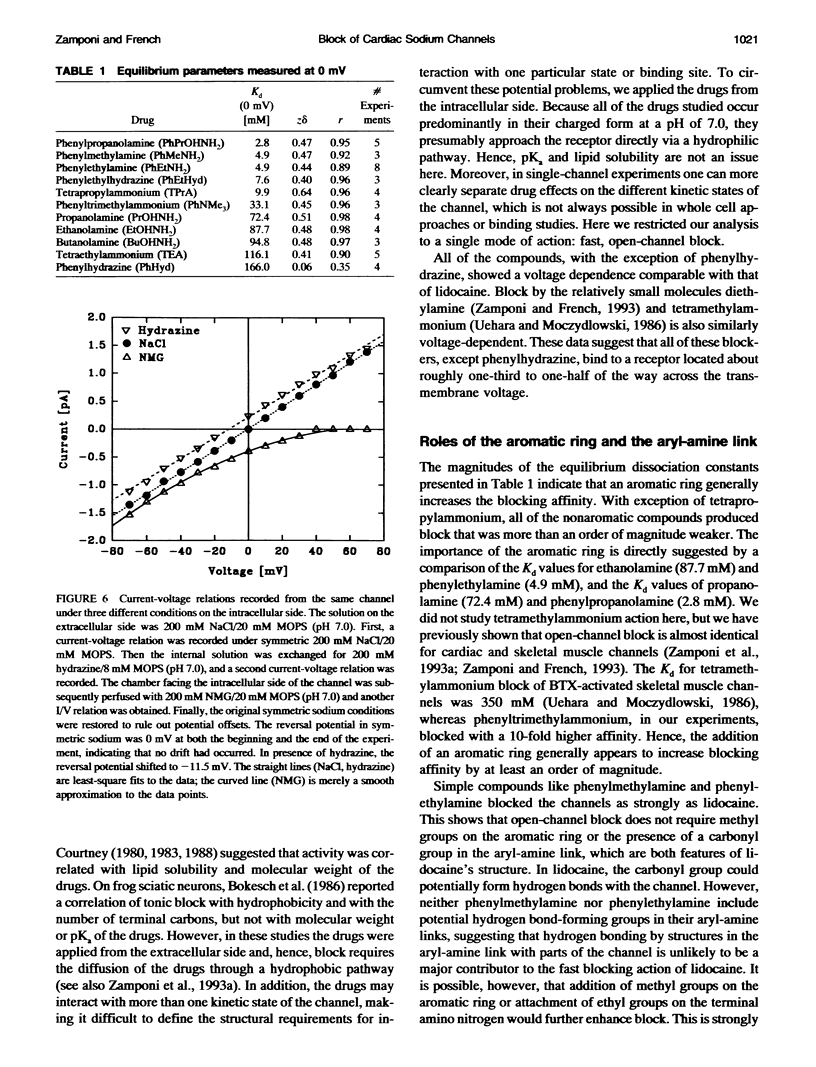
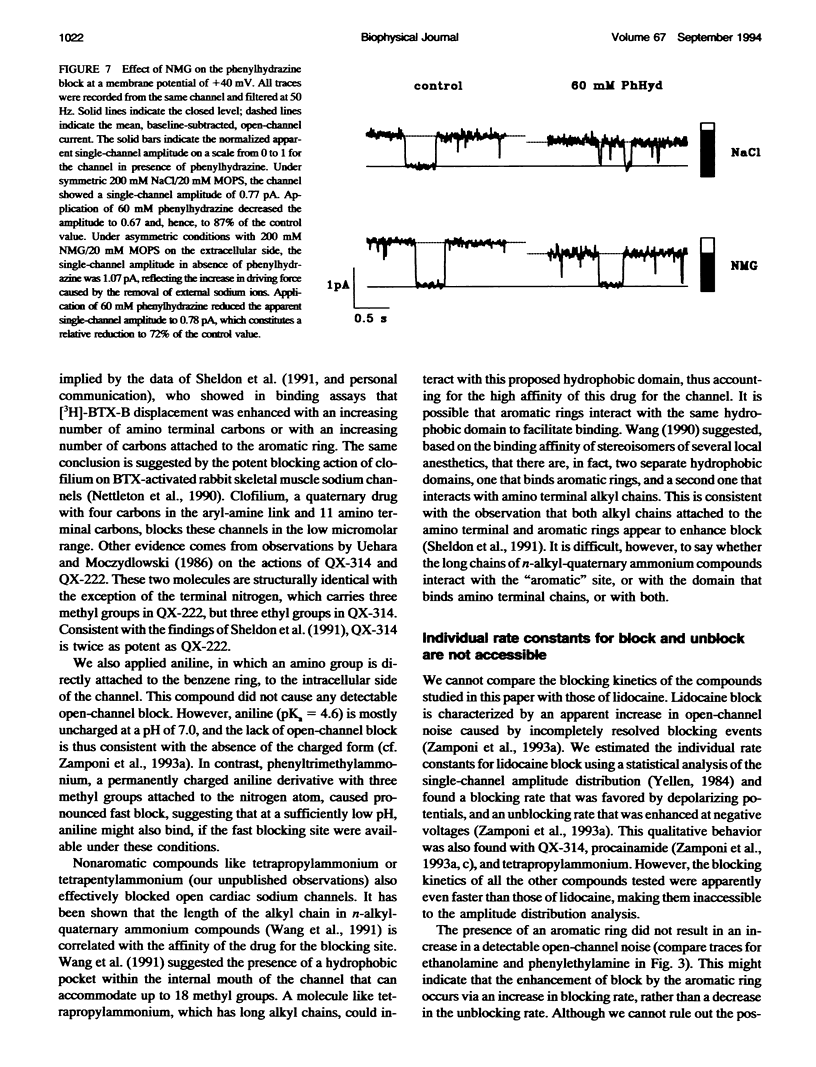
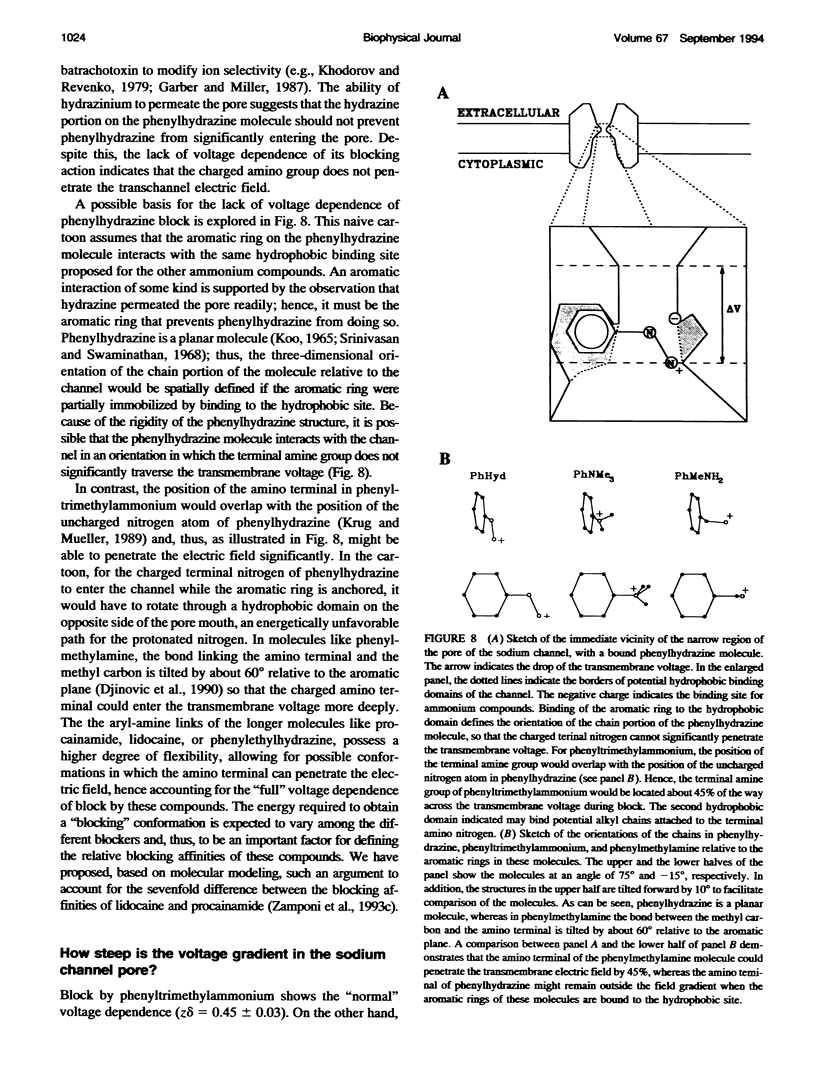
Many drugs block sodium channels from the cytoplasmic end (Moczydlowski, E., A. Uehara, X, Guo, and J. Heiny. 1986. Isochannels and blocking modes of voltage-dependent sodium channels. Ann. N.Y. Acad. Sci. 479:269-292.). Lidocaine, applied to either side of the membrane, induces two blocking modes, a rapid, voltage-dependent open-channel block, and a block of the inactivated channel that occurs on a 1000-fold slower timescale. Here we describe the actions of several lidocaine-related amines on batrachotoxin(BTX)-activated bovine cardiac sodium channels incorporated into planar lipid bilayers. We applied blocking amines from the intracellular side and examined the structural determinants of fast, open-channel block. Neither hydroxyl nor carbonyl groups, present in the aryl-amine link of lidocaine, were necessary, indicating that hydrogen bonding between structures in the aryl-amine link and the channel is not required. Block, however, was significantly enhanced by addition of an aromatic ring, or by the lengthening of aliphatic side chains, suggesting that a hydrophobic domain strengthens binding while the amine group blocks the pore. For most blockers, depolarizing potentials enhanced block, with the charged amine group apparently traversing 45-60% of the transmembrane voltage. By contrast, block by phenylhydrazine was essentially voltage-independent. The relatively rigid planar structure of phenylhydrazine may prevent the charged amino end from entering the electric field when the aromatic ring is bound. The relation between structural features of different blockers and their sensitivity to voltage suggests that the transmembrane voltage drops completely over less than 5 A. We raise the possibility that the proposed hydrophobic binding domain overlaps the endogenous receptor for the inactivation gate. If so, our data place limits on the distance between this receptor and the intrapore site at which charged amines bind.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bokesch P. M., Post C., Strichartz G. Structure-activity relationship of lidocaine homologs producing tonic and frequency-dependent impulse blockade in nerve. J Pharmacol Exp Ther. 1986 Jun;237(3):773–781. [PubMed] [Google Scholar]
- Correa A. M., Latorre R., Bezanilla F. Ion permeation in normal and batrachotoxin-modified Na+ channels in the squid giant axon. J Gen Physiol. 1991 Mar;97(3):605–625. doi: 10.1085/jgp.97.3.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney K. R. Interval-dependent effects of small antiarrhythmic drugs on excitability of guinea-pig myocardium. J Mol Cell Cardiol. 1980 Nov;12(11):1273–1286. doi: 10.1016/0022-2828(80)90071-1. [DOI] [PubMed] [Google Scholar]
- Courtney K. R. Quantifying antiarrhythmic drug blocking during action potentials in guinea-pig papillary muscle. J Mol Cell Cardiol. 1983 Nov;15(11):749–757. doi: 10.1016/0022-2828(83)90334-6. [DOI] [PubMed] [Google Scholar]
- Courtney K. R. Why do some drugs preferentially block open sodium channels? J Mol Cell Cardiol. 1988 Jun;20(6):461–464. doi: 10.1016/s0022-2828(88)80073-7. [DOI] [PubMed] [Google Scholar]
- Ehring G. R., Moyer J. W., Hondeghem L. M. Quantitative structure activity studies of antiarrhythmic properties in a series of lidocaine and procainamide derivatives. J Pharmacol Exp Ther. 1988 Feb;244(2):479–492. [PubMed] [Google Scholar]
- Garber S. S., Miller C. Single Na+ channels activated by veratridine and batrachotoxin. J Gen Physiol. 1987 Mar;89(3):459–480. doi: 10.1085/jgp.89.3.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman L. Internal cations, membrane current, and sodium inactivation gate closure in Myxicola giant axons. Biophys J. 1988 Dec;54(6):1027–1038. doi: 10.1016/S0006-3495(88)83040-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman L. Internal cesium and the sodium inactivation gate in Myxicola giant axons. Biophys J. 1986 Aug;50(2):231–238. doi: 10.1016/S0006-3495(86)83457-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn R., Patlak J., Stevens C. F. The effect of tetramethylammonium on single sodium channel currents. Biophys J. 1981 Nov;36(2):321–327. doi: 10.1016/S0006-3495(81)84734-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodorov B. I., Peganov E. M., Revenko S. V., Shishkova L. D. Sodium currents in voltage clamped nerve fiber of frog under the combined action of batrachotoxin and procaine. Brain Res. 1975 Feb 14;84(3):541–546. doi: 10.1016/0006-8993(75)90771-4. [DOI] [PubMed] [Google Scholar]
- Khodorov B. I., Revenko S. V. Further analysis of the mechanisms of action of batrachotoxin on the membrane of myelinated nerve. Neuroscience. 1979;4(9):1315–1330. doi: 10.1016/0306-4522(79)90159-3. [DOI] [PubMed] [Google Scholar]
- Krueger B. K., Worley J. F., 3rd, French R. J. Single sodium channels from rat brain incorporated into planar lipid bilayer membranes. Nature. 1983 May 12;303(5913):172–175. doi: 10.1038/303172a0. [DOI] [PubMed] [Google Scholar]
- Moczydlowski E., Hall S., Garber S. S., Strichartz G. S., Miller C. Voltage-dependent blockade of muscle Na+ channels by guanidinium toxins. J Gen Physiol. 1984 Nov;84(5):687–704. doi: 10.1085/jgp.84.5.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moczydlowski E., Uehara A., Guo X., Heiny J. Isochannels and blocking modes of voltage-dependent sodium channels. Ann N Y Acad Sci. 1986;479:269–292. doi: 10.1111/j.1749-6632.1986.tb15575.x. [DOI] [PubMed] [Google Scholar]
- Nettleton J., Castle N. A., Wang G. K. Block of single batrachotoxin-activated Na+ channels by clofilium. Mol Pharmacol. 1991 Mar;39(3):352–358. [PubMed] [Google Scholar]
- Oxford G. S., Yeh J. Z. Interactions of monovalent cations with sodium channels in squid axon. I. Modification of physiological inactivation gating. J Gen Physiol. 1985 Apr;85(4):583–602. doi: 10.1085/jgp.85.4.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauf C. L. Tetramethylammonium ions alter sodium-channel gating in Myxicola. Biophys J. 1983 Mar;41(3):269–274. doi: 10.1016/S0006-3495(83)84437-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider M. F., Dubois J. M. Effects of benzocaine on the kinetics of normal and batrachotoxin-modified Na channels in frog node of Ranvier. Biophys J. 1986 Sep;50(3):523–530. doi: 10.1016/S0006-3495(86)83490-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldon R. S., Hill R. J., Taouis M., Wilson L. M. Aminoalkyl structural requirements for interaction of lidocaine with the class I antiarrhythmic drug receptor on rat cardiac myocytes. Mol Pharmacol. 1991 May;39(5):609–614. [PubMed] [Google Scholar]
- Tanguy J., Yeh J. Z. Batrachotoxin uncouples gating charge immobilization from fast Na inactivation in squid giant axons. Biophys J. 1988 Oct;54(4):719–730. doi: 10.1016/S0006-3495(88)83007-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uehara A., Moczydlowski E. Blocking mechanisms of batrachotoxin-activated Na channels in artificial bilayers. Membr Biochem. 1986;6(2):111–147. doi: 10.3109/09687688609065446. [DOI] [PubMed] [Google Scholar]
- Villarroel A., Alvarez O., Oberhauser A., Latorre R. Probing a Ca2+-activated K+ channel with quaternary ammonium ions. Pflugers Arch. 1988 Dec;413(2):118–126. doi: 10.1007/BF00582521. [DOI] [PubMed] [Google Scholar]
- Wang G. K. Binding affinity and stereoselectivity of local anesthetics in single batrachotoxin-activated Na+ channels. J Gen Physiol. 1990 Nov;96(5):1105–1127. doi: 10.1085/jgp.96.5.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G. K., Simon R., Wang S. Y. Quaternary ammonium compounds as structural probes of single batrachotoxin-activated Na+ channels. J Gen Physiol. 1991 Nov;98(5):1005–1024. doi: 10.1085/jgp.98.5.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G. K., Wang S. Y. Altered stereoselectivity of cocaine and bupivacaine isomers in normal and batrachotoxin-modified Na+ channels. J Gen Physiol. 1992 Dec;100(6):1003–1020. doi: 10.1085/jgp.100.6.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West J. W., Patton D. E., Scheuer T., Wang Y., Goldin A. L., Catterall W. A. A cluster of hydrophobic amino acid residues required for fast Na(+)-channel inactivation. Proc Natl Acad Sci U S A. 1992 Nov 15;89(22):10910–10914. doi: 10.1073/pnas.89.22.10910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodhull A. M. Ionic blockage of sodium channels in nerve. J Gen Physiol. 1973 Jun;61(6):687–708. doi: 10.1085/jgp.61.6.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yellen G. Ionic permeation and blockade in Ca2+-activated K+ channels of bovine chromaffin cells. J Gen Physiol. 1984 Aug;84(2):157–186. doi: 10.1085/jgp.84.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue D. T., Lawrence J. H., Marban E. Two molecular transitions influence cardiac sodium channel gating. Science. 1989 Apr 21;244(4902):349–352. doi: 10.1126/science.2540529. [DOI] [PubMed] [Google Scholar]
- Zamponi G. W., Doyle D. D., French R. J. Fast lidocaine block of cardiac and skeletal muscle sodium channels: one site with two routes of access. Biophys J. 1993 Jul;65(1):80–90. doi: 10.1016/S0006-3495(93)81042-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamponi G. W., Doyle D. D., French R. J. State-dependent block underlies the tissue specificity of lidocaine action on batrachotoxin-activated cardiac sodium channels. Biophys J. 1993 Jul;65(1):91–100. doi: 10.1016/S0006-3495(93)81043-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamponi G. W., French R. J. Dissecting lidocaine action: diethylamide and phenol mimic separate modes of lidocaine block of sodium channels from heart and skeletal muscle. Biophys J. 1993 Dec;65(6):2335–2347. doi: 10.1016/S0006-3495(93)81292-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamponi G. W., French R. J. Transcainide causes two modes of open-channel block with different voltage sensitivities in batrachotoxin-activated sodium channels. Biophys J. 1994 Sep;67(3):1028–1039. doi: 10.1016/S0006-3495(94)80568-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamponi G. W., Sui X., Codding P. W., French R. J. Dual actions of procainamide on batrachotoxin-activated sodium channels: open channel block and prevention of inactivation. Biophys J. 1993 Dec;65(6):2324–2334. doi: 10.1016/S0006-3495(93)81291-8. [DOI] [PMC free article] [PubMed] [Google Scholar]


